Abstract
Background
Tear proteomic analysis has become an important tool in medical and veterinary research. The tear collection method could influence the tear protein profile. This study aims to evaluate the protein profiles of dog tears collected using microcapillary tubes (MT), Schirmer tear strips (ST), and ophthalmic sponges (OS).
Methods
The tear samples were collected using MT, ST, and OS. Tear protein profiles were analyzed using two-dimensional electrophoresis (2-DE) and the different protein spots’ expression was compared. Fourteen protein spots were identified using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Results
Tear protein concentrations ranged from 2.80 to 4.03 μg/μL, with no statistically significant differences among collection methods. Protein expression in each collection method differed in terms of both the number and intensity of the spots. There were 249, 327, and 330 protein spots found from tears collected with MT, ST, and OS, respectively. The proteins albumin, haptoglobin, and lactoferrin identified from OS were found to have higher spot intensities than other methods of collection. The use of MT demonstrated the downregulation of nine proteins.
Conclusions
The recent study supported that tear protein analysis is affected by different tear collection methods. Although ST is commonly used for tear collection, it provides insufficient information to study particular tear proteins.
Similar content being viewed by others
Background
Tear film, a thin liquid layer that completely covers the ocular surface, is the first barrier between the outer environment and the eyes. The main functions include moistening and lubricating the ocular surface, providing oxygen and electrolytes to the cornea, improving the refractive power of the eye, and protecting the eye from a potentially damaging environment [1]. Tears are generally classified into three layers. The outer lipid is crucial for the film’s stability and prevents the underlying aqueous layer from rapidly evaporating. The middle aqueous layer contains electrolytes, proteins, and small-molecule metabolites. The inner mucin layer interacts with the corneal epithelial surface. Tear mucus composed of water and mucin glycoproteins serves to maintain barrier function and wettability of the hydrophobic surface epithelial cell membranes, provide a matrix for lacrimal secreted factors, and minimize friction from blinking [2,3,4]. Many biologically active molecules have been found in tears [5]. The protein composition of the tear film can be altered by various local and systemic diseases [1]. Tear protein analysis can facilitate the identification of new biomarkers, a better understanding of disease etiology, and the development of new diagnostic tools and therapies [6]. In addition to its clinical utility, the identification of biomarkers in tear film may be useful in developing new pharmacologically active molecules [7]. Nowadays, tear protein analysis is used for diagnosis and following disease status in the medical and veterinary fields. In humans, tear protein biomarkers are used to determine dry eye syndrome [8], glaucoma [9], and Parkinson’s disease [10]. In veterinary medicine, tear protein biomarkers are used to determine dry eye syndrome [11], and various cancers including transmissible venereal tumor, mammary gland adenocarcinoma, and skin malignant melanoma [12].
For the tear protein analysis process, the most appropriate tear collection method is crucial in tear protein study. There are two tear sampling methods performed on animals, including direct sampling (microcapillary glass tubes) and indirect sampling (Schirmer tear strips and ophthalmic sponges) [6, 13,14,15]. It is evident that the various methods have different advantages and limitations [16]. Moreover, it is unclear what the ideal method of tear collection is for minimizing protein degradation and providing the important information for proteomic study. Schirmer’s tear test-1 or ST is the routine method for clinical ocular examination and dry eye diagnosis in dogs [3, 17]. ST is performed by inserting the folded end of the strip into the lower conjunctival fornix for 1 minute without the use of topical anesthetics [18]. Normal STT-1 values in dogs range from 15 to 25 mm/min [19]. In comparison to the other methods, ST has a lower coefficient of variability in tear protein content. It can provide the reliability and reproducibility of the data [16]. Despite the fact that ST has been considered convenient and easy, it can cause strong irritation of the conjunctiva, and trigger reflex tearing [20, 21]. The MT is the most commonly reported technique to collect tear film. This method is extensively described in humans, dogs, rabbits, mice, and rats. MT can be performed by placing a blunt capillary tube at the corner of the eye, within the tear lake, for around 5 minutes [1]. It is possible to obtain unaltered tear samples by avoiding reflex tearing from ocular irritation. Thus, the tear quality is similar to the basal tear. However, it takes a long time, produces a small volume, and is difficult to collect [6, 16]. The OS is available in different material types such as cellulose, polyvinyl acetal (PVA), polyester, and polyurethane [16]. For tear collection, OS is held against the lacrimal gland or placed beneath the lower eyelid for a given period of time [16]. The OS provides rapid absorbance and has a high absorptive capacity. Consequently, OS is suitable for further use as a blank tear in bioanalytical assays. However, this method is invasive, can promote reflex tearing, and might alter the composition of the tear film [15, 16].
Previous studies have shown differences in protein expression when using various protein analysis and collection methods [13, 17, 22], but no studies have investigated the difference in expression of healthy dog tears using 2-DE gel electrophoresis. The primary objective of this study was to evaluate the protein profiles of dog tears collected using three different tear collection methods: MT, ST, and OS.
