Abstract
Background
Single low-dose primaquine (SLDPQ) effectively blocks the transmission of Plasmodium falciparum malaria, but anxiety remains regarding its haemolytic potential in patients with glucose-6-phopshate dehydrogenase (G6PD) deficiency. We, therefore, examined the independent effects of several factors on haemoglobin (Hb) dynamics in falciparum-infected children with a particular interest in SLDPQ and G6PD status.
Methods
This randomised, double-blind, placebo-controlled, safety trial was conducted in Congolese and Ugandan children aged 6 months–11 years with acute uncomplicated P. falciparum and day (D) 0 Hbs ≥ 6 g/dL who were treated with age-dosed SLDPQ/placebo and weight-dosed artemether lumefantrine (AL) or dihydroartemisinin piperaquine (DHAPP). Genotyping defined G6PD (G6PD c.202T allele), haemoglobin S (HbS), and α-thalassaemia status.
Multivariable linear and logistic regression assessed factor independence for continuous Hb parameters and Hb recovery (D42 Hb > D0 Hb), respectively.
Results
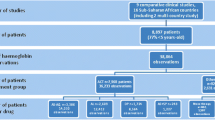
One thousand one hundred thirty-seven children, whose median age was 5 years, were randomised to receive: AL + SLDPQ (n = 286), AL + placebo (286), DHAPP + SLDPQ (283), and DHAPP + placebo (282). By G6PD status, 284 were G6PD deficient (239 hemizygous males, 45 homozygous females), 119 were heterozygous females, 418 and 299 were normal males and females, respectively, and 17 were of unknown status.
The mean D0 Hb was 10.6 (SD 1.6) g/dL and was lower in younger children with longer illnesses, lower mid-upper arm circumferences, splenomegaly, and α-thalassaemia trait, who were either G6PDd or heterozygous females. The initial fractional fall in Hb was greater in younger children with higher D0 Hbs and D0 parasitaemias and longer illnesses but less in sickle cell trait. Older G6PDd children with lower starting Hbs and greater factional falls were more likely to achieve Hb recovery, whilst lower D42 Hb concentrations were associated with younger G6PD normal children with lower fractional falls, sickle cell disease, α-thalassaemia silent carrier and trait, and late treatment failures. Ten blood transfusions were given in the first week (5 SLDPQ, 5 placebo).
Conclusions
In these falciparum-infected African children, posttreatment Hb changes were unaffected by SLDPQ, and G6PDd patients had favourable posttreatment Hb changes and a higher probability of Hb recovery. These reassuring findings support SLDPQ deployment without G6PD screening in Africa.
Trial registration
The trial is registered at ISRCTN 11594437.
Similar content being viewed by others
Background
Since the 2012 WHO recommendation to use single low-dose primaquine (SLDPQ) as an elimination tool for multidrug, especially artemisinin, resistant Plasmodium falciparum (ARPf) [1], several studies have shown its transmission blocking efficacy and ability to reduce gametocyte carriage [2,3,4,5,6].
ARPf has been present in SE Asia for the past 15 years and has seen significant declines in efficacy of the partner drugs used in artemisinin-based combination treatments (ACTs) [7,8,9,10,11]. ARPf is now gaining a foothold in eastern Africa [12, 13] with concomitant evidence of a decline in the sensitivity of lumefantrine, a commonly used ACT [14, 15]. SLDPQ offers an opportunity to counter this threat, but concern remains that SLDPQ could increase the risk of life-threatening haemolysis and blood transfusions (primaquine’s most feared toxicity) in patients with glucose-6-phosphate dehydrogenase deficiency (G6PDd) with a consequential loss of credibility of this strategy by malaria control programmes (MCPs).
In sub-Saharan Africa, G6PD A-, caused by the c.202C → T mutation in the G6PD gene, is the most common variant with reported rates ranging from ~ 2–3% in The Gambia [16, 17]) to ~ 10% in Uganda [18], ~ 17% in Burkina Faso [19], and ~ 24% in Nigeria [20]. G6PDd is, thus, an important consideration for MCPs.
Plasmodium falciparum malaria itself causes haemolysis that may result in anaemia at presentation or following treatment with the mean Hb reaching its nadir concentration on day 2 [21, 22]. Accordingly, the highest risk of blood transfusion occurs early, within the first 4–7 days in falciparum-infected children < 5 years [23,24,25]. Reported blood transfusion rates were 1.5% (10/679) in ACT-treated children in the Democratic Republic of Congo [23], 1.4% (10/702) in Rwandan children treated with chlorproguanil-dapsone-artesunate (CDA) or amodiaquine-sulphadoxine-pyrimethamine [24], rising, in a Kenyan study, to 11% (13/119) in G6PDd children (1– < 15 years) vs. 0.5% (1/200) in heterozygous females treated with CDA or CD alone [25]. In western Thailand, the rate was 1% (55/5253) in children and adults given various treatments, principally, mefloquine alone or combined with artesunate [26].
