Abstract
Background
Little is known about the safety and efficacy of discontinuing antiplatelet therapy via LMWH bridging therapy in elderly patients with coronary stents implanted for > 12 months undergoing non-cardiac surgery. This randomized trial was designed to compare the clinical benefits and risks of antiplatelet drug discontinuation via LMWH bridging therapy.
Methods
Patients were randomized 1:1 to receive subcutaneous injections of either dalteparin sodium or placebo. The primary efficacy endpoint was cardiac or cerebrovascular events. The primary safety endpoint was major bleeding.
Results
Among 2476 randomized patients, the variables (sex, age, body mass index, comorbidities, medications, and procedural characteristics) and percutaneous coronary intervention information were not significantly different between the bridging and non-bridging groups. During the follow-up period, the rate of the combined endpoint in the bridging group was significantly lower than in the non-bridging group (5.79% vs. 8.42%, p = 0.012). The incidence of myocardial injury in the bridging group was significantly lower than in the non-bridging group (3.14% vs. 5.19%, p = 0.011). Deep vein thrombosis occurred more frequently in the non-bridging group (1.21% vs. 0.4%, p = 0.024), and there was a trend toward a higher rate of pulmonary embolism (0.32% vs. 0.08%, p = 0.177). There was no significant difference between the groups in the rates of acute myocardial infarction (0.81% vs. 1.38%), cardiac death (0.24% vs. 0.41%), stroke (0.16% vs. 0.24%), or major bleeding (1.22% vs. 1.45%). Multivariable analysis showed that LMWH bridging, creatinine clearance < 30 mL/min, preoperative hemoglobin < 10 g/dL, and diabetes mellitus were independent predictors of ischemic events. LMWH bridging and a preoperative platelet count of < 70 × 109/L were independent predictors of minor bleeding events.
Conclusions
This study showed the safety and efficacy of perioperative LMWH bridging therapy in elderly patients with coronary stents implanted > 12 months undergoing non-cardiac surgery. An alternative approach might be the use of bridging therapy with half-dose LMWH.
Trial registration
ISRCTN65203415.
Similar content being viewed by others
Background
An increasing number of patients are prescribed single antiplatelet therapy for the prevention of myocardial infarction (MI) and coronary stent thrombosis more than 12 months after the placement of bare metal stents (BMSs) and drug-eluting stents (DESs) [1, 2]. Patients with coronary stents who are taking antiplatelet drugs and who require non-cardiac surgery or invasive procedures are commonly encountered, and their perioperative management is an important consideration.
In patients who undergo percutaneous coronary intervention (PCI) with stenting, there is a delicate balance between the risk of cardiovascular or thrombotic events and the potential risk of bleeding complications. The impact of discontinuing antiplatelet agents in patients with implanted stents undergoing non-cardiac surgery is debated, with previous studies showing conflicting results [3, 4]. Discontinuation of antiplatelet therapy has the potential to increase the risk of major perioperative adverse cardiovascular events, with stent thrombosis being the most feared because of its high associated morbidity and mortality [3, 5]. The continuation of antiplatelet therapy is recommended for patients who are at a moderate-to-high risk of cardiovascular events, while discontinuation is recommended in low-risk patients [6]. However, in the POISE-2 trial, perioperative use of aspirin had no significant effect on the combined risk of death or non-fatal MI in patients undergoing non-cardiac surgery, but it did increase the risk of bleeding [7].
Bridging therapy with low-molecular-weight heparin (LMWH) is usually recommended in patients who require interruption of anticoagulation before surgery [8,9,10]. Although platelets are the primary players in stent thrombosis, coagulation also plays a role [11]. To avoid thrombotic events during surgery while minimizing the perioperative bleeding risk, clinicians often administer LMWH in clinical practice to “bridge” patients undergoing non-cardiac surgery until the previous antiplatelet treatment can be resumed [4, 12]. However, in patients with stents who require bridging via non-cardiac surgery, the European Society of Cardiology guidelines discourage the use of LMWH because this approach might be associated with a greater bleeding risk [13]; however, this approach still needs to be validated in a randomized clinical study.
With aging, the onset of comorbidities and impairments in cardiac, renal, and hepatic function not only increase the incidence of perioperative ischemic cardiovascular or thrombotic events, but they also increase the risk of major bleeding. The optimal management of these complex patients has [14] not been determined, and data from randomized controlled trials are lacking. Randomized controlled trials comparing the use of bridging therapy with no bridging therapy in patients with atrial fibrillation have shown a higher bleeding risk without a change in the incidence of thromboembolic events [15, 16]. Increasing concern has been raised that bridging therapy increases the risk of bleeding in patients without reducing the risk of thromboembolism. However, little is known about the safety and efficacy of discontinuing antiplatelet therapy via LMWH bridging therapy in older patients with coronary stents implanted for > 12 months undergoing non-cardiac surgery. The present randomized controlled trial aimed to shed some light on this debated topic.
