Abstract
Background
Antimicrobial resistance (AMR) in bacterial isolates from food producing animals not only challenges the preventive and therapeutic strategies in veterinary medicine, but also threatens public health. Genetic elements placed on both chromosome and plasmids could be involved in AMR. In the present study, the associations of genomic backbone and plasmids with AMR were evaluated. We also provided some primary evidences that which genetic lineages potentially host certain groups of plasmids.
Results
In the current study, 72 avian pathogenic Escherichia coli (APEC) strains were examined. Isolates resistant to tetracycline and trimethoprim-sulfamethoxazole (87.5%; each), and harboring blaTEM (61.1%) were dominant. Moreover, phylogroup D was the most prevalent phylogroup in total (23.6%), and among multidrug-resistant (MDR) isolates (14/63). The most prevalent Inc-types were also defined as follows: IncP (65.2%), IncI1 (58.3%), and IncF group (54.1%). Significant associations among phylogroups and AMR were observed such as group C to neomycin (p = 0.002), gentamicin (p = 0.017) and florfenicol (p = 0.036). Furthermore, group D was associated with blaCTX. In terms of associations among Inc-types and AMR, resistance to aminoglycoside antibiotics was considerably linked with IncP (p = 0.012), IncI1 (p = 0.038) and IncA/C (p = 0.005). The blaTEM and blaCTX genes presence were connected with IncI1 (p = 0.003) and IncFIC (p = 0.013), respectively. It was also shown that members of the D phylogroup frequently occured in replicon types FIC (8/20), P (13/47), I1 (13/42), HI2 (5/14) and L/M (3/3).
Conclusions
Accorging to the results, it seems that group D strains have a great potential to host a variety of plasmids (Inc-types) carrying different AMR genes. Thus, based on the results of the current study, phyogroup D could be a potential challenge in dealing with AMR in poultry. There were more strong correlations among Inc-types and AMR compared to phylotypes and AMR. It is suggested that in epidemiological studies on AMR both genomic backbone and major plasmid types should be investigated.
Similar content being viewed by others
Background
Colibacillosis, an important poultry bacterial infection caused by Avian Pathogenic Escherichia coli (APEC), is responsible for major losses in the poultry industry worldwide. Despite the fact that it has been identified for more than a century, poultry colibacillosis remains as one of the most important poultry diseases that leads to mortality, reduction in productivity, and economic losses [1]. Unlike intestinal Escherichia coli pathotypes, APEC members mainly cause extraintestinal infections in poultry and the pathogenicity of these strains has not been clearly elucidated. In most studies, APEC pathogenicity has been attributed to the presence and expression of different virulence factors such as: surface antigens, fimbriae, intimin, colicin, heat-sensitive hemagglutinin, iron acquisition systems, serum resistance, toxins and etc. [2,3,4]. Since there is no single genetic determinant to identify APEC, efforts have been made to identify minimal predictors for diagnostic purposes. Accordingly, panel of five genes including: iroN (salmochelin), iutA or aerJ (aerobactin), ompT (outer membrane protease), iss (serum resistance) and hlyF (toxin) has been widely accepted for APEC virulotyping [5, 6].
Therapeutic, metaphylactic and prophylactic purposes are the main reasons of wide application of antibiotics in the poultry industry. The antibiotics usage in poultry farms around the world does not follow a single pattern. However, tetracyclines, macrolides, sulfonamides and aminoglycosides are recorded as the most common prescribed antibiotics in different regions [7,8,9]. The misuse of antibiotics has been contributed to emerging and spread of antimicrobial resistance (AMR), and evolution of multidrug-resistant (MDR) pathogens resulting in poor treatment and public health concerns. Genetic poential of resistance to antibiotics may acquire by mutation or horizontal gene transfer (HGT) [10]. Plasmids carrying AMR genes are effective transmitters of this feature in microbial populations which are preserved and propagated under the selective pressure resulting from the indiscriminate use of antibiotics [11, 12]. It is belived that plasmid-mediated AMR transfer has a key role in increasing the population of MDR strains among Enterobacterales [13]. As an example, common presence of mobile genetic elements responsible for resistance to β-lactam antibiotics, tetracyclines and sulfonamides in APEC has been recorded [14, 15]. Resistance to β-lactam antibiotics due to the production of extended-spectrum beta-lactamase (ESBL) would be one of the most concerning AMR which leads to inactivation of a broad spectrum of antibiotics such as: penicillins, third and fourth generation of cephalosporins, and monobactam [16]. Moreover, it has been suggested that poultry industry acts as a reservoir for ESBL-producing E. coli [17].
Considering the importance of plasmids in bacterial biology, the identification and classification of plasmids can be highly informative. The first method proposed for classification of plasmids used the plasmid incompatibility phenomenon [18]. Actually, plasmid incompatibility (Inc) is defined as the inability of two dependent plasmids to survive for a long time in a common cell [19]. After that, a PCR-based method for classifying plasmids has been developed, known as PBRT (PCR-Based Replicon Typing). This method is able to identify the main plasmid families in Enterobacterales [20]. It has been shown that certain plasmids are related to transferring specific antibiotic resistance to particular bacterial clones like Escherichia coli ST131 clade C2/H30Rx and IncFII plasmids carrying blaCTX-M [10]. It should be noted that other traits such as: virulence, enhanced fitness and metabolism of rare substances could also be acquired through plasmids [21].
The effect of genetic background in acquisition of antibiotic resistance has also been described. While some members of phylogenetic groups A and D are prone to acquire resistance against third-generation cephalosporins, B2 strains are more susceptible [22]. The most common method for identifying phylogroups is a multiplex PCR which is able to categorize Escherichia coli isolates into eight phylogenetic groups (A, B1, C, E, D, F, B2 and E. Clades). Importantly, phylotyping is a valuable method because it is believed that E. coli strains are not randomly distributed throughout the bacterial populations. Members of same group tend to have similar characteristics in pathogenicity, niche, and resistance [23]. Although a vast majority of research has been investigated AMR along with phylotypes, the information about AMR relation to easily spreadable plasmid types has been overlooked.
