Abstract
Background
The co-design of health care enables patient-centredness by partnering patients, clinicians and other stakeholders together to create services.
Methods
We conducted a systematic review of co-designed health interventions for people living with multimorbidity and assessed (a) their effectiveness in improving health outcomes, (b) the co-design approaches used and (c) barriers and facilitators to the co-design process with people living with multimorbidity. We searched MEDLINE, EMBASE, CINAHL, Scopus and PsycINFO between 2000 and March 2022. Included experimental studies were quality assessed using the Cochrane risk of bias tool (ROB-2 and ROBINS-I).
Results
We screened 14,376 reports, with 13 reports meeting the eligibility criteria. Two reported health and well-being outcomes: one randomised clinical trial (n = 134) and one controlled cohort (n = 1933). Outcome measures included quality of life, self-efficacy, well-being, anxiety, depression, functional status, healthcare utilisation and mortality. Outcomes favouring the co-design interventions compared to control were minimal, with only 4 of 17 outcomes considered beneficial. Co-design approaches included needs assessment/ideation (12 of 13), prototype (11 of 13), pilot testing (5 of 13) (i.e. focus on usability) and health and well-being evaluations (2 of 13). Common challenges to the co-design process include poor stakeholder interest, passive participation, power imbalances and a lack of representativeness in the design group. Enablers include flexibility in approach, smaller group work, advocating for stakeholders’ views and commitment to the process or decisions made.
Conclusions
In this systematic review of co-design health interventions, we found that few projects assessed health and well-being outcomes, and the observed health and well-being benefits were minimal. The intensity and variability in the co-design approaches were substantial, and challenges were evident. Co-design aided the design of novel services and interventions for those with multimorbidity, improving their relevance, usability and acceptability. However, the clinical benefits of co-designed interventions for those with multimorbidity are unclear.
Similar content being viewed by others
Background
There is increasing awareness that health services and healthcare institutions designed for acute conditions do not adequately serve patients with multiple long-term conditions, often termed multimorbidity [1]. Consequently, many healthcare systems have adopted integrative care models for greater continuity and care coordination. Integrative care models prioritise patient-centric care and shift from a specialist-led mindset to a generalist-led care approach. The goal is that clinicians provide more holistic care than condition-specific care, with the hope that patients are empowered to understand and actively self-manage their conditions.
Despite the shift to integrative care, evidence on the effectiveness of interventions for those with multimorbidity is limited. In recent systematic reviews, inconsistent findings were found for organisational change (i.e. case management) and patient-level interventions (i.e. self-management support) for multimorbidity or comorbidity [2, 3]. Interventions that targeted common risk factors or functional difficulties appeared most promising, but more research is needed to confirm these results. Notably, the review by Smith et al. [3] also emphasises the importance of intervention design informed by stakeholder perspectives, for example, through participatory design methodologies [4].
Co-design, a participatory design methodology, is an increasingly common approach that facilitates the design of patient-centred services [4]. In general, co-design in healthcare involves active partnerships between patients, families, caregivers and care providers (among other stakeholders) to design a product together [5, 6]. The co-design process is typically iterative, involving multiple rounds of development and evaluation before reaching an outcome. By adopting a user-centred approach, co-design should ensure that healthcare interventions align with the needs, preferences and values of the people they aim to serve.
There are numerous examples of co-designed healthcare interventions, including self-management strategies, decision support systems and entire care models [7,8,9]. Studies suggest that co-designed interventions in healthcare improve outcomes, for example, increased patient satisfaction, improved care processes and safety, reduced medical errors, improved patient knowledge, enhanced service delivery and cost savings [10,11,12,13,14]. However, despite the popularity of co-design, the quality of evidence is relatively poor [15].
This review aimed to assess the impact of co-designed interventions for patients with multimorbidity and understand the experiences of co-design. Accordingly, we sought studies of co-designed health interventions for patients with multimorbidity which assessed (a) their effectiveness in improving health outcomes, (b) the approaches used and (c) what barriers and facilitators to the co-design process with people living with multimorbidity.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and was registered on the PROSPERO database (ID: CRD42022330172). The PRISMA checklist can be found in Additional file 1. We looked to identify projects developing and testing a co-designed intervention targeting people living with multimorbidity or comorbidity. From these projects, data on health outcomes, the design approach and barriers and facilitators to the design process were extracted, if reported.
