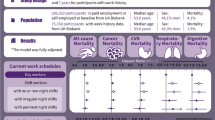
Abstract
Background
Little is known about the effects of night shifts and their interactions with genetic factors on chronic obstructive pulmonary disease (COPD). In this study, we aim to investigate relationships between long-term night shift work exposure and COPD risk, and assess modification effects of genetic predisposition.
Methods
A total of 277,059 subjects who were in paid employment or self-employed were included in the UK Biobank. Information on current and lifetime employment was obtained, and a weighted COPD-specific genetic risk score (GRS) was constructed. We used Cox proportional hazard models to investigate associations between night shift work and COPD risk, and their interaction with COPD-specific GRS.
Results
The cohort study included 277,059 participants (133,063 men [48.03%]; mean [SD] age, 52.71 [7.08] years). During a median follow-up of 12.87 years, we documented 6558 incidents of COPD. From day work, irregular night shifts to regular night shifts, there was an increased trend in COPD incidence (P for trend < 0.001). Compared with day workers, the hazard ratio (HR) and 95% confidence interval (CI) of COPD was 1.28 (1.20, 1.37) for subjects with rarely/sometimes night shifts and 1.49 (1.35, 1.66) for those with permanent night shifts. Besides, the longer durations (especially in subjects with night shifts ≥ 10 years) and increasing monthly frequency of night shifts (in workers with > 8 nights/month) were associated with a higher COPD risk. Additionally, there was an additive interaction between night shifts and genetic susceptibility on the COPD risk. Subjects with permanent night shifts and high genetic risk had the highest risk of COPD (HR: 1.90 [95% CI: 1.63, 2.22]), with day workers with low genetic risk as a reference.
Conclusions
Long-term night shift exposure is associated with a higher risk of COPD. Our findings suggest that decreasing the frequency and duration of night shifts may offer a promising approach to mitigating respiratory disease incidence in night shift workers, particularly in light of individual susceptibility.
Similar content being viewed by others
Background
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterized by consistent restriction of airflow along with varying degrees of obstructive bronchiolitis and parenchymal destruction [1,2,3]. It is estimated that over 350 million people have COPD worldwide, of whom about 3.2 million die each year, making it the third leading cause of mortality [4,5,6]. Although tobacco smoking has been considered the most important cause of COPD [7], recent evidence has indicated that approximately half of global COPD cases are thought to be caused by non-tobacco-related risk factors [1, 7]. Therefore, understanding pathogenesis, especially identifying novel risk factors contributing to COPD, should be emphasized.
With the progress of socioeconomic development in industrialized countries, shift work encompassing evening, night, and rotating shifts has become more prevalent worldwide [8, 9]. It is reported that approximately 15–20% of the working population in Europe and the USA are engaged in work that involves night shifts [10]. Since night shifts can result in a mismatch between actual and biological sleep times, disrupt circadian rhythm, impair sleep quality, and affect work-life balance [11,12,13], the harmful health effects of night shifts have become a growing concern in recent decades. A considerable number of studies indicated that the night shift was linked with an increased risk of cardiovascular diseases [13,14,15], metabolic disorders [11, 12, 16], infection susceptibility [17,18,19,20], and cancer [20,21,22,23]. Additionally, the circadian timing system drives daily changes in airway caliber, airway resistance, respiratory symptoms, mucus hypersecretion, and immune-inflammatory responses [24,25,26]. Therefore, we postulate that alteration in circadian patterns may result in airway damage and impaired lung function, ultimately contributing to the development of chronic airway diseases, such as COPD [24,25,26,27,28,29,30]. However, the relationships between long-term exposure to night shift work and COPD risk have not yet been addressed to date. Most importantly, no previous studies have documented the interaction effects of genetic susceptibility on relationships between night shifts on COPD incidence.
To fill these knowledge gaps, we used a dataset from the UK Biobank to investigate the relationships between long-term exposure to night shift work and COPD risk among 277,059 participants. In addition, we integrated information on in-depth lifetime employment to examine the impact of the duration and frequency of night shifts on COPD risk. For gene-environment interactions, we constructed a COPD-specific genetic risk score (GRS) to assess the interaction between genetic predisposition and night shifts on COPD risk.