In the present study, 72 APEC strains which have been isolated from poultry in recent years, were examined in terms of virulence-associated genes (VAG), plasmid (Inc) types, phylogenetic lineages and drug resistance (phenotypic AMR and ESBL genes). The results of this study can lead to a better understanding of the distribution and importance of genomic backbone and plasmid content in APEC strains isolated from poultry and its relationship with the spreading patterns of drug resistance.
Results
Antibiotic susceptibility testing
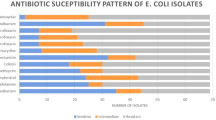
A total of 72 Escherichia coli isolates were investigated for susceptibility to ten antibiotics of eight categories (tetracycline, trimethoprim-sulfamethoxazole, amoxicillin-clavulanic acid, neomycin, florfenicol, enrofloxacin, gentamicin, cefazolin, colistin and furazolidone) by agar-disk diffusion method. According to the results of antibiogram test, the highest rates of antimicrobial resistance were observed against tetracycline (63/72; 87.5%) and trimethoprim-sulfamethoxazole (63/72; 87.5%), followed by enrofloxacin (59/72; 81.94%) and florfenicol (41/72; 56.94%). In addition, the lowest rates of bacterial resistance belonged to colistin (0/72, 0%) and cefazolin (3/72, 4.17%). Moreover, 63 isolates (87.5%) were considered MDR strains based on being resistant to three or more classes of antibiotics.
Molecular detection of ESBL-producing E. coli, revealed the presence of blaTEM, blaCTX and blaOXA genes alone or in combination with each other in 49 (68.05%) strains. The prevalences of genes are as follows: blaTEM (44/72; 61.1%), blaCTX (23/72; 31.94%) and blaOXA (1/72; 1.38%). No isolate was detected positive for blaSHV. Moreover, 24 (33.33%) strains were phenotypically confirmed as ESBL-producing strains. Table 1 and Fig. 1, represents the results in details.
Heatmap. Genotypes and phenotypes of the APEC strains. Red: presence of genetic elements/not susceptible to antibiotics; Blue: absence of replicon types; Green: absence of genes/susceptible to antibiotics. Phylogroups: A=yellow; B1=red; B2=pink; C=black; D=white; E=light blue; F=gray. Cluster analysis was carried out based on the replicon types
Phylogenetic groups
The 72 analyzed APEC strains were classified into seven phylogenetic groups: A, B1, B2, C, D, E and F. Group D was the most prevalent phylogenetic group (17; 23.61%) while only few strains defined as members of group B2 (4; 5.55%). The distribution of remaining strains in other groups are as follows: A (11; 15.27%), F (11; 15.27%), C (10; 13.88%), E (10; 13.88%), and B1 (9; 12.5%).
APEC virulence-associated genes and virulence score
The five genes iroN, ompT, hlyF, iss and iutA are considered as minimal predictos of APEC virulence-associated genes (VAG) [5]. In the current study, the virulotyping of the isolates were carried out which resulted in seven patterns. Isolates were also scored based on the number of VAG possesed. The virulence scores (VS) of the strains were in the range of 0 to 5. The most prevalent VS was 5 (48/72; 66.66%). Moreover, the most prevalent VAGs were ompT (68/72; 94.4%) and hlyF (68/72; 94.4%). The prevalences of the genes iutA, iroN and iss were defined 67 (93.05%), 58 (80.55%) and 56 (77.77%), respectively.
Plasmid replicon typing
The applied PBRT method is able to identify 18 plasmid replicon types. In the present study, only 13 replicon types were detected in the investigated APEC strains. All isolates possessed at least one typable replicon. Furthermore, plasmid replicon type P was the most frequent type among strains (47/72; 65.28%). The second frequent plasmid replicon type was I1 (42/72; 58.33%) followed by B/O (22/72; 30.56%), FIB and K/B (21/72; 29.17% each), whilst the plasmid replicons T, W, FIIA, Y and X were absent. In addition to frequencies of individual replicons, investigation of the presence of IncF group (FIA, FIB, FIC, FIIA, Frep) are important. More than half of the isolates (39/72; 54.16%) benefited from having one or combination of the members of IncF group. The profiles of IncF group among the isolates are as follows: FIA (2/39); FIB (10/39); FIC (13/39); FIA-FIB (6/39); FIA-FIC (3/39); FIB-FIC (2/39); FIB-Frep (1/39); and FIA-FIB-FIC (2/39).
Moreover, 50 different patterns of plasmid replicon combinations were observed which is represented in Fig. 1. The richest pattern belonged to a B1 member with simultanious presence of seven (B/O-K/B-FIB-FIC-A/C-FIA-HI1) replicons. Moreover, the pattern P-I1 was the most prevalent pattern (7/72; 9.72%).
Distribution of phenotypic AMR and ESBL (phenotype and genotype) among phylogenetic groups
Isolates resistant to trimethoprim-sulfamethoxazole, tetracycline, enrofloxacin, florfenicol, neomycin and furazolidone were found in all phylogroups, while resistant isolates to gentamicin, amoxicillin-clavulanic acid and cefazolin were detected in certain phylogenetic groups. Moreover, no notable assosiation was observed among MDR and phylogroups.
Overall, the phylogenetic group D was the most resistant phylotype. Resistance to nine antibiotics was detected in this group (all the tested antibiotics except colistin). Members of D also had the most participation in resistance to enrofloxacin (15/59; 25.42%), cefazolin (2/3; 66.7%), tetracycline (14/63; 22.2%) and trimethoprim-sulfamethoxazole (15/63; 23.8%). Actually, resistance to cefazolin were observed only in D and F groups. Moreover, groups B2 and C had no resistant isolates to amoxicillin-clavulanic acid as well. The results of AMR distribution among phylogenetic groups are represented in details in Table 2.
