Abstract
Background
Maternal gut microbiota and metabolites are associated with their offspring’s health. Our previous study showed that maternal body fat percentage increased from days 45 to 110 of gestation in a Huanjiang mini-pig model. Thus, this study aimed to investigate the changes in gut microbiota composition and microbial metabolite profile of sows from days 45 to 110 of gestation.
Results
Twenty-four Huanjiang mini-pigs with average body weight were assigned for sample collection during early- (day 45 of pregnancy), mid- (day 75 of pregnancy), and late-pregnancy (day 110 of pregnancy). The results showed that the relative abundances of Clostridium_sensu_stricto_1, Romboutsia, Turicibacter, and Streptococcus in jejunal contents were higher at day 110 than those at day 45 or 75 of gestation. In the ileum, the relative abundance of Streptococcus was higher (P < 0.05) at day 110 of gestation, as well as the metabolism function of the jejunal and ileal microbiota. The ileal butyrate and acetate concentrations were higher at days 45 and 110 of gestation, respectively. In the colon, the concentrations of cadaverine and spermine were higher (P < 0.05) at days 45 and 110 of gestation, respectively. Metabolomic analyses demonstrated that the metabolic pathways, including D-glutamine and D-glutamate metabolism, phenylalanine/tyrosine/tryptophan biosynthesis, and alanine/aspartate/glutamate metabolism changed during gestation.
Conclusion
Collectively, our results showed that gut microbiota composition and microbial metabolites changed dramatically from early to late pregnancy in a Huanjiang mini-pig model. These findings will provide new targets in formulating maternal nutritional interventions to alleviate the adverse effects during pregnancy on offspring health outcomes.
Similar content being viewed by others
Background
Maternal physiological and biological changes during pregnancy can highly influence the growth and development of conceptus, resulting in long-term health problems in the offspring [1]. Gut microbiota impacts body physiology and is associated with the etiology of various diseases, including obesity, type 2 diabetes [2], and insulin resistance [3]. Evidence showed that maternal metabolism was associated with the changes of gut microbiota composition [4]. In addition, pregnancy metabolic syndrome is associated with the changes of gut microbiota composition in the third trimester of pregnancy [5]. These findings indicated that gut microbiota presented an important role in maternal metabolism during gestation.
Intestinal microbiota shapes host physiology through their metabolites. These metabolites, such as short-chain fatty acids (SCFAs) and bioamines, play important roles in host physiology [6]. The SCFAs are mainly produced by colonic microbiota from dietary carbohydrates and proteins [7] and are associated with metabolic syndromes [8]. For instance, butyrate protects against diet-induced fat accumulation by increasing energy expenditure [9], and acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndromes [10]. Therefore, evaluating the influence of the microbiota composition on host metabolism will help to reveal the relationship between gut microbiota, metabolites, and their host.
Intestinal microbiota and metabolites play important roles in different physiological states during pregnancy. Research evidence showed that the composition of gut microbiota and metabolites are inconsistent in mammals during pregnancy [11]. However, changes in intestinal microbiota and metabolites during different stages of pregnancy are still unknown. Pig has served as one of the animal models in clinical medicine applications on human pregnancy [12]. Therefore, we used 16 S rRNA sequencing and metabolomics technologies to investigate the gut microbiota composition and identify microbial metabolite markers from days 45 to 110 of gestation in a pig model.
Materials and methods
Animals and housing management
A total of 24 primiparous Huanjiang mini-pigs with average initial body weight (BW) of 30 kg were obtained from a pig farm located in Huanjiang County, Guangxi Province, China. The sows were randomly assigned to one of eight pens, with three sows per pen. The animals were fed a diet formulated according to the recommendations of the Chinese National Feeding Standard for Swine (Additional file 1: Table S1). All animals were housed in 2 × 3 m pens with cement-sclerified flooring. Each pen was equipped with a feeder and a nipple drinker. All sows had ad libitum access to drinking water and were fed three times daily (about 2% of BW) [13].