Multimorbidity and comorbidity
Multimorbidity is a broad term defined as the coexistence of two or more chronic conditions [16]. A related term, comorbidity, refers to a patient with an index condition in combination with other condition(s) [16]. We included papers that considered both situations in this review.
Search strategy
MEDLINE, EMBASE, CINAHL, Scopus and PsycINFO were searched from January 2000 up to 15 March 2022 using a combination of MeSH terms and keywords around the following themes: multimorbidity and co-design. We developed the search strategy with an information specialist. Additional file 2 contains the search strategy for MEDLINE.
Inclusion and exclusion criteria
We considered all quantitative and qualitative studies, regardless of study design, according to the screening criteria in Table 1. Articles were excluded if they were not peer-reviewed, they were not published in English, or the article was not primary research.
Study selection
Citations were downloaded and managed in EndNote X9. Seven researchers conducted an independent preliminary screening of titles and abstracts using the inclusion and exclusion criteria. To improve screening consistency amongst the researchers, the first hundred articles were screened by all. The group then met to discuss queries and align on screening disagreements. The process helped to refine the eligibility criteria and screening alignment. Studies with unclear eligibility were discussed as a group to reach a consensus. Studies that met the inclusion criteria underwent full-text screening by three researchers. Each article was independently dual-screened, and eligibility disagreements were resolved through discussion with a fourth researcher. Where studies were unclear, attempts were made to contact the main author to obtain more detailed information on the project.
Data extraction
Four researchers performed the data extraction for the final sample of included studies. A second researcher checked the data extraction accuracy, and discrepancies were discussed and resolved. Extracted data items include study and population characteristics, intervention details, information on the co-design process, facilitators of and barriers to the co-design process and health and well-being outcome measures (e.g. clinical outcomes, health-related quality of life). The extraction sheet was piloted on two papers and refined before full data extraction. This helped the team to understand whether data items could be extracted and in what format, and whether there was additional relevant data the team should consider.
Quality assessment
We used the updated Cochrane risk of bias tool (ROB-2) for randomised controlled trials (RCTs) and ROBINS-I for non-randomised studies [17, 18]. ROB-2 rates the risk of bias arising from the randomisation process, deviations from the intended intervention, missing outcome data, measurement of the outcome and selective reporting. Signalling questions are used to establish bias within each domain. ROBINS-I rates the risk of bias according to seven domains: confounding, selection bias, classification of interventions, deviation from intended intervention, missing data, outcome measurement and selected reporting. One researcher independently assessed the risk of bias; a second reviewer quality-checked the assessment and any disagreements were discussed until consensus.
Data synthesis
Health and well-being outcomes from RCTs and controlled non-randomised studies were tabulated. Co-design approaches are discussed narratively, and facilitators and barriers to the co-design process were extracted and organised according to the co-design framework outlined by Pirinen [19]. The framework organises the facilitators and barriers of co-design into five domains: collaboration, origination, processes, implementation and methods.
Results
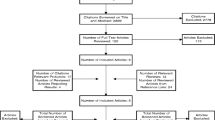
We identified 16,291 reports, including 1915 duplicates, which we removed. Independent screening of the remaining 14,376 reports led to a further exclusion of 14,260 reports. Following the full-text screening, we excluded 100 reports leaving 13 reports included (Fig. 1). The most common reasons for exclusion were due to projects targeting single conditions or problems [20, 21], or using a non-co-design methodology to develop an intervention. In several cases, the intervention development process was not reported [22]. In others, a co-design approach was reported, but people living with multimorbidity were not involved in the design process [23], or were only engaged to understand the issue (needs assessment), demonstrating no evidence of sustained stakeholder involvement [24]. Finally, some studies were early in the development process, proposing future co-design work that had yet to be undertaken [25].
Study characteristics
Table 2 presents the characteristics of the 13 included projects. Projects were from Europe (n = 9), Australia (n = 2), Canada (n = 1) and the USA (n = 1). Eight projects targeted adults with multimorbidity, the remainder targeted a principal chronic condition in combination with other chronic conditions. Principal conditions included stroke (n = 2), mental illness (n = 1), chronic obstructive pulmonary disease (n = 1) and diabetes (n = 1).