Methods
This study was reported using the Consolidated Standards of Reporting Trials (CONSORT) guidelines [17] (Additional File 1: CONSORT Checklist).
Study design and oversight
The trial (ISRCTN65203415) was a randomized placebo-controlled trial. The study was approved by the ethics committee of Chinese PLA General Hospital. The clinical coordinating group was responsible for study coordination, patient randomization, and therapy assignment. The data coordinating group was responsible for maintenance of the study database, data validation, and data analysis.
Patients
Patients who met the following inclusion criteria were included in the trial: (1) ≥ 75 years of age, (2) underwent PCI with stents > 12 months before non-cardiac surgery, (3) treated with antiplatelet therapy for ≥ 1 year, and (4) were undergoing elective surgery or other elective invasive procedures that required interruption of antiplatelet therapy.
Patients’ characteristics included general demographics, clinical covariates, laboratory values, comorbidities, type of surgery, perioperative medication, and PCI information. The surgical procedures were categorized according to surgical risk based on the definition of the Revised Cardiac Risk Index (RCRI) [18].
Patients were excluded if they met one or more of the following: (1) < 75 years of age; (2) had taken antiplatelet therapy for < 12 months; (3) were scheduled for surgery with local anesthesia; (4) were planned for cardiac surgery; (5) had major cardiac ischemic events and/or bleeding within the previous 6 weeks; (6) had a mechanical heart valve, some of whom were receiving both oral anticoagulant therapy and antiplatelet therapy; (7) had a platelet count of < 100 × 103/mm3. The patients were recruited from Chinese PLA General Hospital, and all patients provided written informed consent. Figure 1 shows the patient selection process.
Procedures
Randomization was performed using a computer-generated random number list in a 1:1 ratio in block sizes of six patients. The patients were randomly assigned to receive LMWH bridging therapy with dalteparin sodium (2500 IU administered subcutaneously twice daily) or no bridging therapy (a matching subcutaneous placebo). Dalteparin sodium and placebo were prepared by the pharmacist. A randomized sequence list was also constructed in which the subject number was linked to the study medication number. Gao G.L., Mao Q.X., Li T.Z., Su Y.H., and Ma C. generated the random allocation sequence; Xu L.N., Cheng W.J., Wang R., Lu Q.M., Zhang Y., Wang R., and Lu Y. enrolled the patients; and Wang B., He J., Chen S.H., and Chen L. assigned the patients to the interventions.
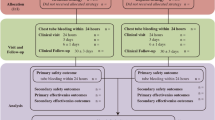
In patients requiring antiplatelet interruption, aspirin and prasugrel were interrupted for 7 days, and clopidogrel and ticagrelor were interrupted for 5 days before the elective surgery/procedure. The last preoperative dose of LMWH was administered 24 h before surgery [19]. LMWH or placebo was resumed 24 h after a surgery/procedure with a low-to-moderate bleeding risk and 48–72 h after a surgery/procedure with a high bleeding risk [6] (Fig. 2). The procedural bleeding risk was assessed based on International Society on Thrombosis and Haemostasis (ISTH) guidance statements [14]. The patients were divided into “high,” “low-to-moderate,” and “minimal” bleeding risk categories based on the expected 30-day postoperative risk of major bleeding (high bleeding risk: ≥ 2%, low-to-moderate bleeding risk: 0–2%, and minimal bleeding risk: 0%) [20].
Data collection
Clinical and surgical variables and laboratory data were collected using standardized reporting forms and assessed for quality. Clinical and surgical variables included baseline characteristics and PCI information of the study population. The following laboratory measurements were collected: hemoglobin, activated partial thromboplastin time (APTT), creatine kinase (CK), CK-MB, myoglobin, lactate dehydrogenase, creatinine, albumin, sodium, N-terminal pro-brain natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT). Biochemical measurements were performed using standard laboratory techniques. CK-MB level was measured by the Abbott AXSYM automatic immune analyzer (Abbott Laboratories, Chicago, IL). Hs-cTNT, creatinine, and NT-proBNP were measured by the Cobas 8000 instrument. NT-proBNP and hs-cTNT were analyzed by the electrochemiluminescence immunoassays “ECLIA.”
Clinical follow-up data were prospectively collected through scheduled clinic evaluations. The patients were followed up by telephone each week, with the final telephone follow-up conducted 30 days after the procedure.