Sample collection and preparation
At days 45 (early-pregnancy, n = 8), 75 (mid-pregnancy, n = 8), and 110 (late-pregnancy, n = 8) of gestation, sows were euthanized using electrical stunning (120 V, 200 HZ) and exsanguination. The jejunal contents (middle portion), ileal and colonic luminal contents from a region 10 cm anterior and posterior to the ileo-cecal valve were collected, respectively [12, 14]. All samples were collected into sterile tubes and stored at −80 °C for further analysis.
DNA extraction and PCR amplification
The total genomic DNA was extracted from the intestinal content samples using HiPure Stool DNA Kits (Magen, Guangzhou, China) according to the manufacturer’s instructions. The concentrations of extracted DNA were measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA). The total DNA of the intestinal contents was diluted to 50 ng/µL and then used to prepare amplicons for high-throughput sequencing.
The V3−V4 hypervariable regions of the bacterial 16 S rRNA gene were amplified as described previously [14]. The PCR reactions volume (10 µL) comprised 4 µL of 5×FastPfu Buffer, 2 µL of 2.5 mM dNTPs, 0.8 µL of each primer (5 µM), 0.4 µL of FastPfu Polymerase, and 2 µL DNA. The PCR reactions were conducted using the following program: 3 min of denaturation at 95 °C; 27 cycles of 30 s at 95 °C, 30 s for annealing at 55 °C, and 45 s for elongation at 72 °C; and a final extension at 72 °C for 10 min. The PCR products were extracted using 2% agarose gel, further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA), and quantified using QuantiFluor™-ST (Promega, USA) according to the manufacturer’s protocol. Purified amplicons were operated using paired-end sequencing on Illumina MiSeq (Illumina, San Diego, USA). The instructions for the platform and manufacturer were from a commercial service provider (Majorbio, Shanghai, China).
Processing of sequencing data
Raw fastq files were demultiplexed, quality-filtered by Trimmomatic (v.0.30), and merged by FLASH (v.1.2.11) with the following criteria: (i) the reads were truncated at any site receiving an average quality score < 20 over a 50 bp sliding window; (ii) primers were exactly matched allowing two nucleotides mismatching and reads containing ambiguous bases were removed; (iii) sequences with overlap > 10 bp were merged according to their overlap sequence. Operational taxonomic units (OTUs) were clustered with a 97% similarity cutoff using UPARSE (v7.1 http://drive5.com/uparse/), and chimeric sequences were identified and removed using UCHIME. The taxonomy of each 16 S rRNA gene sequence was analyzed by the Ribosomal Database Project Classifier algorithm (http://rdp.cme.msu.edu/) against the Silva (SSU123) 16 S rRNA database using a confidence threshold of 70% [15]. The ACE, Chao1, and Simpson indexes for microbiota were used to estimate alpha diversity values. The unadjusted means of OTU level microbial abundances were analyzed using partial least squares discriminant analysis (PLS-DA). The intestinal microbial functions were predicted by the phylogenetic investigation of communities by reconstruction of unobserved states 1 (PICRUSt1) analysis.
Intestinal metabolites analysis
The SCFAs, including acetate, propionate, butyrate, iso-butyrate, valerate, and iso-valerate, were analyzed using gas chromatography (Agilent Technologies 1206, Santa Clara, CA, USA). Bioamines, including putrescine, cadaverine, spermidine, spermine, and tyramine, were measured using high-performance liquid chromatography (Agilent Technologies, Santa Clara, CA, USA) as described previously [13].
1 H NMR spectroscopy analysis
1 H NMR spectra were conducted according to the previous study [16]. Briefly, the 90° pulse length (~ 10.0 ms) was adjusted individually for each sample. The transients were collected into 32 k data points for each sample, with a spectral width of 20 ppm and a recycle delay of 2.0 s. The NMR spectral data scaled to unit variance were analyzed by the orthogonal projection to latent structure discriminant analysis (OPLS-DA) method. Principal component analysis (PCA) was performed to identify differential metabolites among the groups. Each metabolite was assigned for the variable importance in the projection (VIP) value according to the PLS-DA results. Metabolites with fold change VIP value > 1 and P value < 0.05 were used to select the significant differential metabolites between the three gestation stages.