There is growing evidence that SLDPQ is well tolerated across the age spectrum in patients, healthy individuals, and asymptomatic P. falciparum carriers with the African A- and the more severe Southeast Asian G6PD variants [27,28,29,30,31,32,33]. The mean difference in nadir Hb concentrations between SLDPQ treated G6PDd and G6PD normal malaria patients is ~ 1 g/dL [27, 28, 31], but at the higher primaquine doses used by Shekalaghe et al., 0.75 − 1.0 mg/kg, the mean difference was 2 g/dL between the G6PDd and G6PD normal children [34].
Our group recently published the largest study to date on the safety of a bespoke, age-dosed regimen of SLDPQ [35] in young G6PDd children with uncomplicated P. falciparum. SLDPQ was associated with the same blood transfusion rate as placebo (0.9%) and a very similar tolerability profile [36]. Extending our analysis of this study, we sought to determine the independent effects of several factors, including G6PD and sickle cell status, thalassaemia, and SLDPQ on the course of haemoglobin over time.
Methods
Study design, sites, and conduct
Briefly, in this randomised, placebo-controlled trial, children aged 6 months–11 years with uncomplicated P. falciparum, by blood slide or rapid diagnostic test, received either open artemether lumefantrine (AL) or dihydroartemisinin piperaquine (DHAPP), both dosed by weight, and aged-dosed SLDPQ/placebo: 1.25 mg (6 months– < 1 years), 2.5 mg (1–5 years), 5 mg (6–9 years), and 7.5 mg (10–14 years). A subgroup had intense blood sampling for pharmacokinetic (PK) analyses: day (D) 0 hour (H) 0 (baseline) and then at H1, H1.5, H2, H4, H8, H12, and H24 [37].
Genotyping was performed to identify the following: (i) the status of cytochrome (CYP) P450 2D6 [38, 39], (ii) G6PD A- by the G6PD c.202T allele [40], (iii) sickle cell trait (HbAS) and sickle cell disease (HbSS) [41], and (iv) α-thalassaemia caused by the common African-α3.7 deletion in HBAA, where children were classified as heterozygous (-α/αα), homozygous (-α/-α) or normal (αα/αα) [42].
Follow-up extended to D42, during which investigations included symptoms (to detect adverse events), Giemsa-stained blood films, venous blood (D0, 7, 14 and 28) for a complete blood count and routine biochemistry, and HemoCue measured Hb concentrations. The latter were used to define Hb changes over time: D0, 1, 2, 3, 7, 14, 21, 28, 35, and 42; in a subset of patients who were having intense monitoring of their parasite counts, additional samples were taken at D0 H1, H1.5, H2, H4, H6, H8, H12, H36, H60, H84, and H96.
The study sites, the Kinshasa Mahidol Oxford Research Unit (outside Kinshasa) and the Mbale Regional Referral Hospital (Mbale, SE Uganda), are in areas of hyperendemic, perennial malaria transmission of P. falciparum and both sites serve urban and rural populations; children < 5 years are the main risk group for malaria.
Statistical analysis
The analysis population included all patients who received at least one dose of study drug and had at least one posttreatment Hb measurement. As per the WHO, anaemia of any degree was defined as a Hb < 11 g/dL in children < 5 years and < 11.5 mg/dL in those aged 5–11 years [43]; adapting the WHO definition, we defined moderate anaemia as a Hb < 8 g/dL for all ages.
Multivariable linear regression models assessed a range of clinically relevant variables that, collectively, have been assessed in previous studies [21,22,23,24,25,26,27,28,29, 44, 45]; these were age, sex, length of illness, mid upper arm circumference (MUAC), D0 Hb, D0 parasitaemia, splenomegaly, hepatomegaly, G6PD, HbS and α-thalassaemia genotype, ACT, SDLPQ/placebo, Hb fractional fall, and late treatment failure. We assessed their independent effects on the (i) D0 Hb concentration, (ii) initial decline in Hb concentration from D0 to the day of the nadir Hb in the first 14 days, (iii) nadir and D42 Hb concentrations, and (iv) increase in Hb from the nadir to D42 concentration, the total malaria attributable fall in Hb following treatment (MAFt, Additional file 1: Fig S1) [45], excluding variables where needed, e.g. ACT, SLDPQ/placebo, and late treatment failure were not used to assess the baseline Hb. In linear regression models, the slopes and the corresponding 95% confidence intervals for slopes are reported. Collinearity was assessed using the variance inflation factor (VIF) and variables with VIF > 5 would be excluded from the models. However, all variables had low VIFs (all < 3).
The factors associated with Hb recovery, defined as a D42 Hb > D0 Hb, were determined by logistic regression (age, sex, length of illness, MUAC, D0 Hb, D0 parasitaemia, splenomegaly, hepatomegaly, G6PD, HbS and α-thalassaemia genotype, ACT, SDLPQ/placebo, Hb fractional fall and late treatment failure). In these models, the odds ratios and the corresponding 95% confidence intervals for odds ratios are reported.
The times to Hb recovery by G6PD status were determined by the Kaplan–Meier survival approach, and potential explanatory variables for times to Hb recovery were explored by the Cox proportional hazards regression model. In these models, we used the scaled Schoenfeld residuals to test the proportional hazards assumption. Variables not meeting the proportional hazards assumptions were excluded from the analysis. The hazards ratios and the corresponding 95% confidence intervals for hazard ratios are reported. P-values have also been reported for all models considered.