Results
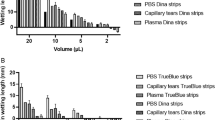
The mean (± SD) protein concentrations in tears collected with MT, ST, and OS were 2.87 ± 0.62, 4.03 ± 1.17, and 3.39 ± 0.69 μg/μL, respectively. There were no statistically significant differences found between the tear collection methods (P- value = 0.22) (Fig. 1).
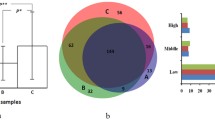
The 2-DE results demonstrated a similar pattern of tear protein profiles in each tear collection method, although the intensities of the protein spots were different. The gel image program detected 249, 327, and 330 protein spots from MT, ST, and OS, respectively. Statistically significant differences among 14 protein spots were candidates for protein annotations. For determining the changes of protein spots among different tear collection methods, ST was used as the base line spot intensity. The 12 protein spots were upregulated and two protein spots were downregulated in OS compared to ST. For the MT collection method, one protein spot was upregulated and nine protein spots were downregulated when compared to ST (Fig. 2). The LC-MS/MS results (Table 1) demonstrated that 11 proteins were identified from 14 candidate protein spots (Fig. 3).
The study demonstrated the downregulation of actin, cytoplasmic 1 (P-value = 0.005), albumin (P-value = 0.334), alpha fetoprotein (P-value = 0.634), haptoglobin (P-value = 0.334), induced myeloid leukemia cell differentiation protein Mcl-1 homolog (P-value = 0.334), keratin 75 (P-value = 0.00003), lactoferrin (P-value = 0.640), lipocalin 1 (P-value = 0.005), and stratifin (P-value = 0.024), when collecting the tear using MT compared to ST. When tears were collected using OS, proteins such as actin, cytoplasmic 1 (P-value = 0.006), albumin (P-value = 0.005), aldehyde dehydrogenase, dimeric NADP-preferring (P-value = 0.005), alpha fetoprotein (P-value = 0.000014), haptoglobin (P-value = 0.003), induced myeloid leukemia cell differentiation protein Mcl-1 homolog (P-value = 0.00004), lactoferrin (P-value = 0.022), NCCRP1, F-box associated domain containing (P-value = 0.013), and stratifin (P-value = 0.156), were upregulated.
The possible interactions and line thickness in Fig. 4 refer to the strength of data support. The protein-protein interaction analysis can observe that proteins of albumin, haptoglobin, alpha fetoprotein, lactotransferrin, actin, cytoplasmic 1, aldehyde dehydrogenase, dimeric NADP-preferring, induced myeloid leukemia cell differentiation protein Mcl-1 homolog, and stratifin have strong interaction relationships.
The pictures show protein-protein interactions and the statistical significance of the candidate proteins using STRING analysis. Alb = albumin, Hp = haptoglobin, Afp = alpha fetoprotein, Ltf = lactotransferrin, Actb = actin, cytoplasmic 1, Aldh3a1 = aldehyde dehydrogenase, dimeric NADP-preferring, Mcl1 = induced myeloid leukemia cell differentiation protein Mcl-1 homolog, and Sfn = stratifin
Discussion
Research for tear proteins in animals is valuable since it could lead to molecular biological investigation and the identification of a new biomarker for ocular and systemic diseases [6, 14, 23, 24]. Tear film is a body fluid widely used for proteomic studies because tears are non-invasively accessible. Due to the limited volume of tear film, pooled tear samples from several subjects can be used to overcome existing challenges [10, 16]. Nowadays, mass spectrometry allows for simultaneous accurate mass measures, in addition to the determination of the structural properties of molecules via tandem MS. Using chromatographic methods in conjunction with MS for protein identification provides the most complete view of a proteome distribution relative to isoelectric point (pI), molecular weight (MW), abundance, and interactions (i.e., protein–protein complex) [13]. Since the different tear collection methods affect proteomics in tear film, the results of proteomics studies using different tear collection methods are not directly comparable, and it is important to consider the potential impact of the collection method on protein concentration and expression [21, 25]. Green-Church (2008) examined the effect of the tear collection methods on the human tear proteome using a combination of one-dimensional (1-DE) and two-dimensional (2-DE) gel electrophoresis and mass spectrometry-based techniques. The result demonstrated that serum albumin expression from ST is greater than from MT [13]. Lamagna (2020) found the expression of aquaporin-1 from the OS collection was higher than from the ST in healthy dogs when using Western blot analysis [17]. Tear proteins have the potential as biomarkers for ocular and systemic diseases in humans and animals. For example, albumin and actin were used as biomarkers of cancers in dogs [12], and alpha fetoprotein was used as a tumor-specific biomarker (for example, in hepatocellular carcinoma) in humans [26], while lactoferrin and lysozyme were used as biomarkers of mucosal immune competence in humans [27], and lipocalin-1 was a candidate as a biomarker of dry eye syndrome [8], etc. Consequently, the findings of this study can be used to establish a proper tear collection method for any tear protein biomarkers in the future.