From ten antibiotics, only the distribution of neomycin (p = 0.006) showed significant difference in relation to different phylogroups. Other noticeable results are the considerable correlations between group C and resistance to neomycin (10/35; 28.57%; p = 0.002), gentamicin (4/10; 40%; p = 0.017) and florfenicol (9/41; 21.95%; p = 0.036).
From three ESBL genes (blaTEM, blaCTX, blaOXA) detected in the current study, only blaCTX showed significant difference (p = 0.000) in distribution among phylogenetic groups. The gene blaCTX (p = 0.000) and phenotypically ESBL-producing strains (p = 0.018) were also considerably related to phylogroup D. For correlation coefficients see Supplementary 1.
Distribution of phenotypic AMR isolates and ESBL (phenotype and genotype) among plasmid replicon types
Isolates resistant to enrofloxacin, tetracycline and trimethoprim-sulfamethoxazole were distributed among all the replicon types, while resistance to gentamicin, cefazolin, amoxicillin-clavulanic acid, neomycin, florfenicol and furazolidone were related to some Inc-types. Members of IncP were the most prevalent resistant isolates to all the tested antibiotics. Along with IncP, replicon type I1 had also the majority of resistent isolates to amoxicillin-clavulanic acid (6/10) and florfenicol (25/41). Table 3, represents distribution of AMR isolates among plasmid replicon types in details.
In the current study, resistance to aminoglycoside antibiotics was considerably linked with IncP, IncI1 and IncA/C. Actually, replicon types P (p = 0.012) and I1 (p = 0.038) had significant presence in gentamicin resistance, and IncA/C (p = 0.005) significantly reflected resistance to neomycin. Moreover, statistical analysis clarified other significant relations (p < 0.05) among replicon types and antibiotic resistance. The IncHI2 were considerably prevalent (p = 0.020) among resistant isolates to amoxicillin-clavulanic acid. In contrast to positive relations, there were significant associations and negative correlations between isolates possessed IncL/M, IncFIC or IncFIM replicon types and some of the antibiotic resistance. These relations are as follows: IncL/M and tetracycline (p = 0.039), IncL/M and sulfamethoxazole-trimethoprim (p = 0.039), IncFIC and neomycin (p = 0.000), IncFIC and florfenicol (p = 0.007). The association between IncFIA and enrofloxacin was marginally significant (p = 0.050). Moreover, IncFIA (p = 0.050) and IncL/M (p = 0.039) were negatively related to MDR.
Significant relations among ESBL genes (blaTEM, blaCTX, blaOXA) and replicon types were detected. Genes blaTEM and blaCTX were positively connected with IncI1 (p = 0.003) and IncFIC (p = 0.013), respectively. On the other hand, blaCTX had a tendency to participate in IncA/C (p = 0.006) and IncFIA (p = 0.050). Furthermore, phenotypically confirmed ESBL strains had notable associations with IncFIA (p = 0.048) and IncI1 (p = 0.048). For correlation coefficients see Supplementary file 1.
Association of plasmid replicon types and phylogenetic groups
The replicon types P and FIB were distributed among all the detected phylogenetic groups. Members of phylogenetic group D were the most prevalent isolates in replicon types FIC (8/20), P (13/47), I1 (13/42), HI2 (5/14) and L/M (3/3).
It should be noted that statistical analysis revealed no significant difference in distribution of plasmid replicon types among phylogenetic groups; except for IncFIC (p = 0.000), IncI1 (p = 0.000) and IncHI2 (p = 0.015). Significant associations with positive or negative correlations were observed among replicons and phylogenetic groups. Memebers of F group had considerable tendency for replicon types FIC (8/11; 72.72%; p = 0.001) and HI2 (5/11; 45.45%; p = 0.032), while strains belonged to C were associated with I1 (10/10; 100%; p = 0.004) replicon. Moreover, high presence of IncA/C in group A (5/11; 45.45%; p = 0.022) and IncL/M in group D (3/17; 17.64%; p = 0.011) were observed. Table 4, presents distribution of plasmid replicon types among phylogroups in details. Furthermore, correlation coefficients are available in Supplementary 1.
Relations among VAG, VS, AMR, ESBL genes, phylogroups, and replicons
Interestingly, most of the notable (p < 0.05) associations amog VAG and VS with AMR, ESBL genes, phylogroups and Inc-types were negatively correlated, except for iss and IncI1 (p = 0.020), VS and tetracycline (p = 0.003), and VS and FIB (p = 0.050). Other considerable relations and correlation coefficients are available in Supplementary 1.
Discussion
Emergence of AMR isolates in food producing animals is not only a challenge to the veterinay preventive and therapeutic strategies, but also a serious public health concern. Genetic factors responsible for AMR to different classes of antibiotics are located on both transferable plasmids and chromosomes (in forms of transposons, integrons and genetic cassets), but plasmids have a pivotal role in HGT of AMR in bacterial populations especially in Enterobacterales [24]. Thus, plasmid contents and types are issues of interest which were mostly neglected in AMR studies.
In the present study, comparisons between genetic backbone (phylotypes), plasmid types (Inc-type) and virulence genotypes with AMR were made to evaluate which relates better to AMR. We also provided some primary evidences that which genetic lineages potentially host certain groups of plasmids. Since the genetic basis of resistance to different antimicrobials are quite diverse, we have chosen the phenotypic resistance to some classes of commonly prescribed antibiotics in animals and humans. Moreover, molecular investigation of common ESBL genes and VAG carried out to see if a notable associations could be find.