Statistical analysis
The alpha diversity indices of the intestinal microbiota communities and colonic microbial metabolites were analyzed using a one-way analysis of variance and Duncan’s multiple-range post-hoc test in SAS (SAS Institute, Inc., Cary, NC). The Student’s t-test was performed to compare the two groups. The relative abundances of microbial communities at the phylum and genus levels were analyzed using the Kruskal Wallis test. The correlations between microbiota and metabolic parameters were analyzed using Pearson’s linear correlation coefficient. Differences between means were considered to be statistically significantly different when P < 0.05, and 0.05 ≤ P < 0.10 were considered a trend.
Results
Body weight and diversity of the intestinal microbiota communities
The body weight of sows increased as gestation extended (Table 1). After size filtering, quality control, and chimera removal, 1,997,373 high-quality reads were obtained. The average read length was 440 bp. All samples were normalized, and the OTU table within each sample was rarefied to 22,701 sequence reads based on the sample rarefaction curves and Shannon curves (Additional file 2: Fig. S1). A Venn diagram was used to investigate the core microbiota presented in intestinal contents during different gestation stages (Additional file 2: Fig. S2). The results showed that 555, 321, and 984 OTUs were shared in jejunal, ileal, and colonic contents, respectively.
The normalized sequence reads were used to calculate richness and diversity indices. As gestation extended, the ACE and Chao1 indices were increased (P < 0.05) in jejunal and ileal contents (Fig. 1A−F). In colonic contents, the Simpson index trended to decrease (P = 0.06) along with gestation extended (Fig. 1G−I).
The PLS-DA was used to analyze the differences among the three groups. As shown in Fig. 1J, samples from jejunal and ileal contents were clustered together. For colonic contents, the samples collected at days 45, 75, and 110 of gestation were separated from each other.
Composition of intestinal microbiota communities
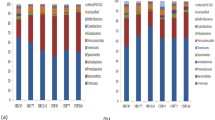
At the phylum level, the most dominant microbiota in the jejunum and ileum were Firmicutes, and those in the colon were Firmicutes and Bacteroidetes (Fig. 2A). The relative abundances of Firmicutes and Actinobacteria were lower (P < 0.05), whereas the relative abundances of Bacteroidetes and Spirochete were higher (P < 0.05) in the colon than in the jejunum or ileum (Fig. 2B−D). In the ileum, the relative abundance of Tenericutes was increased (P < 0.05), and Firmicutes trended to decrease (P = 0.06) from days 45 to 110 of gestation (Fig. 2C).
Microbiota communities at the phylum level. (A) Distribution of microbiota at the phylum level. Phyla with proportion < 0.01 were grouped in others. Comparison of relative microbiota abundance in jejunal (B), ileal (C), and colonic (D) contents at the phylum level. Differences were analyzed using Kruskal Wallis test; *P < 0.05; n = 7−8 per group.
At the genus level, the most dominant microbiota in the jejunum and ileum were Lactobacillus and Clostridium, and those in the colon were Bacteroidales_S24-7_group, Lachnospiraceae XPB1014_group, and Lactobacillus (Fig. 3A). In the jejunum, the relative abundances of Clostridium_sensu_stricto_1, Romboutsia, Turicibacter, and Streptococcus were increased (P < 0.05), while the relative abundance of Megasphaera was decreased (P < 0.05) from days 45 to 110 of gestation (Fig. 3B). In the ileum, the relative abundance of Streptococcus was higher (P < 0.05) at day 110 than that at days 45 and 75 of gestation (Fig. 3C). In the colon, the relative abundances of [Eubacterium]_coprostanoligenes_group and Streptococcus were increased (P < 0.05), while the relative abundances of Clostridium_sensu_stricto_1 and Ruminococcaceae_UCG-014 were decreased (P < 0.05) from days 45 to 110 of gestation (Fig. 3D).
Microbiota communities at the genus level. (A) Relative abundance of the 10 most abundant genera in different groups. Comparison of relative abundance in jejunal (B), ileal (C), and colonic (D) contents at the genus level. Relative abundance of the 15 most abundant genera in different groups were listed. Differences were analyzed using Kruskal Wallis test; *P < 0.05; n = 7−8 per group.