This was the first study to investigate and compare three different tear collection methods: microcapillary tube, Schirmer tear strip, and ophthalmic sponge. The findings of this study can be used to establish a proper tear collection method for future research on any interesting proteins. In this study, the ophthalmic sponges can absorb a larger volume of tears than other methods. Ophthalmic sponges exist in different material types, including polyvinyl alcohol and cellulose sponge materials [16]. Different material types display different properties of absorption. Polyvinyl alcohol sponge material (such as Merocel) with 100% open pores and no dead-end regions that retain residues is very absorbent and rapidly [28]. The natural cellulose sponge material, such as Weck-Cel sponges, is six times more absorbent than filter paper and maintains stiffness during the absorption process. Additionally, micropockets within Weck-Cel sponge materials could hold starch or sulfate residues [29]. Therefore, washing with varied solutions to eliminate the residues must occur when using these sponges. Some of the starch residue might be caught in the sponge’s polymer structure, where dissolving solutions cannot reach it. This makes removing the residues from the final sponge product difficult and may be utilized for cytokine separation from the sponge. Several cytokines and chemokines were found in tear samples collected with the Merocel sponge [28]. Additionally, the IgE value of tears recovered from capillaries was slightly lower than that of sponges. It is unclear whether these variations are due to differences in the adsorption of tear IgE or by evaporation from the larger surface area of the sponge during transportation [30]. Therefore, collecting tears with sponges that are greater in terms of absorption might reduce the fluid evaporation issue.
The tear samples from all dog groups that were collected with ST had the highest protein concentration, followed by the OS and MT. No statistically significant differences were found in each dog group and each of the tear collection methods. The low concentration of tear protein collected from MT is caused by small volume collection and less irritation to the ocular surface [16]. Additionally, while OS and ST were being used, some parts of this equipment were in contact with epithelial cells. Therefore, OS and ST retain ocular surface protein that may contaminate tear samples after the tear extraction process [1, 22, 31].
According to a recent study, the protein profiles from each tear collection method had a similar pattern but differed in terms of both the number and the intensity of the protein spots. Actin cytoplasmic 1, albumin, aldehyde dehydrogenase, dimeric NADP-preferring, alpha fetoprotein, haptoglobin, keratin 75, lactoferrin, lipocalin 1, and stratifin were among the 9 proteins previously found in the tear film [10, 20, 32]. We found some proteins that differed from different tear collection methods such as induced myeloid leukemia cell differentiation protein Mcl-1 homolog and NCCRP1, F-box associated domain containing NCCRP1, F-box associated domain containing.
Actin is the most abundant protein in the cytoplasm of animal cells. Its cellular functions range from organelle trafficking, regulation of cell shape, pathogen motility, cell migration, regulation of gene transcription, to the production of filaments that form cross-linked networks in the cytoplasm of cells [33,34,35]. Actin, cytoplasmic 1 was found in primary open angle glaucoma patients’ tears that could be involved in retina homeostasis [9]. Albumin has been detected in the tear film of humans [36], dogs [20], roadside hawks [37], and horses [38], etc. The main function of serum albumin is to bind to various substrates, including water, Ca2+, Na+, K+, fatty acids, hormones, bilirubin, and drugs. It can also limit the use of iron and the growth of bacteria [39]. Besides, inflammation of the ocular surface is associated with the presence of albumin in tear films. A previous study found that serum albumin in tear film increased significantly with each grade of conjunctivitis severity, with no differences between ST and MT [20]. De Freitas et al. (2008) found the increase in albumin levels in the tear film of dogs with cancer and identified them as the cancer biomarkers [12]. Furthermore, as previously described in dogs and other species, ocular disease disrupts the blood-tear barrier, allowing leakage of plasma compounds into the tear film. Aldehyde dehydrogenase, dimeric NADP-preferring (Aldh3a1) is a member of the ALDH superfamily of proteins that catalyze the NAD(P)-dependent oxidation of a wide range of endogenous and exogenous aldehydes [40]. Aldh3a1 constitutes the major fraction of the water-soluble protein in bovine and other mammalian corneas [41]. It has been reported to play a role in preventing ultraviolet light-induced corneal damage, which is consistent with the anti-apoptotic and cell growth-regulating roles of lens-crystallins [42]. The previous study revealed Aldh3a1 was significantly upregulated in pterygium and further increased in recurrent pterygium patients [40]. In addition, Aldh3a1 is highly expressed in non-small cell lung cancer (adenocarcinoma and squamous cell carcinoma) and in tobacco smokers versus nonsmokers [43]. Alpha fetoprotein is a serum