A subgroup analysis (n = 258) is presented of patients with PK data to assess the effects of (i) the maximum primaquine concentration (Cmax), (ii) primaquine exposure, i.e. area under the drug concentration time curve from 0 to the last PK sample (AUC0-t), and (iii) mg/kg dose and CYP2D6 activity score, inferred from the CYP2D6 genotype [46]; Cmax and AUC0-t were determined by non-compartmental analysis. All statistical analyses were carried out using Stata v17 (Stata Corporation, Texas, USA) and were two sided. A P-value < 0.05 denoted statistical significance.
Results
Between December 18, 2017, and October 7, 2019 (Mbale Regional Referral Hospital, Uganda), and July 17, 2017, and October 5, 2019 (Kinshasa Mahidol Oxford Research Unit, Kinshasa, DRC), 1137 children were recruited: 598 (52.6%) in Uganda and 539 (47.4%) in DRC. Baseline characteristics were comparable across all arms (Table 1). The median age and weight were 5 years and 17 kg, and the boy to girl ratio was 1.4:1. The total number of infants (aged 6 to < 12 months) was 55 (4.8%). Almost all children were of normal nutritional status (7 had moderate malnutrition and 1 severe acute malnutrition). G6PD status was established in 1120/1137 (98.5%) children; heterozygous α-thalassaemia was found in a little under half of children and homozygous α-thalassaemia in < 10%. HbAS was identified in 14.5%. There were two early treatment failures only in the AL and AL + placebo arms (2/542, 0.4%) and 101/542 (18.6%, AL arm) and 27/539 (5.1%, DHAPP arm) late treatment failures.
The mean Hb fell initially to reach its mean nadir concentration on D2 and recovered by D14 in all children and when stratified by G6PD status (Fig. 1); the nadir Hb occurred as early as 6 h in 4.7% of children (54/1137, Fig. 2). A total of 3/1137 (0.3%) of children dropped their Hb to < 5 g/dL; all were transfused.
Factors associated with baseline, nadir, and fractional fall in Hb
The baseline Hb was lower in younger children with a longer illness history, a lower MUAC, splenomegaly, who were homozygous α-thalassaemia, and either G6PDd or heterozygous females (Table 2). Although HbSS was associated with a significantly lower baseline Hb concentration, this result should be interpreted with caution because only 3 children were HbSS. The nadir Hb was higher with a higher baseline Hb, increasing age, and HbAS and lower in those with longer illness duration and higher baseline parasitaemias (Table 2).
The median fractional fall in Hb was 11.7% (IQR 6.8 − 17.5) with maximum falls of 37% (heterozygous females), 40.4% (G6PDd), and 57.9% (G6PD normal); these falls were greater in younger children with higher D0 Hbs and D0 parasitaemias and longer illnesses but less in HbAS (Table 2). The D0 Hb had the greatest influence on the fractional fall, but HbAS had a greater protective effect than increasing age.
Factors associated with D42 Hb concentration and Hb recovery
A more robust MAFt response was seen in older G6PDd children with a longer illness duration and higher D0 Hb and D0 parasitaemia but was lower with a greater fractional fall in Hb and sickle cell disease (n = 3) vs. Hb AA. A higher D42 Hb concentration was seen in older G6PDd children with a greater fractional fall but was lower in patients with sickle cell disease (vs. Hb AA), heterozygous and homozygous α-thalassaemia, and those with late treatment failure. By D42, 807 of 1066 (75.7%) children recovered their Hb and were more likely to be older with G6PDd, lower D0 Hbs, and greater Hb fractional falls.
The time to Hb recovery ranged from 6 h − 42 days for a median of 14 (IQR 2–21) days and 7 (2 − 21) days in the G6PDd group. However, there was an interaction between baseline Hb and G6PD status on the time to haemoglobin recovery, suggesting a differential time to recovery in the G6PD-deficient group that depended on Hb concentration (Additional file 2: Tab S1). Therefore, we stratified these analyses by the presence/absence of anaemia of any degree to understand better the effect of the observed significant interaction. The time to Hb recovery was significantly faster in the G6PDd children without anaemia, but there was no difference by G6PD status in the anaemic group (Fig. 3). This was confirmed in the Cox proportional hazards regression model, which also showed that there were no other explanatory variables (Additional file 2: Tab S1). MUAC, fractional fall, splenomegaly, and log baseline parasitaemia violated the proportional hazards assumption in the univariate analyses and were excluded from the multivariable analysis (all were significant in the univariate analysis).
Being on SLDPQ was not an independent explanatory factor for the time to and probability of Hb recovery by D42 and the D42 Hb concentration.
Effect of primaquine pharmacokinetics and activity score
For all the postbaseline parameters of haemoglobin dynamics, there was no effect of primaquine when modelled either as a categorical factor, i.e. ACT + SLDPQ vs. ACT alone in all patients (Table 2), or in the PK subgroup (Additional file 3: Tab S2) when expressed as the mg/kg dose with CYP2D6 activity score (Additional file 3: Tab S3-8), AUC0-t (Additional file 3: Tab S9-14), or as the Cmax (Additional file 3: Tab S15-20).