Intestinal metabolite profiles
The results of SCFA concentrations in intestinal contents are presented in Table 1. In the jejunum, the acetate concentration was lower (P < 0.05) at day 75 than that at day 45 of gestation. The ileal acetate concentration increased (P < 0.05) linearly while the butyrate concentration decreased (P < 0.05) from days 45 to 110 of gestation. Moreover, the colonic acetate concentration decreased (P < 0.05) from days 45 to 110 of gestation.
The results of bioamine concentrations in the jejunal, ileal, and colonic contents are listed in Table 2. In the jejunum, the putrescine concentration was lower (P < 0.05), whereas the spermidine and spermine concentrations were higher (P < 0.05) at day 110 of gestation. In the ileum, the putrescine concentration was lower (P < 0.05) at day 110 than that at days 45 and 75 of gestation. In the colon, the cadaverine concentration was higher (P < 0.05) at day 45 than that at days 75 and 110 of gestation, whereas the spermine concentration was lower (P < 0.05) at day 110 than that at days 45 and 75 of gestation.
Correlation between metabolites and microbiota
In the jejunum (as shown in Fig. 4A), the putrescine concentration negatively correlated with the relative abundances of Terrisporobacter, Clostridium_sensu_stricto_1, and Turicibacter; while spermidine concentration positively correlated with the relative abundances of Terrisporobacter, Clostridium sensu_stricto_1, Turicibacter, and family Peptostreptococcaceae. The spermine concentration negatively correlated with the relative abundances of Megasphaera and Olsenella.
Pearson’s linear correlation heatmap of microbial metabolites and dominant genera in jejunal (A), ileal (B), and colonic (C) contents. * in green grid indicates a negative correlation (P < 0.05) between the relative abundance of the microbiota and microbial metabolites, whereas in the red grid indicates a positive correlation (P < 0.05).
In the ileum (as shown in Fig. 4B), the acetate concentration positively correlated with the relative abundances of Streptococcus, Corynebacterium_1, Turicibacter, family Peptostreptococcaceae, and Bifidobacterium, but negatively correlated with the relative abundance of Lactobacillus.
In the colon (as shown in Fig. 4C), the cadaverine concentration negatively correlated with the relative abundances of Streptococcus and [Eubacterium] _coprostanoligenes_group. The putrescine concentration negatively correlated with the relative abundance of unclassified_f_Lachnospiraceae. The iso-butyrate concentration positively correlated with the relative abundances of Christensenellaceae_R-7_group, Treponema_2, and Ruminococcaceae_UCG-005. In addition, the iso-valerate concentration positively correlated with the relative abundance of Christensenellaceae_R-7_group.
Function prediction of microbiota communities
The PICRUSt1 analysis was performed to determine the metabolic function differences for jejunal and ileal microbiota. The results showed that eight metabolism functions, including energy production and conversion, amino acid transport and metabolism, inorganic ion transport and metabolism, carbohydrate transport and metabolism, nucleotide transport and metabolism, coenzyme transport and metabolism, lipid transport and metabolism, and secondary metabolites biosynthesis, transport, and catabolism were increased (P < 0.05) from days 45 to 110 of gestation (Fig. 5A−D). However, there was no significant change (P > 0.05) in metabolism functions in the colon of pigs during different gestation stages (Fig. 5E−F).