Methods
Study design
The UK Biobank is a large-scale prospective cohort study that included > 500,000 community-dwelling adults from the UK between 2006 and 2010 [31]. The study design and the protocol of the UK Biobank have been described in detail elsewhere [31].
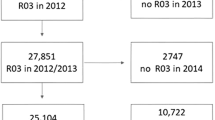
In this study, our analyses focused on participants who were in paid employment or self-employed (n = 287,073). Among them, subjects with missing covariates (including age, body mass index [BMI], Townsend deprivation index, smoking status, alcohol drinking status, and sleep duration [n = 3340]) were excluded. For the analysis of incident COPD, we also excluded subjects with COPD at baseline (n = 6674). Overall, 277,059 participants were analyzed in the primary analysis, and 222,909 European subjects were included in the subsequent gene-environment interaction analysis. Out of those, 74,559 subjects have complete lifetime employment information from the follow-up in 2015 through online questionnaires [32], and a subgroup of 62,311 participants are available for genetic analysis (Fig. 1).
Shift work assessment
Each participant received an employment information survey, in which they were asked whether their current employment involved shift work. If the answer was yes, subjects were further inquired to choose whether the job involved night shifts, which means a work schedule that involves work through the normal sleeping hours (from 00:00 to 06:00). Based on this information and previous studies [14, 16], we categorized all subjects into “day workers,” “shift, but rarely/some night shifts,” and “usual/permanent night shifts”.
In the assessment of lifetime employment, subjects reported each job ever worked, the number of years in each job, and the number of night shifts per month each job entailed [16]. The detailed assessment method has been described in previous studies [14,15,16]. Based on the above information, we calculated the lifetime duration (years) and average frequency (number/per month) of night shifts for each participant [14,15,16].
Outcome ascertainment
Prevalent cases of COPD were derived by using self-reported information, primary care data, death register records, and hospital admission data through linkage to the UK National Health Services register with the International Classification of Diseases Version 10 (ICD-10) codes as J40-J44 [33]. To avoid the interference of asthma [34], we further collected information on asthma diagnosis, which was coded as J45 based on the ICD-10. In this study, COPD incidence refers to newly diagnosed cases.
Genetic risk score (GRS) construction
The explicit procedure of the genotype, quality control, and imputation of the UK Biobank study has been well described before [35]. Based on a recent genome-wide association study [36], we selected 22 independent single nucleotide polymorphisms (SNPs) to construct the weighted COPD-specific GRS for each individual. The details about the selected SNPs are provided in Additional file 1: Table S1. The weighted GRS was calculated by using the summing formula [36]:
Covariates
Demographic data including age, sex, ethnicity (white/others), BMI, the Townsend deprivation index, sleep duration (hours), smoking status, alcohol drinking status, physical activity, and chronotype were collected. Chronotype was categorized as morning type, intermediate type, and evening type [14]. As the proportion of participants choosing “do not know,” “prefer not to answer,” or did not answer chronotype was over 10%, we classified these populations as “not reported” in the primary analysis and then excluded them in the sensitivity analyses. Physical activity was assessed by the validated Short International Physical Activity Questionnaire (IPAQ), and the final scores were divided into three levels of physical activity (“low,” “moderate,” and “high”) [15]. Similarly, the rest of the participants were grouped as “not reported” in the primary analysis and were excluded in the following sensitivity analyses.
Statistical analyses
We used Cox proportional hazard models to estimate the association between night shift work and the risk of incident COPD, with the results reported as hazard ratios (HRs) and 95% confidence intervals (CIs). Person years were aggregated from baseline until the date of death or diagnosis, loss of follow-up, or end of follow-up, whichever occurred first. In the primary analysis, model 1 showed crude associations, while age and sex were adjusted in model 2. Model 3 additionally included ethnicity, BMI, Townsend deprivation index, sleep duration, smoking status, alcohol drinking, IPAQ activity group, and chronotype. Model 4 included asthma in addition to covariates in model 3. In all models, “day workers” were set as the reference. We also performed stratified analyses by sex, age, BMI, and chronotype to investigate associations of night shift work with COPD risk in different socioeconomic subgroups.