Hb dynamics in children with normal Hbs or moderate anaemia at baseline
There were 407 patients without baseline anaemia and 76 with moderate anaemia (Hb < 8 g/dL). G6PD status is detailed in Fig. 4. There was a very small, initial decline in the mean Hb and more rapid recovery and robust mean MAFt in the moderately anaemic children, with a greater response in the G6PDd group.
Mean haemoglobin changes over time with 95% confidence intervals for all children with normal baseline haemoglobin concentrations or moderate anaemia and shown by G6PD status. There were 407 patients without baseline anaemia, defined as a haemoglobin ≥ 11 g/dL for children < 5 years and ≥ 11.5 mg/dL for 5–11 years: G6PD normal = 284 (70%), G6PD heterozygous females = 42 (10%), G6PDd males and females = 74 (18%), and 7 with missing G6PD data. Patients with moderate anaemia (haemoglobin < 8 mg/dL) numbered 76: G6PD normal = 44 (58%), G6PD heterozygous females = 9 (12%), G6PDd males and females = 22 (29%), and 1 with missing G6PD data
Blood transfusions
There were 10 blood transfusions (0.9%) in the first week, equally distributed between SLDPQ and placebo, and one transfusion on D31 in a patient with recurrent parasitaemia (Table 3). The 3 children with Hbs < 5 g/dL had fractional falls of 23 to 58%. None of the HbAS or HbSS children were transfused.
Discussion
MCPs with residual anxiety regarding the safety of SLDPQ should be reassured by the substantial evidence from our earlier randomised, placebo-controlled trial, which showed that the toxicity of age-dosed SLDPQ was similar to that of placebo and significantly reduced gametocyte carriage [36]. Moreover, despite the resulting higher PQ exposure [37] compared to SLDPQ in asymptomatic P. falciparum carriers [47], SLDPQ was well tolerated and, as we show here, had no deleterious effects on any of the posttreatment Hb parameters.
In this analysis, we detail the factors influencing Hb dynamics to guide clinicians and MCPs on identifying particular patients who may be at increased risk of developing clinically significant and, possibly, life-threatening anaemia and those who may not recover their Hb by 6 weeks.
At baseline, children with a lower Hb concentration were younger, had longer illness durations, lower MUACs, an increased prevalence of splenomegaly, and were more frequently affected by either homozygous α-thalassaemia, HbSS, or G6PDd (either as heterozygous females or hemizygous males/homozygous females). Consistent with previous clinical descriptions [48], baseline Hb appeared lowest among children with HbSS, although the small numbers affected precluded meaningful further analysis. Given the high frequency of HbSS of ~ 1–2% at birth [49, 50], the low frequency of HbSS among children recruited to this trial (3/1137; 0.26%) is striking, particularly given our recent observation that ~ 30% of children recruited to a recent trial of severe anaemia (one site was Mbale) had HbSS [51]. This low HbSS rate can be explained by several factors. Children with Hbs < 6 g/dL were excluded; HbSS-affected children [51, 52], like those with HbAS [53], are innately resistant to P. falciparum infections but suffer high rates (> 50%) of early-life mortality [54].
Some of our findings, notably age, length of illness, and splenomegaly, were also significant factors for baseline Hb or anaemia in African patients, especially in children < 5 years [21], in children and adults in Thailand [26], and in adults in Indonesia [45]. We found no association between baseline Hb and baseline parasite count in contrast to WWARN who reported an inverse relationship until a parasitaemia of 10,000/μL, followed by a positive relationship [22], whilst Zwang et al. reported a weak positive relationship between baseline Hb and baseline parasitaemia in children < 5 years (mean 0.08 g/dL/year) but an inverse relationship in patients aged ≥ 5 years [21].
The majority of patients with uncomplicated falciparum infections experience an initial fall in Hb and reach their nadir concentration on day 2 [21, 22] and the first posttreatment week is the time of highest risk for a blood transfusion [23,24,25, 36, 55]. Therefore, the fractional decline and the nadir Hbs are important parameters to consider in conjunction with clinical signs of severity before deciding the need for a blood transfusion. Previous work has also identified hyperparasitaemia (≥ 150,000/μL) [23] and giving CDA or CD to G6PDd males and heterozygous females [24, 25] as risk factors for a blood transfusion.
We found that a lower nadir Hb was associated with a lower baseline Hb in younger children with longer illnesses and higher baseline parasite counts; these factors were also associated with greater fractional falls in Hb except that a higher baseline Hb was associated with a greater fractional fall. Importantly, SLDPQ, G6PDd, and α-thalassaemia genotypes were not explanatory factors for both parameters. HbAS was associated with a slightly higher nadir Hb and lower fractional fall compared to those with normal Hbs that may be related to greater pretreatment haemolysis and, therefore, fewer red cells are available to haemolyse after treatment is given. Our findings concur with previous work showing that younger age, higher baseline Hbs and parasitaemias, and length of illness are significantly associated with a greater mean fractional fall in Hb [21, 22, 26, 27, 45], although Zwang et al. found age was not a factor [21].