Metabonomic analysis
A 1 H NMR spectroscopy analysis was conducted to analyze the metabolite profiles in intestinal contents. The PCA score plots (Additional file 2: Fig. S3) showed distinct separation in the jejunum, ileum, and colon. The PLS-DA model showed that samples from the three gestation stages (days, 45 vs. 75 and 75 vs. 110) were well-separated (Fig. 6A−F). The concentrations of 17 metabolites, including leucine, isoleucine, valine, alanine, citrulline, arginine, lysine, proline, methionine, glutamate, pyruvate, succinate, glutamine, asparagine, choline, threonine, and tyrosine were decreased (P < 0.05) in the jejunum from day 45 to day 75 of gestation (Fig. 7A). In addition, the concentrations of leucine, isoleucine, valine, alanine, citrulline, arginine, lysine, proline, methionine, and tryptophan were decreased (P < 0.05) at day 45 of gestation compared to those at day 110 of gestation (Fig. 7A). From days 45 to 110 of gestation, the concentrations of isoleucine, methionine, glutamate, glutamine, pyruvate, and aspartate were decreased (P < 0.05) in the ileum (Fig. 7B). The concentrations of leucine, isoleucine, arginine, proline, methionine, glutamate, pyruvate, glutamine, aspartate, asparagine, choline, glycine, creatine, histidine, and tyrosine were increased (P < 0.05) in the colon from day 75 to day 110 of gestation (Fig. 7C).
Metabolic pathway analysis
MetaboAnalyst 4.0 was performed to explore the metabolic pathways in intestinal contents from early to late gestation stages. The identified metabolites with P < 0.05 and VIP > 1 were used to perform pathway analysis (Additional file 1: Tables S2−4). A series of metabolic pathways were affected by the gestation stage. In the jejunum, the pathways with significant interferences were D-glutamine and D-glutamate metabolism, taurine and hypotaurine metabolism, alanine/aspartate/glutamate metabolism, and pyruvate metabolism (Fig. 8A−B). The primary metabolic pathways impacted in the ileum were D-glutamine and D-glutamate metabolism, phenylalanine/tyrosine/tryptophan biosynthesis, glycolysis or gluconeogenesis, alanine/aspartate/glutamate metabolism, and arginine and proline metabolism (Fig. 8C−D), and those in the colon were D-glutamine and D-glutamate metabolism, phenylalanine/tyrosine/tryptophan biosynthesis, valine/leucine/isoleucine biosynthesis, and alanine/aspartate/glutamate metabolism (Fig. 8E−F).
Discussion
Gut microbiota and metabolites exert important roles in maternal metabolism and physiology [17, 18]. Moreover, gut microbiota and metabolites are also associated with the changes of maternal physiology during pregnancy and play important roles in the growth and development of the fetus, and thus influence the offspring’s health later in life [19]. Therefore, the present study was conducted to investigate the changes in gut microbiota composition and microbial metabolites in sows from early (day 45) to late (day 110) gestation stages. We found that the microbiota richness and diversity in the jejunum and ileum increased from days 45 to 110 of gestation and identified several bacteria and metabolites using a microbiota-metabolome analysis. Moreover, our findings also demonstrated that acetate concentration increased in the ileum but decreased in the colon from days 45 to 110 of gestation. These findings suggested that gestation stages markedly altered the intestinal microbial community and metabolic profiles, which might be involved in maternal physiological and biological changes at late gestation and long-term health problem in the offspring.
The higher microbial richness and diversity are generally considered to be beneficial for the overall health and productivity of animals. In the present study, the richness and diversity of jejunal and ileal microbiota increased from days 45 to 110 of gestation, as evidenced by the ACE, Chao1, and Simpson indices. However, gestation stages exerted no effects on colonic microbiota richness, with no differences observed in the ACE and Chao1 indices. These findings were in line with a previous study, which reported that the gestation stage did not alter colonic microbiota richness in gestating sows [13]. By contrast, several studies showed that the diversity and richness of fecal microbiota were lower at the end than those at the beginning of pregnancy [11, 20]. This discrepancy might be explained partially by differences in the animal model; however, further studies are needed to confirm the exact reason. The animal model used in the present study was the sow, whereas that used in studies of Koren et al. [11] and Kennedy et al. [20] were human and rat, respectively. The PLS-DA showed that the microbiota composition in jejunal and ileal contents at days 45, 75, and 110 of gestation were clustered together but separated from the colonic samples, suggesting that the small intestine and colon showed different microbiota composition. In addition, the colon samples were separated in a time-dependent manner, indicating that microbiota composition in the colon presented enormous dynamic changes throughout the gestation.