In addition, we used Cox proportional hazards models to estimate the constructed GRS in relation to COPD incidence, and the restricted cubic spline model (RCS) was further applied to examine the dose–response relationships. To test whether genetic risk modified the effect of night shifts on the risk of incident COPD, we first divided participants into subgroups by genetic risk (low vs. high) based on the median value of COPD-specified GRS. Then, we included a product term of COPD-specific GRS (low vs. high) with their current shift work status or lifetime night shift work duration or average monthly frequency of night shifts in the models, respectively. In the above calculations, “day workers” with low genetic risk were set as the reference. We further used the relative excess risk to interaction (RERI) and the attributable proportion (AP) as the measure of interaction on the additive scale.
To examine the robustness of the results, we carried out the following sensitivity analyses: (1) excluding incident cases that occurred within the first year, (2) excluding participants with asthma, (3) excluding participants with all missing covariates.
All analyses were performed using R software, version 4.2.1 (R Foundation for Statistical Computing). All P values were two-sided, and P < 0.05 was considered to indicate statistical significance.
Results
Among 277,059 subjects, the proportions of “day workers,” “shift, but rarely/some night shifts,” and “usual/permanent night shifts” were 82.83%, 13.37%, and 3.80%, respectively (Table 1). Night shift workers were more likely to be male, younger, current smokers, and have higher BMI and shorter sleep duration in comparison to day workers. The baseline characteristics of subjects according to the monthly frequency and duration of night shifts are presented in Additional file 1: Table S2-3. The comparison of baseline characteristics in the participants with or without genetic data is shown in Additional file 1: Table S4.
During a median of 12.87 years of follow-up, we documented 6558 incidents of COPD. In fully adjusted models (Table 2, model 4), we observed that night shift work was significantly related to a higher risk of COPD (all P for trend < 0.001). Compared with day workers, the HR (95% CI) of COPD was 1.28 (1.20, 1.37) in shift workers who rarely or sometimes undertook night shifts and 1.49 (1.35, 1.66) in those with regular night shift work. When we excluded COPD cases within the first year (Additional file 1: Table S5), asthma (Additional file 1: Table S6), or all missing covariates (Additional file 1: Table S7), the effect estimates were consistent with the main analyses. Stratified analyses further demonstrated that the observed associations seem to be more pronounced among females, individuals with age ≥ 60 years, and those with BMI < 25 kg/m2 (Additional file 1: Table S8), although the interaction effects were statistically non-significant (P for interaction > 0.05). We further analyzed the relationships between chronotype and COPD risk in this study population. We observed that subjects who were evening persons had a higher risk of COPD compared with those describing themselves as intermediate chronotypes. The HR (95% CI) for COPD in those reporting being an evening person was 1.09 (1.01, 1.19) in fully adjusted models (Additional file 1: Table S9).
In fully adjusted models, we observed that the longer durations of night shift work were associated with a higher risk of COPD (Table 3, model 4). Compared with day workers, the HR (95% CI) of COPD was 1.17 (1.00, 1.38) among subjects reporting < 10 years of night shifts; and the higher risk was found in subjects who underwent night shift work over 10 years (HR 1.23 [95% CI 1.03, 1.46]). In addition, we observed that workers who undertook > 8 nights/month had the highest COPD risk (HR 1.41 [95% CI 1.19, 1.68]) (Table 4). In sensitivity analyses, all the above results were consistent with the main analyses (Additional file 1: Table S5-7).