glycoprotein with structural and physico-chemical properties similar to albumin. It is also present in small quantities in adults under normal conditions. Its biological role in embryonic and carcinogenesis remains obscure. The investigation of alpha fetoprotein for usage as a tumor-specific biomarker has been reported [44, 45], for example in hepatocellular carcinoma in humans [26]. Haptoglobin is an acute phase protein expressed through the activity of interleukin-6 [46]. A previous study found that haptoglobin in tear film increased with the severity of ocular lesions [10]. A higher serum haptoglobin level was found in calves infected with Eimeria Zuernii than in the control, which may be in response to an increased demand for haptoglobin in the repair process as a result of haemolysis. An inflammatory stimulus will upregulate the hepatic synthesis of haptoglobin [47]. Induced myeloid leukemia cell differentiation protein Mcl-1 homolog, also known as Bcl2-L-3, is encoded by MCL1. Bcl2-L-3 is an apoptosis-regulating protein in the B-cell lymphoma 2 family. It plays a key role in promoting cell survival [48,49,50]. The previous study demonstrated that Bcl2-L-3 prevented cell necrosis and has implications for the treatment of human hepatocellular carcinoma [51]. Keratin 75 is expressed primarily in hair follicles, nail beds, and lingual papillae, and was recently discovered in dental enamel. Keratins organize into heavily cross-linked networks of intermediate filaments, which provide mechanical rigidity to the cells and play important roles in cell-cell contacts as components of the desmosomal complexes [52]. The keratin proteins are usually present in the epidermal layer of skin and often not in the tear film in human. Therefore, the possible presence of keratin in tears could be caused by the habit of eye rubbing in people experiencing eye discomfort [53]. For tear lactoferrin, it was first reported by Masson in 1966 [54]. It is produced in the acinar cells of the lacrimal gland [55] and is present in the normal tears of mammals, including humans, cattle, bison, rabbits, dogs, cats, mice, koalas, and guinea pigs [56]. It has both anti-infective properties by suppressing bacterial growth by binding free iron, which is necessary for bacterial growth and preventing viral particles from entering cells, as well as anti-inflammatory properties by decreasing complement activation and scavenging free radicals [4]. Tear lipocalin-1 is one of the major tear proteins in humans [57]. Like lactoferrin, lipocalin is produced and secreted by the acinar cells [4]. It has many functions in tears, such as antimicrobial activity by binding to microbial siderophores, regulation of tear viscosity, anti-inflammatory activity, and endonuclease inactivation of viral DNA [3, 58]. Lipocalin-1 was a candidate as a biomarker of dry eye syndrome. Immune mediated dacryoadenitis is the most common etiology of dry eye syndrome. There is a progressive lymphocytic infiltration of the lacrimal gland that damages secretory tissues and decreases aqueous and tear protein (such as lipocalin-1) production [2, 16, 59]. NCCRP1, F-box associated domain containing is a type III membrane receptor protein that was isolated from the NCC of catfish and zebrafish. NCCRP-1 is a proline rich protein that has 2 glycosylation sites. Eighteen percent of the amino acids are serine, threonine, or tyrosine and function as potential phosphorylation sites [60]. NCCRP-1 plays a crucial role in the immune system by lysing tumor target cells, protozoan parasites, and virus-infected cells [61]. The 14–3-3 protein sigma, also known as stratifin, is particularly abundant in the stratified epithelium [62]. In addition, stratifin was reported in the tear film of humans and dogs. Stratifin has a crucial role in governing corneal epithelial cell differentiation and promotes cell cycle arrest when DNA is damaged [10, 63].
The current study demonstrated that albumin, Aldh3a1, alpha-fetoprotein, Bcl2-L-3, Hp, lactoferrin, and NCCRP1, F-box associated domain containing increase significantly in OS compared to ST and MT. Due to the size and hardness of the OS and ST, while using these methods, conjunctival injury may cause the serum proteins to leak into the tears through the blood-tear barrier [64]. Additionally, surface protein contamination could occur when using these methods [1, 22, 31]. Actin, cytoplasmic 1, keratin 75, lipocalin-1, and stratifin decrease significantly in MT compared to ST. Additionally, actin, cytoplasmic 1, and stratifin also decrease significantly in MT compared to OS. MT obtains a small volume of tear fluid and has an effect on the low concentration of tear components [16]. Thus, the protein concentration from MT could be lower than from other methods.
Conclusion
The recent study supported that tear protein analysis is affected by different tear collection methods. The expression of tear protein in OS is higher than in other methods. Although ST is commonly used for clinical ocular examination and tear collection, it provides insufficient information to study particular tear proteins. From this research, we obtained knowledge of tear protein analysis using different tear collection methods, and this is the first step towards future studies about tear protein biomarkers of ophthalmic and systemic diseases in dogs.