Interventions had variable aims, such as improvement of a specific aspect of care, i.e. care coordination, communication, shared decision making or care transition. Other interventions supported self-management, were novel treatments or were designed to assess the quality-of-care delivery. Technology was also leveraged in some cases, including web portals, artificial intelligence and apps.
Assessment of health and well-being outcomes
Out of the 13 projects included, two (an RCT n = 134 and cohort study n = 1933) reported on the health and well-being effects of the interventions [30, 37]. One further RCT only reported baseline data, so no outcome data could be extracted [32]. Outcome measures included quality of life, functional status, healthcare utilisation and mortality. In the RCT (Table 3) [37], only one out of eight measures favoured the intervention group (negative well-being subscale of the well-being scale-12). In the observational study [30], activities of daily living (relative risk (RR) and confidence interval (CI) 0.74 (0.58, 0.95)), healthcare utilisation (RR and CI 0.45 (0.29, 0.70)) and risk of adverse outcome (RR and CI 0.72 (0.60, 0.87)) (a composite of death, decline in activities of daily living function and high healthcare demand post admission) favoured the co-designed intervention in the frail cohort. No differences were found in the non-frail cohort.
Risk of bias
Mercer et al. [37] were rated as having a low risk of bias in all domains except outcome measurement, which was rated as moderate. Heim et al. [30] were rated as having a high risk of confounding bias; a moderate risk of bias in the selection, outcome measurement and reporting domains; and a low risk of bias in all other domains.
Co-design approaches used
Co-design stages included needs assessment/ideation (12 of 13), prototyping (11 of 13), pilot testing (5 of 13) (i.e. focus on usability) and health and well-being evaluations (3 of 13). All studies involved patients or patient advocates and healthcare professionals in their co-design process, and nine included carers. Less commonly included stakeholders were commissioners, researchers, service managers and policy-makers.
Needs and ideation
For needs and ideation, a myriad of methods were used, including focus group discussions, interviews, literature reviews and expert panels. Less common techniques included clinical observations, questionnaires and a review of existing patient informational leaflets.
Prototyping
Prototyping helped to gather feedback from stakeholders on the proposed solution. Prototypes ranged in sophistication, from simple pen and paper sketches to functional mock-ups. For example, in one study, telehealth vendors were invited to demonstrate their products so stakeholders could assess factors like functionality, ease of use and cost [26]. In another example, a prototype of an autonomous chatbot was tested with simulated interactions in a living lab (i.e. a mock real-world environment) to gauge the user experience and elicit feedback [27]. Most prototypes followed an iterative development process. For example, in one study, a decision support system underwent four prototyping testing and refinement rounds [38]. Prototypes were typically created to evaluate usability [29, 38], readability [30, 31] and content validity [31]. Prototyping also helped to facilitate discussion, which aided the design process and built knowledge among the stakeholders. Evaluation approaches included focus groups or interviews, expert panel reviews, or workshops with discussion.
Pilot testing
Pilot evaluations were conducted in the real-world setting, primarily to test and refine the functionality of the intervention before assessing clinical effects. Pilot study outcomes were both subjective and objective. Examples of subjective outcomes included usability, acceptability and satisfaction [28, 29, 31, 36, 37]. Objective outcome examples included page views and download rates [36]. Some studies conducted more than one pilot evaluation and used several rounds to test and finesse their intervention [28].
Barriers and facilitators of the co-design process
Ten projects reported on the barriers and facilitators of the co-design process. Barriers and facilitators were categorised into four of the five domains outlined by Pirinen [19]. For the fifth domain ‘barriers to the implementation of co-designed solutions’, we identified no findings. Figure 2 provides a summary of the main factors impacting the co-design process.
The most common barrier to co-design is related to participant interactions. Examples included poor stakeholder interest or difficulty maintaining project momentum [28, 36, 39], passive participation [27], or power imbalances between participants [40]. Representativeness of the design group and, correspondingly, the appropriateness of the output was another frequently mentioned barrier to co-design [12, 26, 27, 40]. Other less common co-design barriers included inadequate skills and knowledge of the co-design approach, poor understanding of the problem or solution, logistical challenges (i.e. scheduling and time commitment) and managing conflicting feedback [26, 36].