Study outcomes
All study outcomes were assessed 30 days after the procedure. The primary efficacy endpoint was the composite of ischemic cardiac or cerebrovascular events, defined as perioperative myocardial injury, perioperative MI, cardiac death, and non-fatal ischemic stroke. Perioperative myocardial injury was defined as a postoperative hs-cTnT concentration of 20 to < 65 ng/L, with an absolute change of at least 5 ng/L or an hs-cTnT concentration of ≥ 65 ng/L within 3 days after non-cardiac surgery, without any of the clinical, electrocardiogram (ECG), or imaging criteria [21,22,23,24]. Perioperative acute MI was diagnosed if the hs-cTnT criteria for perioperative myocardial injury were met and accompanied by one or more of the following: ischemic symptoms (e.g., chest pain), ischemic ECG changes, new regional wall motion abnormalities, or coronary thrombus [21,22,23,24]. Non-fatal stroke was defined as any ischemic cerebrovascular disease.
The primary safety outcome was major bleeding defined by one or more of the events defined by the ISTH [15]. The secondary efficacy endpoints were pulmonary embolism, deep vein thrombosis, and death. The secondary safety endpoint was minor bleeding. All study endpoints were independently and blindly adjudicated.
Statistical analysis
The sample size was determined to achieve a precise estimate of the efficacy and safety of the protocol. According to a previous systematic review, the estimated risk of periprocedural thromboembolic events is 0.89% (≈0.9%) and 0.46% (≈0.5%) for the bridged and non-bridged groups, respectively [16]. A sample size of 2384 patients was considered appropriate for the present study because it would yield a 1.0% margin of error at the 95% confidence level (two-sided significance level = 0.05). This sample size would give the study 80% power to detect the expected difference in the rate of periprocedural thromboembolic events. With a 5% allowance for patient withdrawal from the study, the required sample size was 2500 patients. The sample size was calculated online (https://powerandsamplesize.com).
Patients’ data are summarized using descriptive statistics. The normality of the continuous numeric variables is tested before analysis. The data in normal distribution are reported as the mean ± standard deviation and are compared between the bridged and non-bridged groups using Student’s t-test. And the data in non-normal distribution are expressed in quartiles: P50 (P25; P75). The Mann-Whitney U test is used to compare the differences between the two groups. Categorical variables are presented as count (percentage) and were compared between the two groups using the chi-square test or Fisher’s exact test.
The primary efficacy endpoint was the composite of ischemic cardiac or cerebrovascular events at 30 days after the procedure. The primary safety endpoint was major bleeding at 30 days after the procedure. To identify the predictors of clinical outcomes, a multivariable analysis was performed via stepwise logistic regression. Candidate variables or covariates included in the logistic regression model: age (continuous variable), sex (male/female), LMWH bridging, American Society of Anesthesiologists (ASA), body mass index (BMI), medications, comorbidities, type of surgery, preoperative hemoglobin, preoperative platelet, perioperative mean arterial pressure (MAP), creatinine clearance. The results of logistic regression are presented as relative risk (RR) and 95% confidence intervals (CIs). We considered p < 0.05 as statistically significant, and the SPSS 26.0 software was used for all statistical analyses.
Results
Patients’ characteristics
We recruited 2490 patients between January 1, 2023, and November 1, 2023. Of the 2490 patients enrolled in the trial, 14 patients did not participate. The patients’ baseline characteristics are presented in Table 1. A total of 1242 patients were assigned to receive LMWH bridging therapy (bridging group), and 1234 patients were assigned to the placebo (non-bridging) group. The demographic and baseline variables (sex, age, body mass index, comorbidities, RCRI score, medications, procedural characteristics, and preoperative hs-cTnT concentration) were not significantly different between the two groups. The characteristics were well balanced between the two groups.
Of the 2476 patients enrolled in the trial, 1975 (79.77%) were treated with aspirin, and 386 (15.59%) were treated with clopidogrel. Table 2 shows the PCI information of the study population. Compared with the bridging group, the non-bridging group had a significantly higher proportion of patients treated for two-vessel disease (46.76% vs. 41.94%, p = 0.016) and a lower proportion of patients treated for single-vessel disease (39.14% vs. 45.00%, p = 0.003). There was no significant difference in the type of stent used for PCI between the two groups (p = 0.471). The maximum postoperative hs-cTnT concentration in the bridging group was significantly lower than in the non-bridging group (p = 0.000).
Perioperative anticoagulant management
The mean number of drugs administered was 5.69 ± 2.02. There was no significant difference in the mean number of drugs used between the non-bridging group and the bridging group. Patients in the bridging group were administered dalteparin sodium 2500 IU subcutaneously twice daily. Patients who experienced bleeding in the perioperative period required interruption of LMWH therapy. Antiplatelet treatment was restarted 72 h after a surgery/procedure with a low-to-moderate bleeding risk and 7 days after a surgery/procedure with a high bleeding risk at the patient’s usual dose.