Various AMR profiles were observed in the current study and 87.5% of the strains were confirmed as MDR. Moreover, high resistance to tetracycline (63/72, 87.5%), sulfamethoxazole-trimethoprim (63/72, 87.5%) and enrofloxacin (59/72, 81.94%) was defined which is in line with other reports [25, 26]. The presence of larg number of MDR strains and resistant isolates to “critically important antimicrobials” such as enrofloxacin means that taking proper preventive and theraputic strategies based on using antimicrobial agents for colibacillosis needs special considerations, as there are limited available alternatives for “critically important antimicrobials” [27]. Therefore, emerging resistance to the mentioned antibiotics means more obstacles to cure infections in the forthcoming future. In the present study, more than half of the strains (49/72; 68.05%) carried at least one of the ESBL genes: blaTEM, blaCTX and blaOXA. Interestingly, the prevalence of blaTEM was almost two times greater than blaCTX (44 compared to 23). This pattern of distribution of the ESBL genes is different from other studies [28, 29] as it has been shown that blaCTX was the predominant lineage of ESBL genes for over the last two decades [30]. Moreover, in the present study, almost half of the ESBL gene carrying isolates were confirmed as phenotypically ESBL-producing strains. It should be noted that the importance of phenotypically negative ESBL strains has been showen before as strains with unexpressed ESBL genes also has a role in spreading of ESBL genes via HGT or resulting in ESBL phenotype back in certain conditions [31].
In the current study, 72 APEC strains were assigned into seven groups (A, B1, B2, C, D, E, F) with highest frequencies of phylogenetic groups D (17/72; 23.61%) followed by A (11/72; 15.3%) and F (11/72; 15.3%). While group A is related to human commensal strains [32], strains from phylogroup D are associated with human infections which its high prevalence among APEC isolates is concerning [23]. Since the zoonotic risk of chicken-source phylogroup F E. coli and its contribution to spread of MDR E. coli to humans heve been proposed recently [33, 34], the high portion of group F members should not be neglected. Moreover, the observed scattered distribution of APEC strains in different phylogroups may indirectly reflects the commensal nature of APEC strains who broken the host defence and ended up a septicemic infection [1].
In our study, only few significant associations were observed among phylogenetic groups and AMR. The most notable ones were related to the groups C and D. The group C strians had considerable relation to phenotypic resistant to neomycin (10/35; 28.57%; p = 0.002), gentamicin (4/10; 40%; p = 0.017) and florfenicol (9/41; 21.95%; p = 0.036), while group D was highly associated with blaCTX. Furthermore, in the present study, members of different phylotypes showed the same resistance patterns (R-types) and vice versa that indicates phylotyping might not be a robust genotyping strategy to predict AMR except for few antimicrobials. One of the possible explanation could be due to the very general bacterial classification in Clermont’s method based on some conserved chromosomal genes. Furthermore, plasmids which have a pivotal role in AMR are neglected in this typing method.
In the present study, variety of patterns for plasmid replicons (50 different profiles for 72 strains) were observed. Different AMR genes, virulence determinants and fitness factors could be coded by plasmids. Based on PBRT method applied in the present study, among 72 APEC strains, only 13 replicon types were observed. Out of them, IncP (47/72, 65.27%), IncI1 (42/72, 58.3%) and IncF group (FIA, FIB, FIC, FIIA, Frep; 39/72, 54.16%) were the most prevalent plasmid types. High prevalence of IncP among the isolates (47/72, 65.27%) differs the current study from previous reports on APEC, as they have recorded lower values or no presence of P replicon type [6, 35,36,37]. The IncP plasmids are composed of genes responsible for resistance to a wide variety of antibiotic classes, heavy metals and quaternary ammonium compounds in gram negative bacteria. It is believed that the selective pressure of antimicrobial agents or antiseptics in clinical conditions have resulted in spread and maintenance of IncP plasmids [38, 39]. Moreover, it has been demonstrated that IncI1 plasmid type are prevalent among E. coli of the avian hosts as our results support it too [35]. The high frequency of IncF group in commensals, environmental strains, APEC and other pathotypes of E. coli such as STEC, has been mentioned by others as well [35, 40, 41]. However, no presence of IncF group replicons was also reported in blaCMY-2 plasmids in MDR E. coli from poultry [42]. The significant aspects of the IncF group and I1 replicons are their association to virulence (such as fimbriae and type IV pili, respectively) and antibiotic resistance determinants (ESBL genes in particular) which are therapeutic concerns [19, 43].
Relations among plasmid replicon types and AMR have been investigated in numerous studies [19, 24]. The current study defined significant assosiations as well. Resistance to aminoglycosides was considerably linked with IncP, I1 and A/C replicon types which is also mentioned in other studies [24]. Importantly, plasmid replicon types P and A/C are broad-host-range plasmids which can disseminate the aminoglycoside resistance genes among different bacterial hosts [19]. Besides, significant association was observed between resistance to amoxicillin-clavulanic acid and plasmid replicon types HI2. One of the most resistance traits associated with HI2 replicon type is ESBL genes [19]. Along with HI2, different studies have mentioned the association of blaCTX with IncF family, IncA/C, IncL/M replicon types, although the relation between FIC and blaCTX was confirmed in our study [44] . Moreover, it has been proven that IncI1 is associated with several β-lactamases including blaTEM, which our results support it too [45]. Based on our results and other reports, it seems that in the cases of AMR surveys in APEC, it is not necessary to looking for every Inc-types introduced by Carattoli et al., [20], as some of the plasmid Inc-types (IncT, IncW, IncX) have been recorded in low prevalence or absent in most studies. On the other hand, detection of broad-host-range plasmids that could transfer AMR genes among different species like: IncP, IncA/C, IncI1 and IncH, and high prevalent replicon types such as IncI and IncF group would be essential in studies investigating antibiotic resistance, especially plasmid mediated AMR [36, 42, 46].