Methods
Animals
The study was approved by the Ethics Committee on Animal Experimentation of Kasetsart University (ACKU64-VTN-008). Tear samples were collected from 16 healthy dogs of either gender and any breed. The age of the dogs ranged from 1 to 10 years old (mean ± SD = 5.06 ± 2.69 years) and the body weights were 3.1–26.4 kg (mean ± SD = 10.85 ± 6.41 kg). All dogs underwent physical and ocular examination by the veterinarian before tear collection. They were obliged to have normal physical examinations, including body temperature, heart rate, respiratory rate, pulse rate, capillary refill time, lung sounds, and lymph nodes palpation. Ocular examinations included ocular reflex (dazzle reflex, pupillary light reflex, and menace response), Schirmer tear test-1, measures of intraocular pressure, fluorescein staining, and portable slit lamp examination. The results of visual testing, ocular position, and all examinations were normal. The exclusion criteria included the presence of ocular or systemic diseases, recent ocular surgery, and receiving other than general maintenance medications. Informed consent was obtained from all owners before tear collection.
Sample collection
Three different methods of tear collection were performed. No external stimulation and local anesthesia were required while collecting the tears from all tear collection methods. The MT was placed in contact with the inferior lacrimal lake until the tear was full in this microcapillary tube, in generally 5–10 minutes. The ST, made of Whatman paper no.4 (5-mm width × 35-mm length), was inserted into the ventrolateral conjunctival fornix until the wetness was 30 mm, in generally 1–3 minutes. The OS were modified from PVA ophthalmic surgical sponges. The sponge was inserted in the inferior lacrimal lake or beneath the lower eyelid for 1–3 minutes. The tear samples from each method were put into a sterile microtube and kept at − 80 °C until tear extraction. The dogs’ tears were obtained using a MT for the first method. ST and OS were performed for the second and third methods, respectively. Each method was carried out on a separate day.
Tear protein extraction and concentration measurement
Each wetted ST and OS was transferred to 0.2 mL tubes, which were manually punctured at the bottom with an 18-gauge needle. Each tube was placed in a 1.5 mL microtube and centrifuged at 3884×g for 3 minutes at 4 °C. The tear samples were immediately collected at − 80 °C for further analysis. The tears were pooled from four dogs in each group. Each tear pool was incubated in an ultrasonic bath for 30 minutes at 4 °C. The protein concentration was measured using the Bradford assay at a 595 nm wavelength. Bovine serum albumin (BSA) was used as a standard solution.
2-DE
Amounts of 150 μg of pooled tear protein from each group were separated by isoelectric focusing (IEF) for the first dimension. Immobilized dry strips 7 cm in length, pH gradient 3–10 (Cytiva, USA) were used in this study. The first dimensional separation was performed by Ettan IPGphor II (GE Healthcare) using a focusing profile that increased the voltage to 12,000 Vhrs at 20 °C. Then the strips were equilibrated using an equilibration buffer containing 10 mg/mL dithiothreitol (DTT) for 30 minutes, followed by an equilibration buffer containing 25 mg/mL iodoacetamide (IAA) for 30 minutes. Then, 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed on a mini VE vertical electrophoresis system (GE Healthcare) for the second dimension with a constant voltage of 140 V for 2 hrs 10 minutes at room temperature. The gels were stained with Coomassie blue G250 (CBG) with agitation overnight at room temperature whereupon several changes of MilliQ water were used to de-satin the gels. The gels were captured by ImageScanner II (GE Healthcare) and differential protein spots from triplicate gels were detected using Image Master 2-D Platinum version 7.0 (GE Healthcare). The different protein spots’ expression was compared by ANOVA. The 14 protein spots that showed statistically significant differences were submitted for protein identification using LC-MS/MS.
In-gel digestion, LC-MS/MS analysis and database searching
The candidate protein spots were manually excised and then subjected to in-gel digestion and mass spectrometry. Protein spots were in-gel tryptic digested and dehydrated with 100% acetonitrile (ACN). Protein was digested with trypsin solution (10 ng/μL trypsin containing 50% ACN in 25 mM ammonium bicarbonate) for 18 hrs at 37 °C. Digested peptides were extracted with 0.1% formic acid (FA) in 50% ACN and lyophilized. The digested peptides were dissolved in 0.1% FA and then subjected to mass spectrometry analysis for protein identification. The LC-MS/MS systems were operated using Thermo Scientific Dionex Ultimate 3000 RSLCnano System with a captive spray ionization hybrid to Compact™ quadropole time-of-flight (Q-ToF) (Bruker Daltonik, Bremen, Germany). The LC separation was performed in a reversed-phase column of (Acclaim PepMap RSLC Column C18 NanoViper, 75 μm × 150 mm, particle size 2 μm) and protected by a guard column (C18 PepMap100, 300 μm × 5 mm, particle size 5 μm). The mobile phase composed of Solution A (0.1% FA in deionized water) and Solution B (80% ACN in deionized water). Elution of the peptides was separated at a flow rate of 0.3 μL/min under gradient conditions of 2 to 85% B for 50 minutes. The tandem mass spectrometry spectra were generated by Bruker qTOF Control Software. The files were converted to MGF files using Compass Data Analysis version 4.1 (Bruker Daltonik, Bremen, Germany) and searched using Mascot Server (Matrix Science, https://www.matrixscience.com) using the NCBInr database. The parameters for the Mascot search were peptide mass tolerance of 1 kDa; MS/MS ion mass tolerance of 1 Da; maximally one missed cleavage; and tryptic digestion. Only matched proteins with significance scores (P-value < 0.05) were reported.