Few studies have examined the effects of inherited blood disorders on malaria dynamics. Genotypically defined G6PDd and CYP2D6 genotypes were not factors in the initial absolute fall in falciparum-infected Tanzanian patients (median age 6.4 years), who were treated with SLDPQ [56], and α-thalassaemia genotype was not a factor in the D7 fractional fall in Hb in Tanzanian children (median age 4 years) [34]. In a small study from Cambodia, there was no association between the fractional fall in Hb and G6PD Viangchan and HbE status [27].
Posttreatment Hb recovery occurs as the malaria-induced bone marrow dyserythropoiesis and suppression are reversed and the haemolysis of parasitised and non-parasitised red cells ceases [57]. The return of bone marrow function is reflected by the MAFt, the time to Hb recovery, and an increasing reticulocyte count; the latter peaked on D7 in our patients and was independent of G6PD status [36].
Although we observed a rapid median time to Hb recovery (14 days), ~ 25% of children did not recover their Hb. A higher baseline Hb resulted in a longer time to Hb recovery (Fig. 4), consistent with Zwang et al. [21], a lower probability of achieving Hb recovery, and a lower MAFt (Fig. 4); the latter was also reported in a small study of falciparum-infected Papuan adults [45]. By contrast, our multivariable analysis found a higher D0 Hb was associated with a higher, albeit very small, mean increase in MAFt (Table 2), and this discrepancy might be a chance finding. Increasing age, a higher initial fractional fall and being G6PDd were associated with achieving a higher D42 Hb concentration and, in addition to a lower D0 Hb, a greater probability of Hb recovery by D42. G6PDd children had a more rapid time to Hb recovery, only if they had normal baseline Hbs, ~ 70% increased likelihood of D42 Hb recovery compared to G6PD normal children, and achieved a modest increase in mean D42 Hb concentration of 0.2 g/dL.
Despite the apparent disadvantage of a lower baseline Hb, the D42 Hb concentration was not affected, in contrast to a small study of adults from Papua [45]. However, a lower D42 Hb concentration was associated with either α-thalassaemia genotype (potentially reflecting normal physiology), sickle cell disease, or failing treatment; whilst the latter has been previously reported in two studies [26, 45], it was not a factor in Zwang et al.’s meta-analysis of posttreatment anaemia in patients of all ages [21].
Our study had several limitations. The number of children with moderate anaemia was quite low (~ 7%); there were only three children with HbSS (cautioning interpretation of significant findings), and the majority of children had good nutritional status. As a result, we cannot generalise our findings to children in the community with Hb concentrations of 4–6 g/dL and uncomplicated disease, those with HbSS, or those with moderate or severe acute malnutrition. We dosed SLDPQ by age, which is typically associated with broader mg/kg doses compared to weight-based dosing [35], so our findings may differ from studies based on weight-based regimens. Finally, we conducted many analyses, meaning that some significant findings (e.g. the association between the baseline Hb and the MAFt) might have reflected chance.
Conclusions
To conclude, our large study has shown clearly that SLDPQ did not affect any of the posttreatment parameters of Hb dynamics and has provided additional reassuring evidence of its tolerability. Moreover, G6PDd was only associated with one negative finding—a lower mean baseline Hb concentration. Thereafter, it had a positive posttreatment effect on achieving Hb recovery and a higher D42 Hb but, crucially, did not affect the nadir Hb or the initial fall in Hb. Moreover, children with a Hb < 8 g/dL had a robust posttreatment Hb recovery, allaying anxieties that a lower baseline Hb is necessarily harmful.
Our findings strongly support the notion that using SDLPQ in G6PDd patients is safe and does not increase the risk of subsequent blood transfusion. Indeed, equal numbers of G6PDd and G6PD normal patients were transfused and our overall transfusion rate was just under 1% [36]. SLDPQ should be deployed more widely in Africa as part of a global strategy to eliminate ARPf.
Availability of data and materials
We have provided much detailed analysis in this paper. Nevertheless, deidentified individual participant data and relevant supplementary data and documents (e.g., data dictionary, protocol, and participant information sheet) will be available to applicants who provide a sound proposal to the Mahidol Oxford Tropical Medicine Research Unit Data Access Committee (datasharing@tropmedres.ac). A data access agreement will be put in place before sharing.