Thirty-day ischemic and bleeding outcomes
All of the enrolled patients completed the study and provided clinical outcome data. The perioperative clinical outcomes of the patients are shown in Table 3. During the follow-up period, the rate of the combined endpoint in the bridging group was significantly lower than in the non-bridging group (5.79% vs. 8.42%, p = 0.012). The incidence of myocardial injury in the bridging group was significantly lower than in the non-bridging group (3.14% vs. 5.19%, p = 0.011). There was no significant difference between the groups in terms of the rates of acute MI, cardiac death, stroke, and major bleeding. The median time to a major bleeding outcome after the procedure was 5 days (interquartile range 1.0–15.0 days).
Deep vein thrombosis occurred more frequently in the non-bridging group (1.21% vs. 0.4%, p = 0.024), and there was a trend toward more pulmonary embolism in the non-bridging group (0.32% vs. 0.08%, p = 0.177). Minor bleeding occurred in 0.65% of the patients in the non-bridging group and in 1.53% of the patients in the bridging group (p = 0.035), which indicated that LMWH bridging therapy increased the risk of perioperative minor bleeding. However, there was no significant difference between the groups in the incidence of the combined secondary endpoint (p = 0.620).
The multivariable analysis showed that LMWH bridging therapy (RR 3.114 [95% CI 1.124–8.626]), creatinine clearance < 30 mL/min (RR 3.931 [95% CI 1.121–13.787]), perioperative mean arterial pressure < 60 mmHg (RR 1.416 [95% CI 1.041–1.927]), preoperative hemoglobin < 10 g/dL (RR 2.205 [95% CI 1.228–7.109]), and diabetes mellitus (RR 4.901 [95% CI 2.816–13.758]) were significantly associated with an increased risk of 30-day ischemic myocardial events, including MI and myocardial injury (Table 4). LMWH bridging therapy was significantly associated with an increased risk of minor bleeding (RR 6.560 [95% CI 1.748–14.616]) and a decreased risk of deep vein thrombosis (RR 0.119 [95% CI 0.031–0.453]). A preoperative platelet count < 70 × 109/L (RR 1.732 [95% CI 1.036–2.909]) was an independent predictor of minor bleeding events (Table 5).
Discussion
The present study shows that LMWH bridging therapy did not increase the occurrence of the composite outcome of perioperative acute MI, cardiac death, or stroke at 30 days in patients who underwent surgery > 1 year after PCI compared with placebo. However, compared with placebo, bridging therapy decreased the incidence of perioperative myocardial injury after surgery. Compared with the bridging group, the non-bridging group had a significantly higher proportion of patients treated for two-vessel disease and a lower proportion of patients treated for single-vessel disease. This result may be one of the reasons for the higher incidence of perioperative myocardial injury in the non-bridging group. Moreover, LMWH bridging therapy did not increase the risk of perioperative major bleeding, and it decreased the occurrence of perioperative deep vein thrombosis. Perioperative antithrombotic management is based on risk assessment of thromboembolism and bleeding. Previous studies have been published regarding LMWH bridging in patients with implanted coronary stents undergoing surgery, but the results contradict our findings [4, 13, 25, 26]. This inconsistency may be explained as follows. First, the mean time interval between PCI and surgery was 75 months in the present study. However, 30.3% of the included patients underwent surgery within 180 days after PCI. Surgery within 1 year of PCI is associated with an increased risk of perioperative major adverse cardiovascular events (MACEs) [27,28,29,30]. After 1 year of PCI, the rates of death, MI, and stent thrombosis return to baseline [29, 31, 32]. Second, age has long been established as a factor influencing drug pharmacokinetics. Elderly patients have a higher incidence of age-related comorbidities, less lean body mass, and an increased bleeding risk [33,34,35]. Thus, the LMWH dose in the present study was reduced (dalteparin sodium 2500 IU subcutaneously twice daily). Low-dose LMWH is likely to achieve much of the benefit of therapeutic-dose anticoagulation while minimizing the risk of postoperative major bleeding [6, 36]. Third, the present study showed that LMWH can be safely resumed 24 h after surgery/procedures with a low-to-moderate bleeding risk and 48–72 h after surgery/procedures with a high bleeding risk. The appropriate time window for resuming LMWH is very important. If there is no contraindication after fully evaluating the risk of bleeding and thrombosis, perioperative use of low-dose LMWH will help reduce the risk of perioperative lower-limb venous thrombosis.