Protein-protein interaction network analysis
The protein-protein interaction analysis was performed using the STRING database version 11.5 (https://string-db.org). An interaction network analysis was created with proteins of the Mus musculus species to identify the proteins with a medium confidence score of 0.4 for interactions.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 2-DE:
-
Two-dimensional electrophoresis
- ACN:
-
Acetonitrile
- BSA:
-
Bovine serum albumin
- CBG:
-
Coomassie blue G250
- DTT:
-
Dithiothreitol
- FA:
-
Formic acid
- IAA:
-
Iodoacetamide
- IEF:
-
Isoelectric focusing
- LC-MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- MT:
-
Microcapillary tube
- OS:
-
Ophthalmic sponge
- ST:
-
Schirmer tear strip
References
You J, Willcox MD, Madigan MC, Wasinger V, Schiller B, Walsh BJ, et al. Tear fluid protein biomarkers. In: Advances in clinical chemistry, vol. 62: Elsevier; 2013. p. 151–96.
Guidoboni G, Harris A, Sacco R, editors. Ocular fluid dynamics: anatomy, physiology, imaging techniques, and mathematical modeling: Springer Nature; 2019.
Zhou L, Beuerman RW. Tear analysis in ocular surface diseases. Prog Retin Eye Res. 2012;31(6):527–50.
Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res. 2020;197:108115.
Dilly PN. Structure and function of the tear film. Lacrimal gland, tear film, and dry eye syndromes; 1994. p. 239–47.
Sebbag L, McDowell EM, Hepner PM, Mochel JP. Effect of tear collection on lacrimal total protein content in dogs and cats: a comparison between Schirmer strips and ophthalmic sponges. BMC Vet Res. 2018;14(1):1–7.
Winiarczyk M, Winiarczyk D, Banach T, Adaszek L, Madany J, Mackiewicz J, et al. Dog tear film proteome in-depth analysis. PLoS One. 2015;10(12):e0144242.
Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, Yang H, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8(11):4889–905.
Pieragostino D, Agnifili L, Fasanella V, D'Aguanno S, Mastropasqua R, Di Ilio C, et al. Shotgun proteomics reveals specific modulated protein patterns in tears of patients with primary open angle glaucoma naive to therapy. Mol Biosyst. 2013;9(6):1108–16.
Miller I, Schlosser S, Palazzolo L, Veronesi MC, Eberini I, Gianazza E. Some more about dogs: proteomics of neglected biological fluids. J Proteomics. 2020;218:103724.
Disney JL. Tear lacritin concentrations in canine keratoconjunctivitis sicca (Doctoral dissertation: Virginia Tech.
de Freitas Campos C, Cole N, Van Dyk D, Walsh BJ, Diakos P, Almeida D, et al. Proteomic analysis of dog tears for potential cancer markers. Res Vet Sci. 2008;85(2):349–52.
Green-Church KB, Nichols KK, Kleinholz NM, Zhang L, Nichols JJ. Investigation of the human tear film proteome using multiple proteomic approaches. Mol Vis. 2008;14:456.
Posa A, Bräuer L, Schicht M, Garreis F, Beileke S, Paulsen F. Schirmer strip vs. capillary tube method: non-invasive methods of obtaining proteins from tear fluid. Ann Anat. 2013;195(2):137–42.
Sebbag L, Showman L, McDowell EM, Perera A, Mochel JP. Impact of flow rate, collection devices, and extraction methods on tear concentrations following oral administration of doxycycline in dogs and cats. J Ocul Pharmacol Ther. 2018;34(6):452–9.
Sebbag L, Mochel JP. An eye on the dog as the scientist's best friend for translational research in ophthalmology: focus on the ocular surface. Med Res Rev. 2020;40(6):2566–604.
Lamagna B, Ciaramella P, Lamagna F, Di Loria A, Brunetti A, Pelagalli A. Aquaporin 1 (AQP1) expression in healthy dog tears. Animals. 2020;10(5):820.
Gelatt KN, editor. Essentials of veterinary ophthalmology: John Wiley & Sons; 2013.
Chandler JA, van der Woerdt A, Prittie JE, Chang L. Preliminary evaluation of tear production in dogs hospitalized in an intensive care unit. J Vet Emerg Crit Care. 2013;23(3):274–9.
Page L, Allbaugh RA, Mochel JP, Peraza J, Bertram M, Sebbag L. Impact of diurnal variation, sex, tear collection method, and disease state on tear protein levels in dogs. Vet Ophthalmol. 2020;23(6):994–1000.
Ma JY, Sze YH, Bian JF, Lam TC. Critical role of mass spectrometry proteomics in tear biomarker discovery for multifactorial ocular diseases. Int J Mol Med. 2021;47(5):1–5.