Abbreviations
- A:
-
Sub-Saharan Africa
- ACT:
-
Artemisinin-based combination therapy
- AL:
-
Artemether-lumefantrine
- ARPf:
-
Artemisinin resistant Plasmodium falciparum
- AS:
-
Artesunate
- AUC0-t :
-
Area under the drug concentration time curve from 0 to the last PK sample
- CD:
-
Chlorproguanil dapsone
- CDA:
-
Chlorproguanil-dapsone-artesunate
- CI:
-
Confidence interval
- Cmax :
-
Maximum concentration
- D:
-
Day
- DHAPP:
-
Dihydroartemisinin-piperaquine
- IQR:
-
Interquartile range
- G6PDd:
-
Glucose-6-phosphate dehydrogenase deficiency
- Hb:
-
Haemoglobin
- HbAA:
-
Normal haemoglobin
- HbAS:
-
Sickle cell trait
- HbSS:
-
Sickle cell disease
- MAFt:
-
Total malaria attributable fall in Hb following treatment
- MUAC:
-
Mid upper arm circumference
- PK:
-
Pharmacokinetics
- PQ:
-
Primaquine
- SLDPQ:
-
Single low-dose primaquine
- WHO:
-
World Health Organization
- WWARN:
-
Worldwide Antimalarial Resistance Network
References
White NJ, Qiao LG, Qi G, Luzzatto L. Rationale for recommending a lower dose of primaquine as a Plasmodium falciparum gametocytocide in populations where G6PD deficiency is common. Malar J. 2012;11:418.
Dicko A, Brown JM, Diawara H, Baber I, Mahamar A, Soumare HM, et al. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis. 2016;16(6):674–84.
Dicko A, Roh ME, Diawara H, Mahamar A, Soumare HM, Lanke K, et al. Efficacy and safety of primaquine and methylene blue for prevention of Plasmodium falciparum transmission in Mali: a phase 2, single-blind, randomised controlled trial. Lancet Infect Dis. 2018;18(6):627–39.
Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, et al. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis. 2014;14(2):130–9.
Vantaux A, Kim S, Piv E, Chy S, Berne L, Khim N, et al. Significant efficacy of a single low dose of primaquine compared to stand-alone artemisinin combination therapy in reducing gametocyte carriage in Cambodian patients with uncomplicated multidrug-resistant plasmodium falciparum malaria. Antimicrob Agents Chemother. 2020;64(6):e02108.
Stepniewska K, Humphreys GS, Goncalves BP, Craig E, Gosling R, Guerin PJ, et al. Efficacy of single-dose primaquine with artemisinin combination therapy on plasmodium falciparum gametocytes and transmission: an individual patient meta-analysis. J Infect Dis. 2022;225(7):1215–26.
Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359(24):2619–20.
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361(5):455–67.
Leang R, Taylor WR, Bouth DM, Song L, Tarning J, Char MC, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in western Cambodia: dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59(8):4719–26.
Leang R, Ros S, Duong S, Navaratnam V, Lim P, Ariey F, et al. Therapeutic efficacy of fixed dose artesunate-mefloquine for the treatment of acute, uncomplicated Plasmodium falciparum malaria in Kampong Speu, Cambodia. Malar J. 2013;12:343.
Mihreteab S, Platon L, Berhane A, Stokes BH, Warsame M, Campagne P, et al. Increasing prevalence of artemisinin-resistant HRP2-negative malaria in Eritrea. N Engl J Med. 2023;389(13):1191–202.
Uwimana A, Legrand E, Stokes BH, Ndikumana JM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020;26(10):1602–8.
Balikagala B, Fukuda N, Ikeda M, Katuro OT, Tachibana SI, Yamauchi M, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med. 2021;385(13):1163–71.
Tumwebaze PK, Conrad MD, Okitwi M, Orena S, Byaruhanga O, Katairo T, et al. Decreased susceptibility of Plasmodium falciparum to both dihydroartemisinin and lumefantrine in northern Uganda. Nat Commun. 2022;13(1):6353.
Straimer J, Gandhi P, Renner KC, Schmitt EK. High prevalence of Plasmodium falciparum K13 mutations in Rwanda is associated with slow parasite clearance after treatment with artemether-lumefantrine. J Infect Dis. 2022;225(8):1411–4.
Okebe J, Amambua-Ngwa A, Parr J, Nishimura S, Daswani M, Takem EN, et al. The prevalence of glucose-6-phosphate dehydrogenase deficiency in Gambian school children. Malar J. 2014;13:148.
Clark TG, Fry AE, Auburn S, Campino S, Diakite M, Green A, et al. Allelic heterogeneity of G6PD deficiency in West Africa and severe malaria susceptibility. Eur J Hum Genet. 2009;17(8):1080–5.
Walakira A, Tukwasibwe S, Kiggundu M, Verra F, Kakeeto P, Ruhamyankaka E, et al. Marked variation in prevalence of malaria-protective human genetic polymorphisms across Uganda. Infect Genet Evol. 2017;55:281–7.
Ouattara AK, Yameogo P, Traore L, Diarra B, Assih M, Compaore TR, et al. Prevalence, genetic variants and clinical implications of G-6-PD deficiency in Burkina Faso: a systematic review. BMC Med Genet. 2017;18(1):139.
Williams O, Gbadero D, Edowhorhu G, Brearley A, Slusher T, Lund TC. Glucose-6-phosphate dehydrogenase deficiency in Nigerian children. PLoS ONE. 2013;8(7):e68800.
Zwang J, D’Alessandro U, Ndiaye JL, Djimde AA, Dorsey G, Martensson AA, et al. Haemoglobin changes and risk of anaemia following treatment for uncomplicated falciparum malaria in sub-Saharan Africa. BMC Infect Dis. 2017;17(1):443.