The replicon types P and FIB were distributed among all the detected phylogenetic groups. Plasmids belonging to the mentioned replicon types are conjugative plasmids which could be disseminated among bacterial hosts [24]. Moreover, members of phylogenetic group D were the most prevalent isolates in replicon types FIC (8/20), P (13/47), I1 (13/42), HI2 (5/14) and L/M (3/3). Accorging to this, it seems that group D strains have a great potential to host variety of plasmids belonging to different replicon types. Since IncP, IncI1, IncHI2 and IncL/M are associated with different antimicrobial resistance genes [24], it could be a possible explaination for high participation of D members in MDR group of strains (14/63; 22.2%). Associations of plasmid replicon types and phylogroups are not fully described so far. However, the present study revealed notable relations with positive and negative correlations. Significant high presence of IncFIC (8/11; 72.72%; p = 0.001) and IncHI2 (5/11; 45.45%; p = 0.032) in group F, IncI1 (10/10; 100%; p = 0.004) in group C, IncA/C in group A (5/11; 45.45%; p = 0.022), and IncL/M in group D (3/17; 17.64%; p = 0.011) were observed. Another important point is that almost all of the group C strains possess I1 and P replicons. In contrast to positive associations, negative relations were also defined. Members of group B1 significantly lacked IncI1 (0/9; p = 0.000) and group A isolates lacked IncFIC (0/11; p = 0.028) and N (2/11; 18.18%; p = 0.022). In fact, the mentioned associations are preliminarly data and more comprehensive studies are necessary to verify the associations between the genetic backbone (phylogroups) and plasmid contents. However, according to our results, it seems that phylogroups D and C are prone to aquire and share various plasmid mediated genes as they harbor broad-host-range plasmids such as: P, I1 and L/M replicon types.
Conclusions
In the current study, APEC strains were defined as a heterogeneous population. Numerous variations were observed in gentotypic features (phylogenetic groups, virulence types, plasmid replicon types) and AMR (phenotypic and ESBL genes) of the isolates. Comparisons were conducted to evaluate the correlations among genetic criteria with AMR. The results also showed the weakness of phylogenetic grouping in prediction of AMR. Furthermore, it seems that in the cases of AMR surveys in APEC, not all of the plasmid replicon types are equally important. Some of the Inc-types like: IncP, IncI, IncL/M, IncA/C, IncH and IncF group have a higher priority due to their broad-host-range or their high prevalence. We believe that these types should be screened in most AMR studies to define the AMR spread with more clarity. Moreover, further studies are needed to clarify how genetic backbone and plasmid contents are affected by each other.
Methods
Bacterial strains
A panel of 72 non-duplicate E. coli strains used in the present study were randomly selected from microbial collection (Ferdowsi University of Mashhad, Mashhad, Iran). All the isolates had been recovered from clinical cases of avian colibacillosis (heart or liver samples) occurred in Iran. The identity of the strains were confirmed based on the colony morphology, bacterial morphology and gram staining, lactose fermentation in MacConkey agar, catalase and oxidase production, standard biochemical tests including IMViC (indole, methyl red, acetoin production, citrate utilization), triple sugar agar (TSI) and urease production [47]. To revive each isolate, 50 μl of cryopreserved bacteria were streaked on MacConkey agar to achieve a single colony. Then, a single colony was propagated on Luria-Bertani (LB) agar plates for the next steps.
DNA extraction
DNA was extracted according to the boiling method. Briefly, a loop of bacteria from an overnight culture (18–20 hour) was suspend in 400 μl of sterile distilled water. The suspension was boiled in a boiling water bath for 10 min and after cooling on ice buckets, centrifuged at 800×g for 5 minutes. Finally, supernatant was collected and preserved as DNA template for further molecular investigations [1].
Antimicrobial resistance
Phenotypic resistance
To evaluate susceptibility of APEC strains against different classes of antibiotics, standard agar-disk diffusion method (Kirby-Bauer) was performed [48]. All the isolates (n = 72) were screened for 10 antibiotics from eight classes of commonly used antibiotics in veterinary and human therapeutics. The antibiotic disks were as follow: amoxicillin-clavulanic acid (AMC 20/10 μg), tetracycline (TET 30 μg), neomycin (NEO 30 μg), florfenicol (FLO 30 μg), enrofloxacin (ENFX 5 μg), gentamicin (GM 10 μg), trimethoprim-sulfamethoxazole (SXT 1.25/23.75 μg), colistin (CST 10 μg), cefazolin (CFZ 30 μg) and furazolidone (FDZ 100 μg). Final results were achieved based on comparison of diameter of inhibited growth zones to CLSI interpretive criteria [49].
Phenotypic confirmatory disc diffusion test for ESBL production
According to the procedure recommended by CLSI, ceftazidime (CTZ 30 μg) and cefotaxime (CTX 30 μg) alone and with clavulanate (10 μg) were placed on Muller-Hinton plates inoculated with fresh bacterial suspension with a dilution equal to 0.5 McFarland. After 18 h incubation at 37 °C, inhibition zone was measured. ESBL-producer strain was confirmed when a ≥ 5-mm in a zone diameter for either antimicrobial agent tested in combination with clavulanate versus the zone diameter of the agent when tested alone was observed [49].
β- lactamase/ ESBL genes
Molecular detection of some widespread β- Lactamase /ESBL gene families of E. coli was carried out using a triplex PCR reaction for detection of blaTEM, blaSHV, blaOXA and a uniplex PCR for detection of blaCTX-M. Each PCR reactions was performed in a volume of 20 μl containing: 10 μl Taq DNA Polymerase Master Mix RED 2x (Amplicon, Denmark) containing 1.5 mM MgCl2, various concentration of each primers, ultrapure water and 300 ng of template DNA. Primer characteristics and thermal conditions are shown in Table 5. Finally, PCR products were analyzed by electrophoresis using 1.5% (w/v) agarose gel and Green Viewer safe stain (0.01 v/v).
Phylogenetic groups
Phylogenetic groups of the isolates were determined using the updated quadruplex-PCR method developed by Clermont et al., when necessary, additional PCRs were applied to determine the phylotypes as recommended [23]. This method is capable of assigning E. coli isolate to one of the eight phylogenetic groups: A, B1, B2, C, D, E, F, clade I. Moreover, it allows isolates that are members of other cryptic clades (II to V) of Escherichia to be identified.