The presence of coronary artery disease is associated with an increased risk of postoperative cardiovascular complications [37, 38]. In particular, surgical procedures are performed in patients with a history of PCI, in whom the risk of perioperative adverse cardiac events is potentially is potentially high [39, 40]. Many older patients are treated with antiplatelet agents for the secondary prevention of cardiovascular events. However, the administration of antiplatelet agents (most commonly aspirin) increases the risk of major perioperative bleeding. The balance between bleeding and thrombotic risk related to perioperative maintenance of antiplatelet therapy should be outweighed [7, 41, 42]. To date, studies guiding perioperative antiplatelet management in patients with coronary stents have shown inconsistent results. Clinicians often have difficulty choosing a management strategy that allows the surgery to be as safe as possible while minimizing the risk of perioperative cardiac events. Previous studies have reported that antiplatelet therapy confers an increased risk of bleeding [43,44,45,46] and predicts poor outcomes [47]. Real-world clinical practice and published studies have reported perioperative discontinuation of antiplatelet therapy after PCI [3, 48, 49]. At present, no specific recommendations have been made for the use of LMWH for bridging patients on antiplatelet therapy to surgery. In real life, perioperative discontinuation of antiplatelet therapy and bridging with LMWH are common practices. In the present study, perioperative bridging therapy of patients with coronary stents implanted > 12 months before non-cardiac surgery decreased the incidence of myocardial injury and perioperative deep vein thrombosis compared with placebo. Moreover, it did not increase the risk of perioperative acute MI, cardiac death, stroke, or major bleeding. Therefore, an alternative approach might be the use of perioperative bridging therapy with LMWH, which was studied in patients who underwent PCI > 12 months in the present trial.
These ischemic myocardial events were associated with five preoperative risk factors: LMWH bridging, creatinine clearance < 30 mL/min, preoperative hemoglobin < 10 g/dL, diabetes mellitus, and perioperative mean arterial pressure. Bleeding events were associated with four preoperative risk factors: LMWH bridging, creatinine clearance < 30 mL/min, and preoperative platelet count < 70 × 109/L. As shown in previous studies, perioperative adverse events were associated with high-risk patients, such as those with diabetes mellitus [50, 51], preoperative renal insufficiency [52], anemia [53], antiplatelet/anticoagulant therapy [54, 55], and a low platelet count [56]. Clinicians should consider risk factors when deciding whether to discontinue perioperative antiplatelet therapy or use LMWH bridging therapy in the perioperative period.
Defining the trade-off between ischemia and bleeding requires not only an understanding of the thrombotic risk of the patient (usually defined by cardiologists) but also a clear understanding of the unique bleeding risk of each surgical procedure, which requires the professional knowledge of the surgeon. According to existing guidelines, perioperative management of antithrombotic therapy should be discussed between the surgeon and the cardiologist [13, 57, 58].
Several studies have shown that the risk of perioperative MACEs is dependent on the time from PCI to non-cardiac surgery [29, 59, 60]. However, the optimal timing for non-cardiac surgery after PCI still remains controversial. There are data suggesting that DESs may have a greater risk of late stent thrombosis than BMSs beyond 12 months after implantation, particularly in the perioperative period [61].
This study had several limitations. First, this study was a single-center study and most of the included patients were male, which could have led to selection bias. Second, the results should not be applied to patients undergoing cardiac surgery, who were specifically not enrolled in this clinical trial. Third, LMWH has different mechanisms of action to antiplatelet therapy; therefore, it is not officially recommended for bridging because it cannot substitute the effects of aspirin or P2Y12 inhibitors. However, in patients with coronary stents undergoing non-cardiac surgery, LMWH bridging therapy is frequently used in clinical practice until the previous antiplatelet regimen can be resumed. Against this background, the present trial was designed to compare the clinical benefits and risks of discontinuing antiplatelet drugs with LMWH bridging therapy. Fourth, numerous other factors, such as functional status, comorbidity, mental health status [62], and duration of surgery [63], may have confounded the relationship between age and clinical outcomes [64]. Additionally, frailty may affect a patient’s ability to tolerate, survive, and eventually recover from surgical stress [65]. Therefore, the effect of age alone may be difficult to determine unless these other factors are considered. Fifth, the independent predictors of the clinical adverse endpoints are consistent with the results reported in previous literature [50,51,52,53,54,55,56]. Therefore, this study did not evaluate predictive factors or establish a validation queue for cross validation of risk factors to prove the predictive capability.
Conclusions
This randomized placebo-controlled trial provides an update on the practical recommendations for perioperative antithrombotic management in patients treated with coronary stents > 12 months in non-cardiac surgery according to the predicted individual risk of thrombotic complications against the anticipated risk of surgical bleeding. The study demonstrated the safety and efficacy of perioperative LMWH bridging therapy in elderly patients with coronary stents implanted > 1 year before undergoing non-cardiac surgery. Perioperative discontinuation of antiplatelet therapy and bridging with half-dose LMWH is relatively safe and effective.
Availability of data and materials
All of data and materials were presented within the manuscript.