WorldWide Antimalarial Resistance Network Falciparum Haematology Study G. Haematological consequences of acute uncomplicated falciparum malaria: a WorldWide antimalarial resistance network pooled analysis of individual patient data. BMC Med. 2022;20(1):85.
Onyamboko MA, Fanello CI, Wongsaen K, Tarning J, Cheah PY, Tshefu KA, et al. Randomized comparison of the efficacies and tolerabilities of three artemisinin-based combination treatments for children with acute Plasmodium falciparum malaria in the Democratic Republic of the Congo. Antimicrob Agents Chemother. 2014;58(9):5528–36.
Fanello CI, Karema C, Avellino P, Bancone G, Uwimana A, Lee SJ, et al. High risk of severe anaemia after chlorproguanil-dapsone+artesunate antimalarial treatment in patients with G6PD (A-) deficiency. PLoS ONE. 2008;3(12):e4031.
Pamba A, Richardson ND, Carter N, Duparc S, Premji Z, Tiono AB, et al. Clinical spectrum and severity of hemolytic anemia in glucose 6-phosphate dehydrogenase-deficient children receiving dapsone. Blood. 2012;120(20):4123–33.
Price RN, Simpson JA, Nosten F, Luxemburger C, Hkirjaroen L, ter Kuile F, et al. Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001;65(5):614–22.
Dysoley L, Kim S, Lopes S, Khim N, Bjorges S, Top S, et al. The tolerability of single low dose primaquine in glucose-6-phosphate deficient and normal falciparum-infected Cambodians. BMC Infect Dis. 2019;19(1):250.
Mwaiswelo R, Ngasala BE, Jovel I, Gosling R, Premji Z, Poirot E, et al. Safety of a single low-dose of primaquine in addition to standard artemether-lumefantrine regimen for treatment of acute uncomplicated Plasmodium falciparum malaria in Tanzania. Malar J. 2016;15:316.
Bancone G, Chowwiwat N, Somsakchaicharoen R, Poodpanya L, Moo PK, Gornsawun G, et al. Single low dose primaquine (0.25 mg/kg) does not cause clinically significant haemolysis in G6PD deficient subjects. PLoS ONE. 2016;11(3):e0151898.
Chen I, Diawara H, Mahamar A, Sanogo K, Keita S, Kone D, et al. Safety of single-dose primaquine in G6PD-deficient and G6PD-normal males in Mali without malaria: an open-label, phase 1, dose-adjustment trial. J Infect Dis. 2018;217(8):1298–308.
Tine RC, Sylla K, Faye BT, Poirot E, Fall FB, Sow D, et al. Safety and efficacy of adding a single low dose of primaquine to the treatment of adult patients with Plasmodium falciparum Malaria in Senegal, to reduce gametocyte carriage: a randomized controlled trial. Clin Infect Dis. 2017;65(4):535–43.
Bastiaens GJH, Tiono AB, Okebe J, Pett HE, Coulibaly SA, Goncalves BP, et al. Safety of single low-dose primaquine in glucose-6-phosphate dehydrogenase deficient falciparum-infected African males: two open-label, randomized, safety trials. PLoS ONE. 2018;13(1):e0190272.
Stepniewska K, Allen EN, Humphreys GS, Poirot E, Craig E, Kennon K, et al. Safety of single-dose primaquine as a Plasmodium falciparum gametocytocide: a systematic review and meta-analysis of individual patient data. BMC Med. 2022;20(1):350.
Shekalaghe SA, ter Braak R, Daou M, Kavishe R, van den Bijllaardt W, van den Bosch S, et al. In Tanzania, hemolysis after a single dose of primaquine coadministered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase-deficient (G6PD A-) individuals. Antimicrob Agents Chemother. 2010;54(5):1762–8.
Taylor WR, Naw HK, Maitland K, Williams TN, Kapulu M, D’Alessandro U, et al. Single low-dose primaquine for blocking transmission of Plasmodium falciparum malaria - a proposed model-derived age-based regimen for sub-Saharan Africa. BMC Med. 2018;16(1):11.
Taylor WR, Olupot-Olupot P, Onyamboko MA, Peerawaranun P, Weere W, Namayanja C, et al. Safety of age-dosed, single low-dose primaquine in children with glucose-6-phosphate dehydrogenase deficiency who are infected with Plasmodium falciparum in Uganda and the Democratic Republic of the Congo: a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet Infect Dis. 2023;23(4):471–83.
Mukaka M, Onyamboko MA, Olupot-Olupot P, Peerawaranun P, Suwannasin K, Pagornrat W, et al. Pharmacokinetics of single low dose primaquine in Ugandan and Congolese children with falciparum malaria. EBioMedicine. 2023;96:104805.
Puaprasert K, Chu C, Saralamba N, Day NPJ, Nosten F, White NJ, et al. Real time PCR detection of common CYP2D6 genetic variants and its application in a Karen population study. Malar J. 2018;17(1):427.
Dorado P, Caceres MC, Pozo-Guisado E, Wong ML, Licinio J, Llerena A. Development of a PCR-based strategy for CYP2D6 genotyping including gene multiplication of worldwide potential use. Biotechniques. 2005;39(4):571–4.