Reported enablers of co-design most frequently related to the chosen co-design methods, such as being flexible in accommodating schedules and opting for smaller rather than larger group work to facilitate discussions [39, 40]. It was also considered important to establish a conducive environment where stakeholders would actively engage and feel comfortable expressing their views [40]. Often, this involved advocating for stakeholder views and combating group hierarchies to ensure that all voices are heard [27, 40]. Finally, commitment to the process and taking responsibility for decisions helped promote a sense of ownership among participants, facilitating the co-design process [28].
Discussion
In this systematic review of 13 co-designed intervention studies with people living with multimorbidity, we found that only two reported health and well-being outcomes. Furthermore, the effects of the co-designed interventions were minimal; only 4 of 17 outcomes were considered beneficial compared to the control. The co-design development phases included needs assessment/ideation, prototyping, pilot testing (i.e. focus on usability) and health and well-being evaluations. However, not every project went through every phase of co-design. The most commonly reported challenges to the co-design process were related to participant interactions and the inability to engage a breadth of participants during design. Overall, the authors reported that the co-design approach aided in the design of novel services and interventions, improving their relevance, usability and acceptability. However, the clinical benefits of co-designed interventions are unclear.
We found variability in the co-design approaches undertaken in the included projects, such as in the stages of co-deign undertaken, the degree of stakeholder involvement and methodological techniques used during development processes. The lack of a single, uniform conceptualisation of co-design may explain this. It was common for the included projects to utilise different definitions of co-design, which impacts the approach and aim of co-design [41,42,43,44,45]. For example, terms such as ‘equal partnerships’ and ‘together in partnerships’ introduce considerable ambiguity, and inferences may differ. This variability in co-design nomenclature creates significant challenges in executing a genuine co-design approach. Accordingly, despite claiming to use a co-design approach, we excluded many studies for limited stakeholder involvement or minimal stakeholder interaction (i.e. partnership). Researchers can avoid adopting poor methodology by accessing reliable co-design resources to guide their study design [46,47,48].
We considered projects targeting both multimorbidity and comorbidity in our review. An intervention design, addressing those with multiple chronic conditions, presents unique challenges compared to a comorbid intervention design that targets an index condition alongside other conditions. While designing in the context of multimorbidity accounts for the interconnectedness of conditions, difficulties arise in defining and measuring outcomes that have made synthesising evidence and drawing conclusions not straightforward [3]. Efforts to address obstacles to evidence synthesis in multimorbidity research include the development of a core set of indicators for studies in this field [49]. In contrast, intervention design with a comorbid focus, which emphasises a patient’s needs related to an index condition, may be more straightforward but risks overlooking the holistic needs of patients with multiple conditions. Although we could not examine the distinct effects of interventions for those with multimorbidity and comorbidity in this review, as the co-design evidence base grows, this should be re-examined.
In our review, a significant portion of projects leveraged technology. Examples include apps [28, 50], online web portals [27, 29] and other digital media [36]. Those with multimorbidity tend to be older adults, and it is not uncommon for this group to be less accepting or less able to use technology [51]. Furthermore, designing and evaluating technology with older adults can also be difficult [52, 53]. The challenges of designing technology with older adults can be managed by adhering to inclusive design principles such as the Universal Design principles (equitable use and flexibility, perceptibility, tolerance for error, simplicity, low effort and accessibility) [54]. By involving older adults in the co-design process, designers can customise the technological interventions to facilitate adoption and improve ease of routine use.
In our included studies, we found numerous barriers related to the co-design processes. One significant barrier was the presence of pre-existing hierarchies, which hindered collaboration efforts in some projects. For instance, patients or non-professional groups often had less recognition or acknowledgement of their contribution, limiting their active involvement in the co-design process. Some projects found that participants had a poor understanding of co-design and the process involved, which hindered the ongoing work and outputs. Others reported that their projects may have limited generalisability due to a lack of diversity in the participants recruited [15]. For example, two projects struggled with adequate representation of healthcare professionals in their design group, risking the discussion being dominated by other stakeholders [12, 55].