Abbreviations
- APTT:
-
Activated partial thromboplastin time
- ASA:
-
American society of anesthesiologists
- BARC:
-
Bleeding academic research consortium
- BMI:
-
Body mass index
- BMS:
-
Bare metal stent
- CIs:
-
Confidence intervals
- CK:
-
Creatine kinase
- CONSORT:
-
Consolidated standards of reporting trials
- DES:
-
Drug-eluting stents
- ECG:
-
Electrocardiogram
- hs-cTnT:
-
High-sensitivity cardiac troponin T
- ISTH:
-
International society on thrombosis and haemostasis
- LMWH:
-
Low-molecular-weight heparin
- MACE:
-
Major adverse cardiovascular events
- MAP:
-
Mean arterial pressure
- MI:
-
Myocardial infarction
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- PCI:
-
Percutaneous coronary intervention
- RCRI:
-
Revised cardiac risk index
- RR:
-
Relative risk
- ST:
-
Stent thrombosis
References
Luepker RV, Steffen LM, Duval S, Zantek ND, Zhou X, Hirsch AT. Population trends in aspirin use for cardiovascular disease prevention 1980-2009: the Minnesota Heart Survey. J Am Heart Assoc. 2015;4(12).
Miyazaki Y, Suwannasom P, Sotomi Y, Abdelghani M, Tummala K, Katagiri Y, Asano T, Tenekecioglu E, Zeng Y, Cavalcante R, et al. Single or dual antiplatelet therapy after PCI. Nat Rev Cardiol. 2017;14(5):294–303.
Rossini R, Musumeci G, Capodanno D, Lettieri C, Limbruno U, Tarantini G, Russo N, Calabria P, Romano M, Inashvili A, et al. Perioperative management of oral antiplatelet therapy and clinical outcomes in coronary stent patients undergoing surgery. Results of a multicentre registry. Thromb Haemost. 2015;113(2):272–82.
Capodanno D, Musumeci G, Lettieri C, Limbruno U, Senni M, Guagliumi G, Valsecchi O, Angiolillo DJ, Rossini R. Impact of bridging with perioperative low-molecular-weight heparin on cardiac and bleeding outcomes of stented patients undergoing non-cardiac surgery. Thromb Haemost. 2015;114(2):423–31.
Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation. 2009;119(12):1634–42.
Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, Dunn AS, Kunz R. Perioperative management of antithrombotic therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso-Coello P, Kurz A, Villar JC, Sigamani A, Biccard BM, Meyhoff CS, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494–503.
Douketis JD, Berger PB, Dunn AS, Jaffer AK, Spyropoulos AC, Becker RC, Ansell J. The perioperative management of antithrombotic therapy: American college of chest physicians evidence-based clinical practice guidelines (8th Edition). Chest. 2008;133(6 Suppl):299S-339S.
Douketis JD. Perioperative management of warfarin therapy: to bridge or not to bridge, that is the question. Mayo Clin Proc. 2008;83(6):628–9.
Pengo V, Cucchini U, Denas G, Erba N, Guazzaloca G, La Rosa L, De Micheli V, Testa S, Frontoni R, Prisco D, et al. Standardized low-molecular-weight heparin bridging regimen in outpatients on oral anticoagulants undergoing invasive procedure or surgery: an inception cohort management study. Circulation. 2009;119(22):2920–7.
Kristensen SD, Grove EL, Maeng M. Coronary stents and non-cardiac surgery: to bridge or not to bridge? Thromb Haemost. 2015;114(2):211–3.
Murasaki K. Guidelines for management of anticoagulant and antiplatelet therapy in cardiovascular disease (JCS 2009). Nihon Rinsho. 2011;69(Suppl 9):567–71.
Kristensen SD, Knuuti J, Saraste A, Anker S, Botker HE, Hert SD, Ford I, Gonzalez-Juanatey JR, Gorenek B, Heyndrickx GR, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
Spyropoulos AC, Douketis JD, Gerotziafas G, Kaatz S, Ortel TL, Schulman S. Subcommittee on Control of Anticoagulation of the SSCotI: Periprocedural antithrombotic and bridging therapy: recommendations for standardized reporting in patients with arterial indications for chronic oral anticoagulant therapy. J Thromb Haemost. 2012;10(4):692–4.
Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–33.
Kuo HC, Liu FL, Chen JT, Cherng YG, Tam KW, Tai YH. Thromboembolic and bleeding risk of periprocedural bridging anticoagulation: a systematic review and meta-analysis. Clin Cardiol. 2020;43(5):441–9.
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–9.
Douketis JD, Spyropoulos AC, Murad MH, Arcelus JI, Dager WE, Dunn AS, Fargo RA, Levy JH, Samama CM, Shah SH, et al. Perioperative management of antithrombotic therapy: an American College of Chest Physicians clinical practice guideline. Chest. 2022;162(5):e207–43.
Spyropoulos AC, Brohi K, Caprini J, Samama CM, Siegal D, Tafur A, Verhamme P, Douketis JD, Perioperative SSCSo, Critical Care T, et al. Scientific and Standardization Committee Communication: guidance document on the periprocedural management of patients on chronic oral anticoagulant therapy: recommendations for standardized reporting of procedural/surgical bleed risk and patient-specific thromboembolic risk. J Thromb Haemost. 2019;17(11):1966–72.