Each PCR reactions was performed in a volume of 20 μl containing: 10 μl Taq DNA Polymerase Master Mix RED 2x (Amplicon, Denmark) containing 1.5 mM MgCl2, various concentration of each primers, ultrapure water and 300 ng of template DNA. Primer characteristics and thermal conditions are shown in Table 5. Finally, PCR products were analyzed by electrophoresis using 1.5% (w/v) agarose gel and Green Viewer safe stain (0.01 v/v).
APEC virulence genotyping
A total of 72 isolates were screened for five virulence genes (iroN, ompT, hlyF, iss, iutA) known as minimal predictors of APEC virulence [5]. Each PCR reactions was performed in a volume of 20 μl containing: 10 μl Taq DNA Polymerase Master Mix RED 2x (Amplicon, Denmark) containing 1.5 mM MgCl2, 1 μl of each forward and reverse primers, ultrapure water and 300 ng of template DNA. Primer characteristics and thermal conditions are shown in Table 5. Finally, PCR products were analyzed by electrophoresis using 1.5% (w/v) agarose gel and Green Viewer safe stain (0.01 v/v).
Plasmid replicon typing
Plasmid replicon types of the APEC isolates were defined using the method designed by Johnson et al. [35]. This is a simplified method which requires only three multiplex-PCR panels to identify 18 plasmid replicons. The panels are as follow: panel 1: (B/O, FIC, A/C, P, T); panel 2: (K/B, W, FIIA, FIA, FIB, Y); panel 3: (I1, Frep, X, HI1, N, HI2, L/M).
Each PCR reactions was performed in a volume of 20 μl containing: 10 μl Taq DNA Polymerase Master Mix RED 2x (Amplicon, Denmark) containing 1.5 mM MgCl2, various concentration of each primers, ultrapure water and 300 ng of template DNA. Primer characteristics are shown in Table 5. Thermal conditions of PCR reactions were as follows: 5 min at 94 °C; 30 cycles of 30 s at 94 °C, 30s at 60 °C, and 90s at 72 °C; and a final extension of 5 min at 72 °C. Lastly, PCR products were visualized by electrophoresis using 1.5% (w/v) agarose gel and Green Viewer safe stain (0.01 v/v).
Statistical analysis and data visualization
In addition to descriptive review of the results, possible relations among genetic criteria (phylogenetic groups, VGs, replicon types, ESBL genes) with phenotypic AMR were determined by chi-square test and Fisher’s exact test. In cases of significant associations (p < 0.05), correlations were determined using Spearman’s correlation coefficient. In the present study, calculations were performed in SPSS version 16.0 (SPSS Inc., Chicago, USA).
For better ilustration of the results, cluster analysis was performed (UPGMA method) using online tool CIMminer and a heatmap was also generated (http://discover.nci.nih.gov/cimminer).
Availability of data and materials
Most data generated or analyzed during the current study are included in this published article. The other data are available from the corresponding author on reasonable request.
Abbreviations
- APEC:
-
Avian pathogenic Escherichia coli
- AMR:
-
Antimicrobial resistance
- MDR:
-
Multidrug-resistant
- HGT:
-
Horizontal gene transfer
- ESBL:
-
Extended-spectrum beta-lactamase
- Inc:
-
Plasmid incompatibility
- PBRT:
-
PCR-Based Replicon Typing
- VAG:
-
Virulence-associated genes
- VS:
-
Virulence scores
- AMC:
-
Amoxicillin-clavulanic acid
- TET:
-
Tetracycline
- NEO:
-
Neomycin
- FLO:
-
Florfenicol
- ENFX:
-
Enrofloxacin
- GM:
-
Gentamicin
- SXT:
-
Trimethoprim-sulfamethoxazole
- CST:
-
Colistin
- CFZ:
-
Cefazolin
- FDZ:
-
Furazolidone
References
Askari Badouei M, Joseph Blackall P, Koochakzadeh A, Haghbin Nazarpak H, Sepehri MA. Prevalence and clonal distribution of avian Escherichia coli isolates harboring increased serum survival (iss) gene. J Appl Poult Res. 2016;25(1):67–73. https://doi.org/10.3382/japr/pfv064.
Janßen T, Schwarz C, Preikschat P, Voss M, Philipp H-C, Wieler LH. Virulence-associated genes in avian pathogenic Escherichia coli (APEC) isolated from internal organs of poultry having died from colibacillosis. Int J Med Microbiol. 2001;291(5):371–8. https://doi.org/10.1078/1438-4221-0014.
Kathayat D, Lokesh D, Ranjit S, Rajashekara G. Avian pathogenic Escherichia coli (APEC): an overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens. 2021;10(4):467. https://doi.org/10.3390/pathogens10040467.
Subedi M, Luitel H, Devkota B, Bhattarai RK, Phuyal S, Panthi P, et al. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet Res. 2018;14(1):113. https://doi.org/10.1186/s12917-018-1442-z.
Johnson TJ, Wannemuehler Y, Doetkott C, Johnson SJ, Rosenberger SC, Nolan LK. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J Clin Microbiol. 2008;46(12):3987–96. https://doi.org/10.1128/JCM.00816-08.
Newman DM, Barbieri NL, de Oliveira AL, Willis D, Nolan LK, Logue CM. Characterizing avian pathogenic Escherichia coli (APEC) from colibacillosis cases, 2018. PeerJ. 2021;9:e11025. https://doi.org/10.7717/peerj.110251.
Iwu CD, Korsten L, Okoh AI. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: a concern for public health. Microbiologyopen. 2020;9(9):e1035. https://doi.org/10.1002/mbo3.1035.
Xu J, Sangthong R, McNeil E, Tang R, Chongsuvivatwong V. Antibiotic use in chicken farms in northwestern China. Antimicrob Resist Infect Control. 2020;9(1):10. https://doi.org/10.1186/s13756-019-0672-6.
Koirala A, Bhandari P, Shewade HD, Tao W, Thapa B, Terry R, et al. Antibiotic Use in Broiler Poultry Farms in Kathmandu Valley of Nepal: Which Antibiotics and Why? Trop Med Infect Dis. 2021;6(2):47. https://doi.org/10.3390/tropicalmed6020047.