Nättinen J, Aapola U, Jylhä A, Vaajanen A, Uusitalo H. Comparison of capillary and Schirmer strip tear fluid sampling methods using SWATH-MS proteomics approach. Transl Vis Sci Technol. 2020;9(3):16.
Balasubramanian SA, Mohan S, Pye DC, Willcox MD. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012;90(4):e303–9.
Sebbag L, Harrington DM, Mochel JP. Tear fluid collection in dogs and cats using ophthalmic sponges. Vet Ophthalmol. 2018;21(3):249–54.
Neagu AN, Jayathirtha M, Baxter E, Donnelly M, Petre BA, Darie CC. Applications of tandem mass spectrometry (MS/MS) in protein analysis for biomedical research. Molecules. 2022;27(8):2411.
Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214–29.
Hanstock HG, Edwards JP, Walsh NP. Tear lactoferrin and lysozyme as clinically relevant biomarkers of mucosal immune competence. Front Immunol. 2019;10:1178.
Inic-Kanada A, Nussbaumer A, Montanaro J, Belij S, Schlacher S, Stein E, et al. Comparison of ophthalmic sponges and extraction buffers for quantifying cytokine profiles in tears using Luminex technology. Mol Vis. 2012;18:2717.
van Agtmaal EJ, van Haeringen NJ, Bloem MW, Schreurs WH, Saowakontha S. Recovery of protein from tear fluid stored in cellulose sponges. Curr Eye Res. 1987;6(4):585–8.
Tuft SJ, Dart JK. The measurement of IgE in tear fluid: a comparison of collection by sponge or capillary. Acta Ophthalmol. 1989;67(3):301–5.
Farias E, Yasunaga KL, Peixoto RV, Fonseca MP, Fontes W, Galera PD. Comparison of two methods of tear sampling for protein quantification by Bradford method. Pesqui Vet Bras. 2013;33:261–4.
Kaswan RL, Fullard RJ. Components in normal dog tears and tears from dogs with KCS treated. In: Sjögren’s syndrome: state of the art: proceedings of the fourth international symposium, Tokyo, Japan, august 11-13-1993: Demos Medical Publishing; 1994. p. 265.
Drazic A, Aksnes H, Marie M, Boczkowska M, Varland S, Timmerman E, et al. NAA80 is actin’s N-terminal acetyltransferase and regulates cytoskeleton assembly and cell motility. Proc Natl Acad Sci. 2018;115(17):4399–404.
Cuvertino S, Stuart HM, Chandler KE, Roberts NA, Armstrong R, Bernardini L, et al. ACTB loss-of-function mutations result in a pleiotropic developmental disorder. Am J Hum Genet. 2017;101(6):1021–33.
Colin A, Singaravelu P, Théry M, Blanchoin L, Gueroui Z. Actin-network architecture regulates microtubule dynamics. Curr Biol. 2018;28(16):2647–56.
Dor M, Eperon S, Lalive PH, Guex-Crosier Y, Hamedani M, Salvisberg C, et al. Investigation of the global protein content from healthy human tears. Exp Eye Res. 2019;179:64–74.
Raposo AC, Lebrilla CB, Portela RW, Goonatilleke E, Neto FA, Oriá AP. The proteomics of roadside hawk (Rupornis magnirostris), broad-snouted caiman (caiman latirostris) and loggerhead sea turtle (Caretta caretta) tears. BMC Vet Res. 2020;16(1):1–2.
Terhaar HM, Allbaugh RA, Mochel JP, Sebbag L. Serum albumin and total protein concentration in the tear film of horses with healthy or diseased eyes. Vet Ophthalmol. 2021;24(1):20–7.
Veloso JF, Brandão Guedes PE, Lacerda LC, Santana JO, Mora-Ocampo IY, Pirovani CP, et al. Tear film proteome of healthy domestic cats. Vet Med Int. 2021;2021.
Kim SW, Lee J, Lee B, Rhim T. Proteomic analysis in pterygium; upregulated protein expression of ALDH3A1, PDIA3, and PRDX2. Mol Vis. 2014;20:1192.
Nees DW, Wawrousek EF, Robison WG Jr, Piatigorsky J. Structurally normal corneas in aldehyde dehydrogenase 3a1-deficient mice. Mol Cell Biol. 2002;22(3):849–55.
Chen HY, Chou HC, Chang SJ, Liao EC, Tsai YT, Wei YS, et al. Proteomic Analysis of Various Rat Ocular Tissues after Ischemia–Reperfusion Injury and Possible Relevance to Acute Glaucoma. Int J Mol Sci. 2017;18(2):334.
Rossi A, Voigtlaender M, Klose H, Schlüter H, Schön G, Loges S, et al. High aldehyde dehydrogenase levels are detectable in the serum of patients with lung cancer and may be exploited as screening biomarkers. J Oncol. 2019;2019.