At the phylum level, Firmicutes and Bacteroidetes are the most abundant bacteria in porcine intestines. In the present study, Firmicutes was the most dominant phyla in jejunal and ileal contents, and those in colonic contents were Firmicutes and Bacteroidetes, which is consistence with previous studies [12, 13]. Firmicutes and Bacteroidetes are also the dominant phyla in colonic contents in Large × Landrace pig [21]. In addition, a lower Firmicutes relative abundance and Firmicutes/Bacteroidetes ratio were observed in the ileum from days 45 to 110 of gestation, which is in agreement with the changes in body fat percentage of sows [22]. However, earlier studies showed that obesity was associated with the increased Firmicutes relative abundance and Firmicutes/Bacteroidetes ratio [23, 24]. This discrepancy might be explained by differences in the physiology of animals, diets, and tested samples.
The small intestine and colon also showed a marked differences in microbiota type at the genus level. The most dominant genera in jejunal and ileal contents were Lactobacillus and Clostridium, which in colonic contents was norank_f_Bacteroidetes_S24-7_group. In vitro and in vivo studies showed that Lactobacillus could reduce fat storage [25, 26] and protect animals against obese-insulin resistance [27]. In addition, colonization of Lactobacillus in the intestine was beneficial to health and prevented the colonization of opportunistic pathogens [28]. In the present study, the relative abundance of Lactobacillus reduced in the jejunum and ileum ongoing with pregnancy, which indicating that Lactobacillus was associated with changed maternal metabolism during the pregnancy. Obese women had a higher number of Streptococcus mutants compared to normal-weight women [29], and higher Streptococcus colonization was also significantly associated with obesity in children [30]. Here, the higher relative abundance of Streptococcus in the jejunum, ileum, and colon at day 110 of gestation suggested that Streptococcus might contribute to maternal fat accumulation. However, the mechanism still needs further investigation. The increase of the relative abundance of Clostridium_sensu_stricto_1 along with the pregnancy may have connections with the inflammation in the jejunum and ileum [31]. Other studies also showed that some clusters of Clostridium produced butyrate and played a role in anti-inflammation [32, 33]. Based on these previous studies, we suspected that the increased relative abundance of Clostridium_sensu_stricto_1 in the jejunum might contribute to the improvement of jejunal health as gestation extended.
Our previous study indicated that the SCFAs were associated with body fat accumulation [14]. Therefore, we investigated whether intestinal SCFAs concentration changed as gestation extended. The present study showed that the main SCFAs in jejunal and ileal contents were acetate and butyrate, while those in colonic contents were acetate, propionate, and butyrate. Acetate participates in energy generation and lipogenesis for all types of tissues [34]. Butyrate increases energy expenditure and prevents diet-induced fat accumulation and insulin resistance [35]. Consistent with the changes in maternal body fat percentage [24], ileal acetate concentration increased, and butyrate concentration decreased from days 45 to 110 of gestation, indicating that acetate and butyrate in the ileum might contribute to maternal fat accumulation in late pregnancy. Our previous study also showed that butyrate concentration was negatively associated with the body fat content in Duroc × Large White × Landrace pigs [14]. Based on these findings, we speculated that acetate and butyrate might be the main SCFAs involved in maternal fat metabolism during pregnancy.
The gestation stage also leads to different intestinal microbial functions and metabolite profiles. In the present study, microbiota in the small intestine and colon showed different functions, with eight functions increased in the jejunum and ileum, whereas these functions showed no differences in the colon. This discrepancy might be explained by differences in microbiota composition. The most dominant genera in the jejunum and ileum were Lactobacillus and Clostridium, whereas those in the colon were norank_f_Bacteroidetes_S24-7_group, Treponema_2, and Lachnospiraceae_XPB1014_group.