San MA. Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol. 2018;26(12):978–85. https://doi.org/10.1016/j.tim.2018.06.007.
Smets BF, Barkay T. Horizontal gene transfer: perspectives at a crossroads of scientific disciplines. Nat Rev Microbiol. 2005;3(9):675–8. https://doi.org/10.1038/nrmicro1253.
Madec J-Y, Haenni M. Antimicrobial resistance plasmid reservoir in food and food-producing animals. Plasmid. 2018;99:72–81. https://doi.org/10.1016/j.plasmid.2018.09.001.
Citterio B, Andreoni F, Simoni S, Carloni E, Magnani M, Mangiaterra G, et al. Plasmid replicon typing of antibiotic-resistant Escherichia coli from clams and marine sediments. Front Microbiol. 2020;11:1101. https://doi.org/10.3389/fmicb.2020.01101.
Awad A, Arafat N, Elhadidy M. Genetic elements associated with antimicrobial resistance among avian pathogenic Escherichia coli. Ann Clin Microbiol Antimicrob. 2016;15(1):59. https://doi.org/10.1186/s12941-016-0174-9.
Cummins ML, Reid CJ, Roy Chowdhury P, Bushell RN, Esbert N, Tivendale KA, et al. Whole genome sequence analysis of Australian avian pathogenic Escherichia coli that carry the class 1 integrase gene. Microb Genomics. 2019;5(2):e000250. https://doi.org/10.1099/mgen.0.000250.
Dhillon RH-P, Clark J. ESBLs: a clear and present danger? Crit Care Res Pract. 2012;2012:625170. https://doi.org/10.1155/2012/625170.
Apostolakos I, Feudi C, Eichhorn I, Palmieri N, Fasolato L, Schwarz S, et al. High-resolution characterisation of ESBL/pAmpC-producing Escherichia coli isolated from the broiler production pyramid. Sci Rep. 2020;10(1):11123. https://doi.org/10.1038/s41598-020-68036-9.
Datta N, Hedges RW. R factors of compatibility group a. J Gen Microbiol. 1973;74(2):335–6. https://doi.org/10.1099/00221287-74-2-335.
Carattoli A. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother. 2009;53(6):2227–38. https://doi.org/10.1128/AAC.01707-08.
Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63(3):219–28. https://doi.org/10.1016/j.mimet.2005.03.018.
Johnson TJ, Nolan LK. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol Mol Biol Rev. 2009;73(4):750–74. https://doi.org/10.1128/MMBR.00015-09.
Tenaillon O, Skurnik D, Picard B, Denamur E. The population genetics of Comensal. Nat Rev Microbiol. 2010;8:207–17. https://doi.org/10.1038/nrmicro2298.
Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5(1):58–65. https://doi.org/10.1111/1758-2229.12019.
Rozwandowicz M, Brouwer MSM, Fischer J, Wagenaar JA, Gonzalez-Zorn B, Guerra B, et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother. 2018;73(5):1121–37. https://doi.org/10.1093/jac/dkx488.
Jahantigh M, Samadi K, Dizaji RE, Salari S. Antimicrobial resistance and prevalence of tetracycline resistance genes in Escherichia coli isolated from lesions of colibacillosis in broiler chickens in Sistan. Iran BMC Vet Res. 2020;16(1):267. https://doi.org/10.1186/s12917-020-02488-z.
Ayandiran TO, Falgenhauer L, Schmiedel J, Chakraborty T, Ayeni FA. High resistance to tetracycline and ciprofloxacin in bacteria isolated from poultry farms in Ibadan, Nigeria. J Infect Dev Ctries. 2018;12(6):462–70. https://doi.org/10.3855/jidc.9862.
Anonymous. Critically important antimicrobials for human medicine, 6th revision, Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/publications/i/item/9789241515528.
Gundran RS, Cardenio PA, Villanueva MA, Sison FB, Benigno CC, Kreausukon K, et al. Prevalence and distribution of blaCTX-M, blaSHV, blaTEM genes in extended- spectrum β- lactamase- producing E. coli isolates from broiler farms in the Philippines. BMC Vet Res. 2019;15(1):227. https://doi.org/10.1186/s12917-019-1975-9.
Falgenhauer L, Imirzalioglu C, Oppong K, Akenten CW, Hogan B, Krumkamp R, et al. Detection and characterization of ESBL-producing Escherichia coli from humans and poultry in Ghana. Front Microbiol. 2019:9. https://doi.org/10.3389/fmicb.2018.03358.
D’Andrea MM, Arena F, Pallecchi L, Rossolini GM. CTX-M-type β-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol. 2013;303(6):305–17. https://doi.org/10.1016/j.ijmm.2013.02.008.
Zhang Z, Zhai Y, Guo Y, Li D, Wang Z, Wang J, et al. Characterization of Unexpressed Extended-Spectrum Beta-Lactamase Genes in Antibiotic–Sensitive Klebsiella pneumoniae Isolates. Microb Drug Resist. 2018;24(6):799–806. https://doi.org/10.1089/mdr.2017.0018.
de Stoppe NC, Silva JS, Carlos C, MIZ S, Saraiva AM, Ottoboni LMM, et al. Worldwide phylogenetic group patterns of Escherichia coli from commensal human and wastewater treatment plant isolates. Front Microbiol. 2017:8. https://doi.org/10.3389/fmicb.2017.02512.
Zhuge X, Zhou Z, Jiang M, Wang Z, Sun Y, Tang F, et al. Chicken-source Escherichia coli within phylogroup F shares virulence genotypes and is closely related to extraintestinal pathogenic E. coli causing human infections. Transbound Emerg Dis. 2021;68(2):880–95. https://doi.org/10.1111/tbed.13755.