Limitations
Co-design is not defined consistently in the literature and includes a high degree of variability in terminology. Therefore, it is possible that we missed some relevant papers because we did not use all pertinent co-design terms in our literature searches. By extending our selection of search phrases, we attempted to minimise this risk. In addition, due to the lack of a standardised co-design definition, we a priori set criteria to define co-design, such as deciding that participants must be involved in at least two co-design processes. Others may have different interpretations. Thus, we may have excluded relevant articles. Furthermore, we did not include specific conditions in our search strategy, which may mean we missed eligible articles. Finally, we could not draw conclusions on the impact of the co-designed interventions on health and well-being outcomes due to the limited evidence identified. Multiple statistical comparisons within these studies also introduced bias, further complicating the interpretation of their results.
Recommendations for co-design
First, clinicians and researchers engaging in co-design should recognise the complexity and diversity of people with multimorbidity. Patients’ conditions, symptoms, treatment regimens and challenges may vary significantly. Thus, everyone’s unique needs and circumstances need to be considered throughout when undertaking a co-design project. Furthermore, the heterogeneity of people living with multimorbidity requires careful consideration of what stakeholders need to be involved. To ensure inclusivity and comprehensive insights, researchers should strive to involve a wide range of individuals and groups. Second, stakeholder interactions must be managed. Being flexible and using a variety of engagement approaches can help facilitate the encounters between stakeholders. In cases of power imbalance among stakeholders, design teams must advocate for fair representation to ensure that perspectives from all stakeholders are captured. Third, while many co-design guidelines exist [56,57,58], the concept of co-design remains heterogeneous, with no unified guide on reporting or evaluating such studies. There is a need for better standardisation in reporting. The COcreation REsearch Standards (CORES) project is underway to improve reporting standards, and findings will be published on the Equator Network website in the future [59].
Recommendations for multimorbid and comorbid research
Current opinion suggests that an RCT design may be unsuitable for evaluating interventions for those with multimorbidity [2, 3, 60]. Future work should consider pragmatic research designs, which can more adeptly consider intervention complexities and the diversity in people living with multimorbidity. Longitudinal work is also lacking; studies gauging intervention impact over time should be prioritised, in addition to implementation evaluations, to understand real-world dynamics and what works best for whom. Finally, core indicators such as those developed by Smith et al. as part of the Core Outcomes Measures in Effectiveness Trials initiative must be included in studies to facilitate evidence synthesis and policy decisions [49].
Conclusions
Co-design is a participatory design approach that is becoming more prevalent in healthcare to improve services. However, the benefits of co-designed interventions for people with multimorbidity remain unclear. Future efforts should continue to involve stakeholders in healthcare redesign but should also commit to evaluating the impact of co-design interventions. More significant consideration of mental health and specific disease combinations is also needed to account for the complexities of care for those with multimorbidity.
Availability of data and materials
Not applicable.
Abbreviations
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- CORES:
-
The cocreation research standards
- EQ-5D:
-
European Quality of Life index-5 dimensions
- HADs:
-
Hospital anxiety and depression scale
- LTC:
-
Long-term condition
- NR:
-
Not reported
- P3C:
-
Patient-centred coordinated care
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RCTs:
-
Randomised controlled trials
- ROB-2:
-
Risk of Bias Tool-2
- ROBINS-I:
-
Risk Of bias in non-randomised studies of interventions
- RR:
-
Relative risk
- WBQ12:
-
Well-being scale-12
References
Moffat K, Mercer SW. Challenges of managing people with multimorbidity in today’s healthcare systems. BMC Fam Pract. 2015;16(1):129.
Eriksen CU, Kamstrup-Larsen N, Birke H, Helding SAL, Ghith N, Andersen JS, et al. Models of care for improving health-related quality of life, mental health, or mortality in persons with multimorbidity: a systematic review of randomized controlled trials. J Multimorb Comorb. 2022;12:26335565221134017.
Smith SM, Wallace E, O'Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2021(1):CD006560.
Bate P, Robert G. Experience-based design: from redesigning the system around the patient to co-designing services with the patient. Qual Saf Health Care. 2006;15(5):307–10.