Writing Committee for the VSI, Devereaux PJ, Biccard BM, Sigamani A, Xavier D, MTV C, Srinathan SK, Walsh M, Abraham V, Pearse R, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642–51.
Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295–304.
Devereaux PJ, Szczeklik W. Myocardial injury after non-cardiac surgery: diagnosis and management. Eur Heart J. 2020;41(32):3083–91.
Gualandro DM, Puelacher C, Lurati Buse G, Glarner N, Cardozo FA, Vogt R, Hidvegi R, Strunz C, Bolliger D, Gueckel J, et al. Incidence and outcomes of perioperative myocardial infarction/injury diagnosed by high-sensitivity cardiac troponin I. Clin Res Cardiol. 2021;110(9):1450–63.
Broad L, Lee T, Conroy M, Bolsin S, Orford N, Black A, Birdsey G. Successful management of patients with a drug-eluting coronary stent presenting for elective, non-cardiac surgery. Br J Anaesth. 2007;98(1):19–22.
Vicenzi MN, Meislitzer T, Heitzinger B, Halaj M, Fleisher LA, Metzler H. Coronary artery stenting and non-cardiac surgery--a prospective outcome study. Br J Anaesth. 2006;96(6):686–93.
Smilowitz NR, Lorin J, Berger JS. Risks of noncardiac surgery early after percutaneous coronary intervention. Am Heart J. 2019;217:64–71.
Mahmoud KD, Sanon S, Habermann EB, Lennon RJ, Thomsen KM, Wood DL, Zijlstra F, Frye RL, Holmes DR Jr. Perioperative cardiovascular risk of prior coronary stent implantation among patients undergoing noncardiac surgery. J Am Coll Cardiol. 2016;67(9):1038–49.
Cruden NL, Harding SA, Flapan AD, Graham C, Wild SH, Slack R, Pell JP, Newby DE. Scottish Coronary Revascularisation Register Steering C: Previous coronary stent implantation and cardiac events in patients undergoing noncardiac surgery. Circ Cardiovasc Interv. 2010;3(3):236–42.
Wijeysundera DN, Wijeysundera HC, Yun L, Wasowicz M, Beattie WS, Velianou JL, Ko DT. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation. 2012;126(11):1355–62.
Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 2013;310(14):1462–72.
Anwaruddin S, Askari AT, Saudye H, Batizy L, Houghtaling PL, Alamoudi M, Militello M, Muhammad K, Kapadia S, Ellis SG. Characterization of post-operative risk associated with prior drug-eluting stent use. JACC Cardiovasc Interv. 2009;2(6):542–9.
Clark NP. Low-molecular-weight heparin use in the obese, elderly, and in renal insufficiency. Thromb Res. 2008;123(Suppl 1):S58–61.
Pautas E, Gouin I, Bellot O, Andreux JP, Siguret V. Safety profile of tinzaparin administered once daily at a standard curative dose in two hundred very elderly patients. Drug Saf. 2002;25(10):725–33.
Mahe I, Gouin-Thibault I, Drouet L, Simoneau G, Di Castillo H, Siguret V, Bergmann JF, Pautas E. Elderly medical patients treated with prophylactic dosages of enoxaparin: influence of renal function on anti-Xa activity level. Drugs Aging. 2007;24(1):63–71.
Malato A, Saccullo G, Lo Coco L, Caramazza D, Abbene I, Pizzo G, Casuccio A, Siragusa S. Patients requiring interruption of long-term oral anticoagulant therapy: the use of fixed sub-therapeutic doses of low-molecular-weight heparin. J Thromb Haemost. 2010;8(1):107–13.
Kaluza GL, Joseph J, Lee JR, Raizner ME, Raizner AE. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. J Am Coll Cardiol. 2000;35(5):1288–94.
Wilson SH, Fasseas P, Orford JL, Lennon RJ, Horlocker T, Charnoff NE, Melby S, Berger PB. Clinical outcome of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42(2):234–40.
Sabate S, Mases A, Guilera N, Canet J, Castillo J, Orrego C, Sabate A, Fita G, Parramon F, Paniagua P, et al. Incidence and predictors of major perioperative adverse cardiac and cerebrovascular events in non-cardiac surgery. Br J Anaesth. 2011;107(6):879–90.
Holcomb CN, Graham LA, Richman JS, Itani KM, Maddox TM, Hawn MT. The incremental risk of coronary stents on postoperative adverse events: a matched cohort study. Ann Surg. 2016;263(5):924–30.
Merritt JC, Bhatt DL. The efficacy and safety of perioperative antiplatelet therapy. J Thromb Thrombolysis. 2004;17(1):21–7.
Burger W, Chemnitius JM, Kneissl GD, Rucker G. Low-dose aspirin for secondary cardiovascular prevention - cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation - review and meta-analysis. J Intern Med. 2005;257(5):399–414.