To investigate the functional states of gut microbiota, a metabolomics analysis was conducted in the present study. The PLS-DA analysis showed a clear cluster of metabolic profiles between different gestation stages, indicating significant differences in metabolite profiles from days 45 to 110 of gestation. At day 75 of gestation, the concentrations of amino acids were lower in the jejunum. Consistent with the changes in the jejunum, the concentrations of amino acids were decreased in the ileum as gestation extended. The main role of the jejunum and ileum is nutrient digestion and absorption. The decreased amino acid concentrations partly indicated that the digestion and absorption function of the maternal small intestine improved as gestation extended. Our results also indicate that the most influenced metabolic pathways in the intestine are D-glutamine and D-glutamate metabolism. The fetus grows quickly from day 75 of gestation to late gestation, and the mother needs to obtain adequate nutrients at this stage to support the growth and development of the fetus [7]. Therefore, we suspected that the increased metabolism functions of microbiota during late pregnancy might help the mother acquire extra nutrients. Collectively, our study showed that gut metabolites changed dramatically from early to late pregnancy, which might be associated with maternal physiology.
Conclusion
In summary, the present study indicated that the jejunal and ileal microbiota community increased from days 45 to 110 of gestation. As gestation extended, ileal acetate concentration was increased, and butyrate concentration decreased, which were associated with the relative abundances of specific microbial genera. These findings can help us to understand the changes in gut microbiota communities and microbial metabolites from early to late pregnancy, and provide new targets in formulating the nutritional intervention to ameliorate the adverse effects of maternal fat accumulation on offspring health outcomes.
Data availability
The datasets supporting the conclusions of this article can be found in online repositories. The names of the repository and accession number can be found at: Science Data Bank with accession DOI: https://doi.org/10.57760/sciencedb.j00001.00704.
References
Lahti PM, Bhattacharya S, Wild SH, Lindsay RS, Raikkonen K, Norman JE, et al. Consequences of being overweight or obese during pregnancy on diabetes in the offspring: A record linkage study in Aberdeen. Scotl Diabetologia. 2019;62:1412–9.
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99.
Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, et al. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22:516–30.
Nurielohayon M, Neuman H, Koren O. Microbial changes during pregnancy, birth, and infancy. Front Microbiol. 2016;7:1031.
Crusell MKW, Hansen TH, Nielsen T, Allin KH, Ruhlemann MC, Damm P, et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome. 2018;6:89.
Ríoscovián D, Ruasmadiedo P, Margolles A, Gueimonde M, Reyesgavilán CGDL, Salazar N. Intestinal short-chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185.
Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TMS. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes. 2014;38:1525–31.
Delzenne NM, Cani PD. Interaction between obesity and the gut microbiota: Relevance in nutrition. Annu Rev Nutr. 2011;31:15–31.
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–17.
Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–7.
Koren O, Goodrich J, Cullender TC, Spor A, Laitinen K, Bäckhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–80.
Ji YJ, Kong XF, Li HW, Zhu Q, Guo QP, Yin YL. Effects of dietary nutrient levels on microbial community composition and diversity in the ileal contents of pregnant Huanjiang mini-pigs. PLoS ONE. 2017;12:e0172086.
Kong XF, Ji YJ, Li HW, Zhu Q, Blachier F, Geng MM, et al. Colonic luminal microbiota and bacterial metabolite composition in pregnant Huanjiang mini-pigs: Effects of food composition at different times of pregnancy. Sci Rep. 2016;6:37224.
Hu CJ, Li FN, Duan YH, Yin YL, Kong XF. Dietary supplementation with leucine or in combination with arginine decreases body fat weight and alters gut microbiota composition in finishing pigs. Front Microbiol. 2019;10:1767.
Amato KR, Yeoman CJ, Kent A, Righini N, Carbonero F, Estrada A, et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013;7:1344–53.
He QH, Ren PP, Kong XF, Xu WX, Tang HR, Yin YL, et al. Intrauterine growth restriction alters the metabonome of the serum and jejunum in piglets. Mol BioSyst. 2011;7:2147–55.
Heiss CN, Olofsson LE. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J Neuroendocrinol. 2019;31:e12684.
Ziętek M, Celewicz Z, Szczuko M. Short-chain fatty acids, maternal microbiota and metabolism in pregnancy. Nutrients. 2021;13:1244.
Ma C, Gao QK, Zhang WH, Azad MAK, Kong XF. Alteration in the blood parameters and fecal microbiota and metabolites during pregnant and lactating stages in Bama mini pigs as a model. Med Inflamm. 2020; 2020:8829072.