Osborne SP, Radnor Z, Strokosch K. Co-production and the co-creation of value in public services: a suitable case for treatment? Public Manag Rev. 2016;18(5):639–53.
Ward ME, De Brún A, Beirne D, Conway C, Cunningham U, English A, et al. Using co-design to develop a collective leadership intervention for healthcare teams to improve safety culture. Int J Environ Res Public Health. 2018;15(6):1182.
Sanz MF, Acha BV, García MF. Co-design for people-centred care digital solutions: a literature review. Int J Integr Care. 2021;21(2):16.
Kynoch K, Ramis M-A. Experience based co-design in acute healthcare services: a scoping review protocol. JBI Evid Syn. 2019;17(1):3–9.
Green T, Bonner A, Teleni L, Bradford N, Purtell L, Douglas C, et al. Use and reporting of experience-based codesign studies in the healthcare setting: a systematic review. BMJ Qual Saf. 2020;29(1):64–76.
Silvola S, Restelli U, Bonfanti M, Croce D. Co-design as enabling factor for patient-centred healthcare: a bibliometric literature review. Clinicoecon Outcomes Res. 2023;15:333–47.
Dimopoulos-Bick TLOCC, Montgomery J, Szanto T, Fisher M, Sutherland V, Baines H, Orcher P, Stubbs J, Maher L, Verma R, Palmer VJ. “Anyone can co-design?”: a case study synthesis of six experience-based co-design (EBCD) projects for healthcare systems improvement in New South Wales. Australia Patient Exp J. 2019;6(2):93–104.
Knowles S, Hays R, Senra H, Bower P, Locock L, Protheroe J, et al. Empowering people to help speak up about safety in primary care: Using codesign to involve patients and professionals in developing new interventions for patients with multimorbidity. Health Expect. 2018;21(2):539–48.
Bombard Y, Baker GR, Orlando E, Fancott C, Bhatia P, Casalino S, et al. Engaging patients to improve quality of care: a systematic review. Implement Sci. 2018;13(1):98.
David C, Fiona J, Ruth H, Glenn R. What outcomes are associated with developing and implementing co-produced interventions in acute healthcare settings? A rapid evidence synthesis. BMJ Open. 2017;7(7):e014650.
Sumner J, Chong LS, Bundele A, Wei LY. Co-designing technology for aging in place: a systematic review. Gerontol. 2021;61(7):395–409.
Boyd CM, Fortin M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Public Health Rev. 2010;32(2):451–74.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919.
Pirinen A. The barriers and enablers of co-design for services. Int J Des. 2016;10(3):27–42.
Jayes M, Palmer R, Enderby P. Giving voice to people with communication disabilities during mental capacity assessments. Int J Lang Commun Disord. 2021;56(1):90–101.
Korpershoek YJG, Hermsen S, Schoonhoven L, Schuurmans MJ, Trappenburg JCA. User-centered design of a mobile health intervention to enhance exacerbation-related self-management in patients with chronic obstructive pulmonary disease (Copilot): mixed methods study. J Med Internet Res. 2020;22(6): e15449.
Tan A, Far Ho S, Fong YT. Integrated-care clinics: patient management and return to work rates. Int J of Integr Care. 2019;19(4):568.
Elwyn G, Vermunt N. Goal-based shared decision-making: developing an integrated model. J Patient Exp. 2020;7(5):688–96.
Aharaz A, Fabricius PK, Rasmussen JH, Mcnulty HBØ, Cyron A, Bengaard AKP, et al. 6ER-034 Medication deprescribing and follow-up: a survey among subacute multimorbid patients in a multidisciplinary outpatient clinic. Eur J Hosp Pharm. 2021;28(Suppl 1):A171.
Pereira RB, Brown TL, Guida A, Hyett N, Nolan M, Oppedisano L, et al. Consumer experiences of care coordination for people living with chronic conditions and other complex needs: an inclusive and co-produced research study. Aust Health Rev. 2021;45(4):472–84.
Davis SM, Jones A, Jaynes ME, Woodrum KN, Canaday M, Allen L, et al. Designing a multifaceted telehealth intervention for a rural population using a model for developing complex interventions in nursing. BMC Nurs. 2020;19(1):9.