Wang M, Jiang M, Wang Z, Chen R, Zhuge X, Dai J. Characterization of antimicrobial resistance in chicken-source phylogroup F Escherichia coli: similar populations and resistance spectrums between E. coli recovered from chicken colibacillosis tissues and retail raw meats in eastern China. Poult Sci. 2021;100(9):101370. https://doi.org/10.1016/j.psj.2021.101370.
Johnson TJ, Wannemuehler YM, Johnson SJ, Logue CM, White DG, Doetkott C, et al. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl Environ Microbiol. 2007;73(6):1976–83. https://doi.org/10.1128/AEM.02171-06.
Solà-Ginés M, Cameron-Veas K, Badiola I, Dolz R, Majó N, Dahbi G, et al. Diversity of multi-drug resistant avian pathogenic Escherichia coli (APEC) causing outbreaks of Colibacillosis in broilers during 2012 in Spain. PLoS One. 2015;10(11):e0143191. https://doi.org/10.1371/journal.pone.0143191.
Yoon MY, Bin KY, Ha JS, Seo KW, Noh EB, Son SH, et al. Molecular characteristics of fluoroquinolone-resistant avian pathogenic Escherichia coli isolated from broiler chickens. Poult Sci. 2020;99(7):3628–36. https://doi.org/10.1016/j.psj.2020.03.029.
Popowska M, Krawczyk-Balska A. Broad-host-range IncP-1 plasmids and their resistance potential. Front Microbiol. 2013;4:44. https://doi.org/10.3389/fmicb.2013.00044.
Schlüter A, Szczepanowski R, Pühler A, Top EM. Genomics of IncP-1 antibiotic resistance plasmids isolated from wastewater treatment plants provides evidence for a widely accessible drug resistance gene pool. FEMS Microbiol Rev. 2007;31(4):449–77. https://doi.org/10.1111/j.1574-6976.2007.00074.x.
Lyimo B, Buza J, Subbiah M, Temba S, Kipasika H, Smith W, et al. IncF plasmids are commonly carried by antibiotic resistant Escherichia coli isolated from drinking water sources in northern Tanzania. Int J Microbiol. 2016;2016:3103672. https://doi.org/10.1155/2016/3103672.
Lorenz SC, Monday SR, Hoffmann M, Fischer M, Kase JA. Plasmids from Shiga toxin-producing Escherichia coli strains with rare Enterohemolysin gene (ehxA) subtypes reveal pathogenicity potential and display a novel evolutionary path. Appl Environ Microbiol. 2016;82(21):6367–77. https://doi.org/10.1128/AEM.01839-16.
Ferreira JC, Penha Filho RAC, Andrade LN, Berchieri Junior A, Darini ALC. Diversity of plasmids harboring blaCMY-2 in multidrug-resistant Escherichia coli isolated from poultry in Brazil. Diagn Microbiol Infect Dis. 2017;88(4):361–4. https://doi.org/10.1016/j.diagmicrobio.2017.04.014.
Girlich D, Poirel L, Carattoli A, Kempf I, Lartigue M-F, Bertini A, et al. Extended-spectrum beta-lactamase CTX-M-1 in Escherichia coli isolates from healthy poultry in France. Appl Environ Microbiol. 2007;73(14):4681–5. https://doi.org/10.1128/AEM.02491-06.
Bielak E, Bergenholtz RD, Jørgensen MS, Sørensen SJ, Hansen LH, Hasman H. Investigation of diversity of plasmids carrying the blaTEM-52 gene. J Antimicrob Chemother. 2011;66(11):2465–74. https://doi.org/10.1093/jac/dkr331.
Marcadé G, Deschamps C, Boyd A, Gautier V, Picard B, Branger C, et al. Replicon typing of plasmids in Escherichia coli producing extended-spectrum β-lactamases. J Antimicrob Chemother. 2009;63(1):67–71. https://doi.org/10.1093/jac/dkn428.
Papouskova A, Masarikova M, Valcek A, Senk D, Cejkova D, Jahodarova E, et al. Genomic analysis of Escherichia coli strains isolated from diseased chicken in the Czech Republic. BMC Vet Res. 2020;16(1):189. https://doi.org/10.1186/s12917-020-02407-2.
Nataro JP, Bopp CA, Fields PI, Kaper JB, Strockbine NA. Escherichia, Shigella, and Salmonella. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9th ed. Washington, DC: ASM Press; 2007.
Bauer AW, Kirby WM, Sherris JC, Tierch M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol. 1968;45(4):493–6.
CLSI. Performance standards for antimicrobial susceptibility testing. 27th ed. CLSI supplement M100. Wayne: Clinical and Laboratory Standards Institute; 2017.
Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–5. https://doi.org/10.1093/jac/dkp498.
Monstein H-J, Ostholm-Balkhed A, Nilsson MV, Nilsson M, Dornbusch K, Nilsson LE. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS. 2007;115(12):1400–8. https://doi.org/10.1111/j.1600-0463.2007.00722.x.
Acknowledgments
Not applicable.
Funding
This work is supported by Ferdowsi University of Mashhad under the project number 48884. The funding body played no role in the design of the study and collection, analysis, and interpretation of data in writing the manuscript.
Author information
Authors and Affiliations
Contributions
GH and MAB designed the study and GH supervised the study. MT performed the laboratory tests. GH, MAB, MT, HKR analyzed the data. MT wrote the first draft and all authors contributed in revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Author MAD is an unpaid member of the editorial board for the BMC Veterinary Research journal and the manuscript was handled indipendently by other editorial board members. All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary 1.
Correlation coefficients for significant associations (p < 0.05) among genomic criteria (virulence types, phylotypes, plasmid types) with AMR (phenotypic and ESBL genes).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tohmaz, M., Askari Badouei, M., Kalateh Rahmani, H. et al. Antimicrobial resistance, virulence associated genes and phylogenetic background versus plasmid replicon types: the possible associations in avian pathogenic Escherichia coli (APEC). BMC Vet Res 18, 421 (2022). https://doi.org/10.1186/s12917-022-03496-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03496-x