We first noted that higher GRS was significantly and positively related to higher COPD risk in dose–response manners. Each SD increment in GRS was associated with a 9% increase in COPD risk (HR 1.09 [95% CI 1.06, 1.12]) (Additional file 1: Table S10; Fig. S1). Furthermore, we observed significant joint effects between genetic risk and current work schedule on the COPD risk; the overall risk of COPD incidence increased as both genetic risk and night work-related risk increased (Fig. 2). Compared with day workers who had lower genetic risk, individuals with usual/permanent night shift work and high genetic risk had the highest risk of COPD (HR 1.90 [95% CI 1.63, 2.22]). The RERI and AP for interaction between the current work schedule and genetic risk were 0.19 (0.02, 0.36) and 0.12 (0.02, 0.22), respectively. In addition, we also found the cumulative effects between the duration of night shift work and genetic risk on COPD risk (Additional file 1: Fig. S2). Compared with day workers with low genetic risk, subjects with night shift work over 10 years and high genetic risk showed the highest risk of COPD (HR 1.30 [95% CI 1.00, 1.67]) (Additional file 1: Fig. S2). Similarly, we demonstrated significant joint effects of the monthly frequency of night shift work and genetic risk on COPD risk. Workers who undertook over 8 nights/month and high genetic risk had higher COPD risk (HR 1.55 [95% CI 1.21, 1.99]), with day workers with low genetic risk as the reference (Additional file 1: Fig. S3).
Joint effects of genetic risk score (GRS) with current work schedule on COPD risk. AP Attributable proportion, COPD Chronic obstructive pulmonary disease, HR hazard ratio, CI Confidence interval, BMI Body mass index, IPAQ International Physical Activity Questionnaire. RERI Relative excess risk to interaction. Adjusted for age, sex, BMI, Townsend deprivation index, sleep duration, smoking status, alcohol drinking, IPAQ activity group, chronotype, and asthma
Discussion
In this study, we observed that night shifts were significantly associated with an increased risk of COPD. From day work, irregular night shifts to regular night shifts, there was an elevated trend in COPD incidence. Besides, longer durations of night shift work were related to a higher COPD risk. Regarding the frequency of night shifts, we found that the increasing monthly frequency of night shifts was positively related to COPD risk. In particular, there was a significant additive interaction between genetic susceptibility and night shifts. Participants with usual/permanent night shift work and high genetic risk had the highest risk of incident COPD compared to those with day workers who had lower genetic risk.
To the best of our knowledge, this is the first prospective cohort study to investigate the relationships between night shift work and the risk of incident COPD. Although existing literature has suggested that night shift work is related to a number of serious health conditions, such as cardiovascular diseases [13,14,15], metabolic disorders [11, 12, 16], increased infection susceptibility [17,18,19,20], and cancer [20,21,22,23], the adverse effects of the night shifts on the respiratory system have been rarely mentioned. Only a limited number of studies reported that shift work was related to declined lung function [34, 37], higher odds of asthma [34], and increased risk of lung cancer [38, 39]. To some extent, our results may be partly supported by those findings. For example, Maidstone et al. proposed that individuals with night shifts may suffer a greater risk of developing asthma in comparison to those day workers [34]. They further observed that workers with permanent night shifts experienced a significant decline in lung function [34]. Consistently, Nemery et al. found that lung function was unchanged over the morning shift among steelworkers, but those spirometry indices had a pronounced decrease in those performing night shift work [37]. Additionally, Schernhammer et al. reported an increased risk of lung cancer among women who worked rotating night shifts [38]. In this study, we observed that the night shift was a noteworthy and new risk factor for COPD. These findings not only expand our understanding of the harmful respiratory effects of night shifts but also provide valuable evidence for the development of primary prevention strategies for COPD. Besides, we observed that longer durations and monthly frequency of night shift work were important determinants of increased risk of COPD. Similar to our findings, Schernhammer et al. enrolled 78,612 women in the Nurses’ Health Study and demonstrated that the increasing years of night shifts was positively related to lung cancer risk [38]. Maidstone et al. suggested that more frequent nights could contribute to a higher risk of asthma [34]. All these findings provide evidence on detrimental relationships between night shift work and health and give a clue that reducing the frequency and duration of night shift work might be an effective measure to prevent respiratory diseases in night shift workers.