Terentiev AA, Moldogazieva NT. Alpha-fetoprotein: a renaissance. Tumor Biol. 2013;34(4):2075–91.
Rizzo A, Galgano M, Mutinati M, Sciorsci RL. Alpha-fetoprotein in animal reproduction. Res Vet Sci. 2019;123:281–5.
Quaye IK. Haptoglobin, inflammation and disease. Trans R Soc Trop Med Hyg. 2008;102(8):735–42.
Lassen B, Bangoura B, Lepik T, Orro T. Systemic acute phase proteins response in calves experimentally infected with Eimeria zuernii. Vet Parasitol. 2015;212(3–4):140–6.
Hoter A, Rizk S, Naim HY. The multiple roles and therapeutic potential of molecular chaperones in prostate cancer. Cancers. 2019;11(8):1194.
Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584(14):2981–9.
Young AI, Timpson P, Gallego-Ortega D, Ormandy CJ, Oakes SR. Myeloid cell leukemia 1 (MCL-1), an unexpected modulator of protein kinase signaling during invasion. Cell Adh Migr. 2018;12(6):513–23.
Moreau R, Bataller R, Berg T, Zucman-Rossi J, Jalan R. From the Editor’s desk…: June 2018. J Hepatol. 2018;68(6):1107–9.
Yang X, Yamazaki H, Yamakoshi Y, Duverger O, Morasso MI, Beniash E. Trafficking and secretion of keratin 75 by ameloblasts in vivo. J Biol Chem. 2019;294(48):18475–87.
Sussadee M, Rucksaken R, Havanapan PO, Reamtong O, Thayananuphat A. Changes in tear protein profile in dogs with keratoconjunctivitis sicca following topical treatment using cyclosporine A. Vet World. 2021;14(6):1711.
Masson PL, Heremans JF, Dive C. Studies of the proteins of secretions from two villous tumours of the rectum. Digestion. 1966;105(5):270–82.
Gillette TE, Allansmith MR. Lactoferrin in human ocular tissues. Am J Ophthalmol. 1980;90(1):30–7.
Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91(1):35–43.
Yu V, Bhattacharya D, Webster A, Bauskar A, Flowers C, Heur M, et al. Clusterin from human clinical tear samples: positive correlation between tear concentration and Schirmer strip test results. Ocul Surf. 2018;16(4):478–86.
Huang Z, Du CX, Pan XD. The use of in-strip digestion for fast proteomic analysis on tear fluid from dry eye patients. PLoS One. 2018;13(8):e0200702.
Kota SJ. Expression of tear lipocalin and MMPs in the lacrimal gland and their implication in dry eye disease. Florida: Atlantic University; 2003.
Connor MA. Role of a novel pattern recognition receptor in antibacterial innate immunity (Doctoral dissertation): University of Georgia.
Yan X, Yan W, Dai L, Jian J. Expression differences of interferon regulatory factor 3 and non-specific cytotoxic cell receptor protein-1 in grass carp (Ctenopharyngodon idella) after challenges with two genotypes of grass carp reovirus, and analysis of antiviral signaling pathways. Iran J Fish Sci. 2020;19(6):2846–64.
Zanello SB, Nayak R, Zanello LP, Farthing-Nayak P. Identification and distribution of 14.3. 3σ (Stratifin) in the human cornea. Curr Eye Res. 2006;31(10):825–33.
Manicam C, Perumal N, Wasielica-Poslednik J, Ngongkole YC, Tschäbunin A, Sievers M, et al. Proteomics unravels the regulatory mechanisms in human tears following acute renouncement of contact lens use: a comparison between hard and soft lenses. Sci Rep. 2018;8(1):1–5.
Sebbag L, Yan Y, Smith JS, Allbaugh RA, Wulf LW, Mochel JP. Tear fluid pharmacokinetics following oral prednisone administration in dogs with and without conjunctivitis. J Ocul Pharmacol Ther. 2019;35(6):341–9.
Acknowledgements
The authors are thankful to the Faculty of Veterinary Technology, Kasetsart University, Bangkok, and Institute of Molecular Biosciences, Mahidol University, Salaya Campus, Nakhonpathom, for providing the necessary facilities for this study.
Funding
This research is funded by Kasetsart University through the Graduate School Fellowship Program, Kasetsart University, Bangkok, Thailand.
Author information
Authors and Affiliations
Contributions
MS and SR conceived and designed the study. MS, RM and SR carried out the sample collections. NP, PH, and SR performed 2-DE. MS, PH, RR, and SR contributed to laboratory analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee on Animal Experimentation of Kasetsart University with the code of ACKU64-VTN-008. Informed consent was obtained from all owners before tear collection. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ritchoo, S., Havanapan, Po., Phungthanom, N. et al. Analysis and comparison of tear protein profiles in dogs using different tear collection methods. BMC Vet Res 18, 442 (2022). https://doi.org/10.1186/s12917-022-03543-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03543-7