Antithrombotic Trialists C. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial I: Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502.
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, Caixeta A, Feit F, Manoukian SV, White H, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4(6):654–64.
Rossini R, Capodanno D, Lettieri C, Musumeci G, Nijaradze T, Romano M, Lortkipanidze N, Cicorella N, Biondi Zoccai G, Sirbu V, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol. 2011;107(2):186–94.
Ferreira-Gonzalez I, Marsal JR, Ribera A, Permanyer-Miralda G, Garcia-Del Blanco B, Marti G, Cascant P, Martin-Yuste V, Brugaletta S, Sabate M, et al. Background, incidence, and predictors of antiplatelet therapy discontinuation during the first year after drug-eluting stent implantation. Circulation. 2010;122(10):1017–25.
Garg R, Schuman B, Bader A, Hurwitz S, Turchin A, Underwood P, Metzger C, Rein R, Lortie M. Effect of preoperative diabetes management on glycemic control and clinical outcomes after elective surgery. Ann Surg. 2018;267(5):858–62.
Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, Hudson M, Mendoza J, Johnson R, Lin E, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33(8):1783–8.
Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–30.
Wu WC, Schifftner TL, Henderson WG, Eaton CB, Poses RM, Uttley G, Sharma SC, Vezeridis M, Khuri SF, Friedmann PD. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297(22):2481–8.
Banerjee S, Angiolillo DJ, Boden WE, Murphy JG, Khalili H, Hasan AA, Harrington RA, Rao SV. Use of antiplatelet therapy/DAPT for post-PCI patients undergoing noncardiac surgery. J Am Coll Cardiol. 2017;69(14):1861–70.
Capodanno D, Angiolillo DJ. Antiplatelet therapy after implantation of bioresorbable vascular scaffolds: a review of the published data, practical recommendations, and future directions. JACC Cardiovasc Interv. 2017;10(5):425–37.
Chow JH, Chancer Z, Mazzeffi MA, McNeil JS, Sokolow MJ, Gaines TM, Reif MM, Trinh AT, Wellington IJ, Camacho JE, et al. Impact of preoperative platelet count on bleeding risk and allogeneic transfusion in multilevel spine surgery. Spine (Phila Pa 1976). 2021;46(1):E65–72.
Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213–60.
Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123–55.
Egholm G, Kristensen SD, Thim T, Olesen KK, Madsen M, Jensen SE, Jensen LO, Sorensen HT, Botker HE, Maeng M. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol. 2016;68(24):2622–32.
Holcomb CN, Graham LA, Richman JS, Rhyne RR, Itani KM, Maddox TM, Hawn MT. The incremental risk of noncardiac surgery on adverse cardiac events following coronary stenting. J Am Coll Cardiol. 2014;64(25):2730–9.
McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364(9444):1519–21.
Nilsson U, Dahlberg K, Jaensson M. Low preoperative mental and physical health is associated with poorer postoperative recovery in patients undergoing day surgery: a secondary analysis from a randomized controlled study. World J Surg. 2019;43(8):1949–56.
de’Angelis N, Schena CA, Piccoli M, Casoni Pattacini G, Pecchini F, Winter DC, O’Connell L, Carcoforo P, Urbani A, Aisoni F, et al. Impact of operation duration on postoperative outcomes of minimally-invasive right colectomy. Colorectal Dis. 2022;24(12):1505–15.
Guidet B, de Lange DW, Boumendil A, Leaver S, Watson X, Boulanger C, Szczeklik W, Artigas A, Morandi A, Andersen F, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020;46(1):57–69.
Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157.
Acknowledgements
The authors offer their appreciation to all the investigators, study site staff, and patient volunteers who participated in the study.
Funding
This study was supported by the National Clinical Research Center for Geriatric Diseases projects (Funding No. NCRCG-PLAGH-2023003), co-funded by the “National Key R&D Program of China” (Funding No. 2022YFC3602400).
Author information
Authors and Affiliations
Contributions
G.L.G., L.T.Z. and W.B. conceived the study. S.Y.H., M.C., X.L.N., C.W.J., W.R., L.Q.M., and L.Y. performed the data curation. M.Q.X., M.C., Z.Y., L.T.Z., and G.L.G. conducted the analysis. W.B., W.R., H.J., C.S.H., and C.L. drafted the original version of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The present trial was approved by the ethical committee of the Chinese PLA General Hospital (approval number: S2022-664-03). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, B., Su, Y., Ma, C. et al. Impact of perioperative low-molecular-weight heparin therapy on clinical events of elderly patients with prior coronary stents implanted > 12 months undergoing non-cardiac surgery: a randomized, placebo-controlled trial. BMC Med 22, 171 (2024). https://doi.org/10.1186/s12916-024-03391-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03391-2