There are several potential mechanisms underlying the associations between night shift work and COPD risk. Night shifts may trigger circadian disruption, resulting from the mismatch between internal circadian rhythms and external environmental conditions [40]. Subsequently, the changes in sleep–wake cycles and exposure to light–dark patterns can lead to abnormal levels of cortisol, melatonin, and body temperature, which have been associated with COPD [40,41,42,43]. For example, the night shift can alter the circadian rhythm, which has been reported to suppress melatonin biosynthesis [15, 44, 45]. Previous experimental studies have shown that melatonin has a beneficial effect on COPD by attenuating apoptosis and endoplasmic reticulum stress [42]. Therefore, lower melatonin levels caused by disruption of circadian rhythm may be an important pathway that contributes to the observed associations. Additionally, circadian disruption induced by night shifts can disrupt the normal expression of clock gene expression, such as BMAL, CLOCK, PER1, PER2, and PER3 [46]. The oscillation and dysregulated expression of these clock gene expression have been reported to play a role in COPD pathogenesis, including chronic inflammation and imbalanced autophagy level [47]. On the other hand, circadian clock disruption during chronic lung diseases has essential effect in augmented oxidative stress and increased systemic inflammation, as evidenced by increased interleukin-6, C-reactive protein, and tumor necrosis factor [14, 48]. These factors are believed to be responsible for the advancement of COPD [49]. Lastly, unfavorable changes in health behaviors (such as increased smoking and irregular meals), among night shift workers, could also be a potential reason that contributes to COPD.
Significant additive interactions between genetic predisposition and current shift work were observed in the current study. These results support public health efforts to emphasize the harmful impact of night shift work, especially for those individuals who are at high genetic risk. We also observed that the risk of COPD with longer durations and increased monthly frequency was aggravated by high genetic risk, despite the lack of statistical significance in the interaction test. All findings might facilitate the emergence of new strategies for personalized precision prevention of COPD and highlight that further primary prevention strategies of COPD for night shift workers should take into account individual susceptibility.
Strength of our study lies in the prospective design, large sample size, long-term follow-up, and detailed current shift work information. Most importantly, this is the first cohort study to investigate relationships between night shifts and COPD risk. Besides, we further assessed the contribution of genetic factors in the above relationships, which enabled us to precisely determine the effects of night shifts on groups with varying levels of susceptibility. Moreover, we used detailed information on the lifetime of night shift work, which provides original insight and a unique opportunity to study the relationship between night shifts and COPD [14].
Nevertheless, several potential weaknesses also exist in this study. First, causality cannot be determined because of the observational study design. Second, the information on current and former employment history and some of the COPD incidence was assessed by self-report, which may contribute to misclassification. Third, we did not assess the role of melatonin in the observed relationships, thus we could not confirm our findings mechanistically. Fourth, most of the participants in this study were white, which limits the generalizability of our results to other racial/ethnic groups. Lastly, online lifetime employment information only enrolled ~ 70,000 individuals in this study.
Conclusions
In conclusion, night shift work was significantly associated with an increased risk of COPD and maybe a novel risk factor for COPD. Besides, we found that the positive relationships between night shift work and COPD risk could be aggravated by higher COPD-genetic risk.
Availability of data and materials
The UK Biobank resources are available from the authors upon reasonable request and can be accessed through applications on their website (https://www.ukbiobank.ac.uk/).
Abbreviations
- AP:
-
Attributable proportion
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- CI:
-
Confidence interval
- GRS:
-
Genetic risk score
- HR:
-
Hazard ratios
- IPAQ:
-
International Physical Activity Questionnaire
- ICD-10:
-
International Classification of Diseases Version 10
- RCS:
-
Restricted cubic spline
- RERI:
-
Relative excess risk to interaction
- SNPs:
-
Single nucleotide polymorphisms
References
Pavord ID, Yousaf N, Birring SS. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9706):1964 author reply 1965–1966.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–82.