Easton K, Potter S, Bec R, Bennion M, Christensen H, Grindell C, et al. A virtual agent to support individuals living with physical and mental comorbidities: co-design and acceptability testing. J Med Internet Res. 2019;21(5): e12996.
Ekstedt M, Kirsebom M, Lindqvist G, Kneck Å, Frykholm O, Flink M, et al. Design and development of an eHealth service for collaborative self-management among older adults with chronic diseases: a theory-driven user-centered approach. Int J Environ Res Public Health. 2021;19(1).
Gagnon MP, Ndiaye MA, Larouche A, Chabot G, Chabot C, Buyl R, et al. User-centered design for promoting patient engagement in chronic diseases management: the development of CONCERTO. Stud Health Technol Inform. 2020;270:1423–4.
Heim N, Rolden H, van Fenema EM, Weverling-Rijnsburger AW, Tuijl JP, Jue P, et al. The development, implementation and evaluation of a transitional care programme to improve outcomes of frail older patients after hospitalisation. Age Ageing. 2016;45(5):643–51.
Horrell J, Lloyd H, Sugavanam T, Close J, Byng R. Creating and facilitating change for Person-Centred Coordinated Care (P3C): the development of the Organisational Change Tool (P3C-OCT). Health Expect. 2018;21(2):448–56.
Healey EL, Mallen CD, Chew-Graham CA, Nicholls E, Lewis M, Lawton SA, et al. Integrating case-finding and initial management for osteoarthritis, anxiety, and depression into primary care long-term condition reviews: results from the ENHANCE pilot trial. Fam Pract. 2022;39(4):592–602.
Jinks C NE, Liddle J, Healey EL, Evans AL, Chew-Graham CA, Dziedzic KS, Tan VA, Finney AG, Porcheret M, Lawton S, Cooper V, Lewis M, Mallen CD,. Integrating case finding and initial management for osteoarthritis, anxiety and depression into routine primary care nurse-led lon-term condition reviews: Results from the enhance pilot trial. Ann rheum dis. 2015;76(2).
Jinks C, Morden A, Chew-Graham C, Porcheret M, Finney A, Dziedzic K, et al. 046. Integrating care for joint pain and anxiety and depression into reviews for long-term conditions: the enhance study. Rheumatology. 2015;54(suppl_1):i67–i67.
Lo C, Zimbudzi E, Teede H, Cass A, Fulcher G, Gallagher M, et al. Models of care for co-morbid diabetes and chronic kidney disease. Nephrology. 2018;23(8):711–7.
Mehmet M, Roberts R, Nayeem T. Using digital and social media for health promotion: a social marketing approach for addressing co-morbid physical and mental health. Aust J Rural Health. 2020;28(2):149–58.
Mercer SW, Fitzpatrick B, Guthrie B, Fenwick E, Grieve E, Lawson K, et al. The CARE Plus study – a whole-system intervention to improve quality of life of primary care patients with multimorbidity in areas of high socioeconomic deprivation: exploratory cluster randomised controlled trial and cost-utility analysis. BMC Med. 2016;14(1):88.
Porat T, Marshall IJ, Sadler E, Vadillo MA, McKevitt C, Wolfe CDA, et al. Collaborative design of a decision aid for stroke survivors with multimorbidity: a qualitative study in the UK engaging key stakeholders. BMJ Open. 2019;9(8): e030385.
Sadler E, Porat T, Marshall I, Hoang U, Curcin V, Wolfe CDA, et al. Shaping innovations in long-term care for stroke survivors with multimorbidity through stakeholder engagement. PLoS ONE. 2017;12(5): e0177102.
Ottmann G, Laragy C, Allen J, Feldman P. Coproduction in practice: participatory action research to develop a model of community aged care. Syst Pract Action Res. 2011;24(5):413–27.
Schilling I, Gerhardus A. Methods for involving older people in health research-a review of the literature. Int J Environ Res Public Health. 2017;14(12).
Di Lorito C, Bosco A, Birt L, Hassiotis A. Co-research with adults with intellectual disability: a systematic review. J Appl Res Intellect Disabil. 2018;31(5):669–86.
Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al. Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach. Health Technol Assess. 2004;8(15):1–148.
Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. A systematic review of the impact of patient and public involvement on service users, researchers and communities. Patient. 2014;7(4):387–95.
Slattery P, Saeri AK, Bragge P. Research co-design in health: a rapid overview of reviews. Health Res Policy Syst. 2020;18(1):17.
The point of care foundation. Experience-based co-design toolkit UK: The point of care foundation; 2013 [Available from: https://www.pointofcarefoundation.org.uk/resource/experience-based-co-design-ebcd-toolkit/.
Agency for clinical innovation. Connect with me Co-design Guide Australia: agency for clinical innovation; 2023 [Available from: https://aci.health.nsw.gov.au/projects/co-design.
Van Citters A. Experience-Based Co-Design of Health Care Services USA: Institute for Healthcare Improvement; 2017 [Available from: https://www.ihi.org/resources/Pages/Publications/Experience-Based-Co-Design-Health-Care-Services-Innovation-Case-Study.aspx.
Smith SM, Wallace E, Salisbury C, Sasseville M, Bayliss E, Fortin M. A Core Outcome Set for Multimorbidity Research (COSmm). Ann Fam Med. 2018;16(2):132–8.
Ferrucci F, Jorio M, Marci S, Bezenchek A, Diella G, Nulli C, et al. A web-based application for complex health care populations: user-centered design approach. JMIR Hum Factors. 2021;8(1): e18587.
Cheng C, Beauchamp A, Elsworth GR, Osborne RH. Applying the electronic health literacy lens: systematic review of electronic health interventions targeted at socially disadvantaged groups. J Med Internet Res. 2020;22(8): e18476.
Sinabell I, Ammenwerth E. Challenges and recommendations for eHealth usability evaluation with elderly users: systematic review and case study. Univ Access Inf Soc. 2022.
Perzynski AT, Roach MJ, Shick S, Callahan B, Gunzler D, Cebul R, et al. Patient portals and broadband internet inequality. J Am Med Inform Assoc. 2017;24(5):927–32.
Myerson J, West J. Make It Better: how universal design principles can have an impact on healthcare services to improve the patient experience. Dublin: Universal Design in Education; 2015.
Poitras ME, Légaré F, Tremblay Vaillancourt V, Godbout I, Poirier A, Prévost K, et al. High users of healthcare services: development and alpha testing of a patient decision aid for case management. Patient. 2020;13(6):757–66.
Giolitto. L. WACOSS Co-Design Toolkit. Australia: WACOSS; 2016.
The Health Foundation. Health care improvement toolkit. UK: The Health Foundation; 2015. Available from: www.health.org.uk/publications/communications-in-health-care-improvement-a-toolkit.
Point of Care Foundation. EBCD: Experience-based co-design toolkit UK: Point of Care Foundation; 2013. Available from: https://www.pointofcarefoundation.org.uk/resource/experience-based-co-design-ebcd-toolkit/.
Pearce G, Holliday N, Magee P, Christensen F, Darlington E, Bernard S, Olivo M, Vilaça T, Carvalho G, Anastacio Z, Botteri D, Camilli L. Protocol for the development of the CO-creation REporting Standards (CORES) for research. UK: Coventry University, UK; 2023.
Fortin M, Stewart M, Almirall J, Beaupré P. Challenges in multimorbidity research: lessons learned from the most recent randomized controlled trials in primary care. Front Med. 2022;9.
Acknowledgements
We thank Professor Howard Bauchner for his invaluable guidance in shaping this manuscript and his detailed editorial review of the content.
Funding
None.
Author information
Authors and Affiliations
Contributions
JS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: YWL, JS; acquisition, analysis, or interpretation of data: all authors; drafting of the manuscript: JS, CWTN, YWL; critical review of the manuscript for important intellectual content: all authors; statistical analysis: JS; obtained funding: N/A; supervision: YWL and JS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA 2020 Checklist.
Additional file 2.
MEDLINE search strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sumner, J., Ng, C.W.T., Teo, K.E.L. et al. Co-designing care for multimorbidity: a systematic review. BMC Med 22, 58 (2024). https://doi.org/10.1186/s12916-024-03263-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03263-9