Kennedy RC, Fling RR, Robeson MS, Saxton AM, Donnell RL, Darcy JL, et al. Temporal development of gut microbiota in triclocarban exposed pregnant and neonatal rats. Sci Rep. 2016;6:33430.
Liu BS, Zhu XY, Cui YL, Wang WJ, Liu H, Li ZD, et al. Consumption of dietary fiber from different sources during pregnancy alters sow gut microbiota and improves performance and reduces inflammation in sows and piglets. mSystems. 2021;6(1):e00591-20.
Zhu Q, Xie PF, Li HW, Ma C, Zhang WH, Yin YL, et al. Fetal Huanjiang mini-pigs exhibit differences in nutrient composition according to body weight and gestational period. PLoS ONE. 2018;13:e0199939.
Le CE, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6.
Dalby MJ, Ross AW, Walker AW, Morgan PJ. Dietary uncoupling of gut microbiota and energy harvesting from obesity and glucose tolerance in mice. Cell Rep. 2017;21:1521–33.
Huang Y, Zheng Y. The probiotic Lactobacillus acidophilus reduces cholesterol absorption through the down-regulation of Niemann-Pick C1-like 1 in Caco-2 cells. Br J Nutr. 2010;103:473–8.
Linda A, Ying H, Paolo P, Marion KA, Janet H, Jan-Åke G, et al. Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4). PLoS ONE. 2010;e13087.
Wanchai K, Yasom S, Tunapong W, Chunchai T, Eaimworawuthikul S, Thiennimitr P, et al. Probiotic Lactobacillus paracasei HII01 protects rats against obese-insulin resistance-induced kidney injury and impaired renal organic anion transporter 3 function. Clin Sci. 2018;132:1545–63.
Baba E, Nagaishi S, Fukata T, Arakawa A. The role of intestinal microflora on the prevention of Salmonella colonization in Gnotobiotic chickens. Poult Sci. 1991;70:1902–7.
Barkeling B, Linne Y, Lindroos AK, Birkhed D, Rooth P, Rossner S. Intake of sweet foods and counts of cariogenic microorganisms in relation to body mass index and psychometric variables in women. Int J Obes Relat Metab Disord. 2002;26:1239–44.
Ndanu TA, Aryeetey R, Sackeyfio J, Otoo G, Lartey A, Opintan JA, et al. Streptococcus mutans and Lactobacillus species infection in obese and non-obese school children in Accra, Ghana. J Obes Overweight. 2015;1:1–5.
Zhao X, Guo Y, Liu H, Gao J, Nie W. Clostridium butyricum reduce lipogenesis through bacterial wall components and butyrate. Appl Microbiol Biotechnol. 2014;98:7549–57.
Abbeele PVD, Belzer C, Goossens M, Kleerebezem M, Vos WMD, Thas O, et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7:949–61.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8.
Blaut M. Gut microbiota and energy balance: Role in obesity. Proc Nutr Soc. 2015;74:227–34.
Hong J, Jia Y, Pan S, Jia L, Li H, Han Z, et al. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget. 2016;7:56071–82.
Funding
This study was jointly supported by the China Agricultural Research System of MOF and MARA (CARS-35), National Natural Science Foundation of China (31772613), and Special Funds for Construction of Innovative Provinces in Hunan Province (2019RS3022).
Author information
Authors and Affiliations
Contributions
P.X. and X.K. conceived and designed the study. P.X., C.H., M.A.K.A., and Q.Z. performed the experiments. P.X., C.H., and Q.H. processed the data. P.X. and C.H. prepared and drafted the manuscript. M.A.K.A., Q.H., and X.K. revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Animal Care Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China. All animal experiments were conducted following the guideline of the Research Ethics Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences. All methods in this study were also carried out in compliance with the ARRIVE guidelines (https://arriveguidelines.org).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, P., Hu, C., Azad, M.K. et al. Dynamic alteration in the gut microbiota and metabolome of Huanjiang mini-pigs during pregnancy. BMC Vet Res 18, 385 (2022). https://doi.org/10.1186/s12917-022-03477-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03477-0