Leitao Filho FS, Alotaibi NM, Ngan D, Tam S, Yang J, Hollander Z, Chen V, FitzGerald JM, Nislow C, Leung JM, et al. Sputum microbiome is associated with 1-year mortality after chronic obstructive pulmonary disease hospitalizations. Am J Respir Crit Care Med. 2019;199(10):1205–13.
Suzuki M, Makita H, Ostling J, Thomsen LH, Konno S, Nagai K, Shimizu K, Pedersen JH, Ashraf H, Bruijnzeel PL, et al. Lower leptin/adiponectin ratio and risk of rapid lung function decline in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(10):1511–9.
Collaborators GDaI. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222.
Lee H, Sin DD. GETting to know the many causes and faces of COPD. Lancet Respir Med. 2022;10(5):426–8.
Ma J, Hao X, Nie X, Yang S, Zhou M, Wang D, Wang B, Cheng M, Ye Z, Xie Y, et al. Longitudinal relationships of polycyclic aromatic hydrocarbons exposure and genetic susceptibility with blood lipid profiles. Environ Int. 2022;164: 107259.
Liao H, Pan D, Deng Z, Jiang J, Cai J, Liu Y, He B, Lei M, Li H, Li Y, et al. Association of shift work with incident dementia: a community-based cohort study. BMC Med. 2022;20(1):484.
Pilcher JJ, Lambert BJ, Huffcutt AI. Differential effects of permanent and rotating shifts on self-report sleep length: a meta-analytic review. Sleep. 2000;23(2):155–63.
Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V, Group WHOIAFRoCMW. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–6.
Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8(12): e1001141.
Shan Z, Li Y, Zong G, Guo Y, Li J, Manson JE, Hu FB, Willett WC, Schernhammer ES, Bhupathiraju SN. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: results from two large US cohorts of female nurses. BMJ. 2018;363: k4641.
Xiao Z, Xu C, Liu Q, Yan Q, Liang J, Weng Z, Zhang X, Xu J, Hang D, Gu A. Night shift work, genetic risk, and hypertension. Mayo Clin Proc. 2022;97(11):2016–27.
Wang N, Sun Y, Zhang H, Wang B, Chen C, Wang Y, Chen J, Tan X, Zhang J, Xia F, et al. Long-term night shift work is associated with the risk of atrial fibrillation and coronary heart disease. Eur Heart J. 2021;42(40):4180–8.
Yang L, Luo Y, He L, Yin J, Li T, Liu S, Li D, Cheng X, Bai Y. Shift work and the risk of cardiometabolic multimorbidity among patients with hypertension: a prospective cohort study of UK Biobank. J Am Heart Assoc. 2022;11(17): e025936.
Vetter C, Dashti HS, Lane JM, Anderson SG, Schernhammer ES, Rutter MK, Saxena R, Scheer F. Night shift work, genetic risk, and type 2 diabetes in the UK Biobank. Diabetes Care. 2018;41(4):762–9.
Loef B, Dolle MET, Proper KI, van Baarle D, Initiative LCR, van Kerkhof LW. Night-shift work is associated with increased susceptibility to SARS-CoV-2 infection. Chronobiol Int. 2022;39(8):1100–9.
Loef B, van Baarle D, van der Beek AJ, Sanders EAM, Bruijning-Verhagen P, Proper KI. Shift work and respiratory infections in health-care workers. Am J Epidemiol. 2019;188(3):509–17.
Loef B, van der Beek AJ, Hulsegge G, van Baarle D, Proper KI. The mediating role of sleep, physical activity, and diet in the association between shift work and respiratory infections. Scand J Work Environ Health. 2020;46(5):516–24.
Schwarz C, Pedraza-Flechas AM, Lope V, Pastor-Barriuso R, Pollan M, Perez-Gomez B. Gynaecological cancer and night shift work: a systematic review. Maturitas. 2018;110:21–8.
Travis RC, Balkwill A, Fensom GK, Appleby PN, Reeves GK, Wang XS, Roddam AW, Gathani T, Peto R, Green J, et al. Night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. J Natl Cancer Inst. 2016;108(12):djw169.
Dun A, Zhao X, Jin X, Wei T, Gao X, Wang Y, Hou H. Association between night-shift work and cancer risk: updated systematic review and meta-analysis. Front Oncol. 2020;10:1006.
Hansen J. Night shift work and risk of breast cancer. Curr Environ Health Rep. 2017;4(3):325–39.
Giri A, Wang Q, Rahman I, Sundar IK. Circadian molecular clock disruption in chronic pulmonary diseases. Trends Mol Med. 2022;28(6):513–27.
Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock-coupled lung cellular and molecular functions in chronic airway diseases. Am J Respir Cell Mol Biol. 2015;53(3):285–90.
Sundar IK, Yao H, Sellix MT, Rahman I. Circadian molecular clock in lung pathophysiology. Am J Physiol Lung Cell Mol Physiol. 2015;309(10):L1056-1075.
Spengler CM, Shea SA. Endogenous circadian rhythm of pulmonary function in healthy humans. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1038–46.
Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014;28(1):176–94.
Krakowiak K, Durrington HJ. The role of the body clock in asthma and COPD: implication for treatment. Pulm Ther. 2018;4(1):29–43.
Aquino-Santos HC, Tavares-Vasconcelos JS, Brandao-Rangel MAR, Araujo-Rosa AC, Morais-Felix RT, Oliveira-Freitas S, Santa-Rosa FA, Oliveira LVF, Bachi ALL, Alves TGG, et al. Chronic alteration of circadian rhythm is related to impaired lung function and immune response. Int J Clin Pract. 2020;74(10): e13590.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3): e1001779.
De Matteis S, Jarvis D, Darnton A, Hutchings S, Sadhra S, Fishwick D, Rushton L, Cullinan P. The occupations at increased risk of COPD: analysis of lifetime job-histories in the population-based UK Biobank Cohort. Eur Respir J. 2019;54(1):1900186.
Chen L, Cai M, Li H, Wang X, Tian F, Wu Y, Zhang Z, Lin H. Risk/benefit tradeoff of habitual physical activity and air pollution on chronic pulmonary obstructive disease: findings from a large prospective cohort study. BMC Med. 2022;20(1):70.
Maidstone RJ, Turner J, Vetter C, Dashti HS, Saxena R, Scheer F, Shea SA, Kyle SD, Lawlor DA, Loudon ASI, et al. Night shift work is associated with an increased risk of asthma. Thorax. 2021;76(1):53–60.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–9.
Hobbs BD, de Jong K, Lamontagne M, Bosse Y, Shrine N, Artigas MS, Wain LV, Hall IP, Jackson VE, Wyss AB, et al. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat Genet. 2017;49(3):426–32.
Nemery B, Van Leemputten R, Goemaere E, Veriter C, Brasseur L. Lung function measurements over 21 days shiftwork in steelworkers from a strandcasting department. Br J Ind Med. 1985;42(9):601–11.
Schernhammer ES, Feskanich D, Liang G, Han J. Rotating night-shift work and lung cancer risk among female nurses in the United States. Am J Epidemiol. 2013;178(9):1434–41.
Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176(9):751–9.
Joshi A, Sundar IK. Circadian disruption in night shift work and its association with chronic pulmonary diseases. Adv Biol (Weinh). 2023;7(11):e2200292.
Cunningham PS, Jackson C, Chakraborty A, Cain J, Durrington HJ, Blaikley JF. Circadian regulation of pulmonary disease: the importance of timing. Clin Sci (Lond). 2023;137(11):895–912.
He B, Zhang W, Qiao J, Peng Z, Chai X. Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can J Physiol Pharmacol. 2019;97(5):386–91.
Wei P, Li Y, Wu L, Wu J, Wu W, Chen S, Qin S, Feng J. Serum cortisol levels and adrenal gland size in patients with chronic obstructive pulmonary disease. Am J Transl Res. 2021;13(7):8150–7.
Leung M, Tranmer J, Hung E, Korsiak J, Day AG, Aronson KJ. Shift work, chronotype, and melatonin patterns among female hospital employees on day and night shifts. Cancer Epidemiol Biomarkers Prev. 2016;25(5):830–8.
Ulhoa MA, Marqueze EC, Burgos LG, Moreno CR. Shift work and endocrine disorders. Int J Endocrinol. 2015;2015: 826249.
Toffoli B, Tonon F, Giudici F, Ferretti T, Ghirigato E, Contessa M, Francica M, Candido R, Puato M, Grillo A, et al. Preliminary Study on the Effect of a Night Shift on Blood Pressure and Clock Gene Expression. Int J Mol Sci. 2023;24(11):9309.
Hu Y, He T, Zhu J, Wang X, Tong J, Li Z, Dong J. The link between circadian clock genes and autophagy in chronic obstructive pulmonary disease. Mediators Inflamm. 2021;2021:2689600.
Kim SW, Jang EC, Kwon SC, Han W, Kang MS, Nam YH, Lee YJ. Night shift work and inflammatory markers in male workers aged 20–39 in a display manufacturing company. Ann Occup Environ Med. 2016;28:48.
Tkacova R. Systemic inflammation in chronic obstructive pulmonary disease: may adipose tissue play a role? Review of the literature and future perspectives. Mediators Inflamm. 2010;2010: 585989.
Acknowledgements
This research was conducted using the UK Biobank Resource under Application Number 88159. The authors thank all participants and staff of the UK Biobank.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82204002), the Science and Technology Innovation Team (Tianshan Innovation Team) Project of Xinjiang Uygur Autonomous Region (2022TSYCTD0013), and the Fundamental Research Funds for the Central Universities (No. 2020kfyXJJS005). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
J.L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: J.M. and J.L. Acquisition, statistical analysis, or interpretation of data: J.M. and J.L. Drafting of the manuscript: J.L. Critical revision of the manuscript for important intellectual content: L.Y., Y.Y., P.G., Y.X., H.Y., M.X., and Y.J. Statistical analysis, administrative, technical, or material support: L.Y., J.M., J.D., and J.L. Supervision: J.D. and J.L. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethics approval for the UK Biobank was obtained from the North West Multi-center Research Ethics Committee (Ref: 11/NW/0382) (see https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Information on genetic variants associated with COPD. Table S2. Basic characteristics of participants by lifetime duration of night shift work exposure. Table S3. Basic characteristics of participants by average lifetime number of night shift work exposure. Table S4. Basic characteristics between all subjects and those with genetic data. Table S5. Adjusted HRs (95% CIs) of the risk of incident COPD by current work schedule and lifetime night shift work experience after excluding participants with COPD occurred within 1 year of follow-up. Table S6. Adjusted HRs (95% CIs) of the risk of incident COPD by current work schedule and lifetime night shift work experience after excluding participants with asthma. Table S7. Adjusted HRs (95% CIs) of the risk of incident COPD by current work schedule and lifetime night shift work experience after excluding participants with all missing covariates. Table S8. Associations of current shift work schedule and the risk of incident COPD stratified by sex, age, BMI, and chronotype. Table S9. Associations between chronotype and the risk of incident COPD. Table S10. Adjusted HRs (95% CIs) of incident COPD by individual genetic risk score (GRS) of participants of current work schedule. Fig. S1. Association of genetic risk score (GRS) and the risk of incident COPD (n=222,909). Fig. S2. Joint effects of genetic risk score (GRS) with lifetime duration of night shift work exposure on the risk of incident COPD (n=62,311). Fig. S3. Joint effects of genetic risk score (GRS) with average lifetime number of night shift work exposure on the risk of incident COPD (n=62,311).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Yang, L., Yao, Y. et al. Associations between long-term night shift work and incidence of chronic obstructive pulmonary disease: a prospective cohort study of 277,059 UK Biobank participants. BMC Med 22, 16 (2024). https://doi.org/10.1186/s12916-023-03240-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-03240-8