Malaria Genomic Epidemiology N, Malaria Genomic Epidemiology N. Reappraisal of known malaria resistance loci in a large multicenter study. Nat Genet. 2014;46(11):1197–204.
Waterfall CM, Cobb BD. Single tube genotyping of sickle cell anaemia using PCR-based SNP analysis. Nucleic Acids Res. 2001;29(23):E119.
Williams TN, Wambua S, Uyoga S, Macharia A, Mwacharo JK, Newton CR, et al. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005;106(1):368–71.
WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System (2011) Geneva, World Health Organization WHO/NMH/NHD/MNM/11.1).
Khim N, Benedet C, Kim S, Kheng S, Siv S, Leang R, et al. G6PD deficiency in Plasmodium falciparum and Plasmodium vivax malaria-infected Cambodian patients. Malar J. 2013;12(1):171.
Taylor WR, Widjaja H, Basri H, Tjitra E, Ohrt C, Taufik T, et al. Haemoglobin dynamics in Papuan and non-Papuan adults in northeast Papua, Indonesia, with acute, uncomplicated vivax or falciparum malaria. Malar J. 2013;12:209.
Caudle KE, Sangkuhl K, Whirl-Carrillo M, Swen JJ, Haidar CE, Klein TE, et al. Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin Transl Sci. 2020;13(1):116–24.
Goncalves BP, Pett H, Tiono AB, Murry D, Sirima SB, Niemi M, et al. Age, weight, and CYP2D6 genotype are major determinants of primaquine pharmacokinetics in African children. Antimicrob Agents Chemother. 2017;61(5):e02590.
Sadarangani M, Makani J, Komba AN, Ajala-Agbo T, Newton CR, Marsh K, et al. An observational study of children with sickle cell disease in Kilifi, Kenya. Br J Haematol. 2009;146(6):675–82.
Tshilolo L, Aissi LM, Lukusa D, Kinsiama C, Wembonyama S, Gulbis B, et al. Neonatal screening for sickle cell anaemia in the Democratic Republic of the Congo: experience from a pioneer project on 31 204 newborns. J Clin Pathol. 2009;62(1):35–8.
Ndeezi G, Kiyaga C, Hernandez AG, Munube D, Howard TA, Ssewanyana I, et al. Burden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): a cross-sectional study. Lancet Glob Health. 2016;4(3):e195-200.
Uyoga S, Olupot-Olupot P, Connon R, Kiguli S, Opoka RO, Alaroker F, et al. Sickle cell anaemia and severe Plasmodium falciparum malaria: a secondary analysis of the Transfusion and Treatment of African Children Trial (TRACT). Lancet Child Adolesc Health. 2022;6(9):606–13.
Band G, Leffler EM, Jallow M, Sisay-Joof F, Ndila CM, Macharia AW, et al. Malaria protection due to sickle haemoglobin depends on parasite genotype. Nature. 2022;602(7895):106–11.
Willcox M, Bjorkman A, Brohult J, Pehrson PO, Rombo L, Bengtsson E. A case-control study in northern Liberia of Plasmodium falciparum malaria in haemoglobin S and beta-thalassaemia traits. Ann Trop Med Parasitol. 1983;77(3):239–46.
Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2011;41(6 Suppl 4):S398-405.
Maitland K, Kiguli S, Olupot-Olupot P, Engoru C, Mallewa M, Saramago Goncalves P, et al. Immediate transfusion in African children with uncomplicated severe anemia. N Engl J Med. 2019;381(5):407–19.
Mwaiswelo RO, Ngasala B, Msolo D, Kweka E, Mmbando BP, Martensson A. A single low dose of primaquine is safe and sufficient to reduce transmission of Plasmodium falciparum gametocytes regardless of cytochrome P450 2D6 enzyme activity in Bagamoyo district, Tanzania. Malar J. 2022;21(1):84.
White NJ. Anaemia and malaria. Malar J. 2018;17(1):371.
Acknowledgements
We thank the patients and their guardians for participating in the study and the laboratory technicians, nurses, and data managers from both sites as well as MORU’s CTSG for making the study happen.
Funding
This work was cofunded by the UK Medical Research Council, Wellcome, and UK Aid through the Global Health Trials (grant reference MR/P006973/1). The funders had no role in the study design, execution, and analysis and decisions regarding publication. This research was also funded in part by the Wellcome Trust (grant reference 220211). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
TNW is funded through a Senior Research Fellowship from Wellcome (202800/Z/16/Z). KM and POO received funding from Wellcome East African Overseas Programme Award from the Wellcome Trust 203077/Z/16/Z.
Author information
Authors and Affiliations
Contributions
WRJT conceived the study and co-wrote the grant with NJPD. Site PIs were PO-O (Mbale) and MAO (KIMORU). PO-O, MAO, WW, CN, PO, HT, JB, RM, DKK, PON, BBB, CBO, TNW, SU, CF, and KM were responsible for data collection with MAO, CF, POO, TNW, and KM overseeing the study. WRJT, MM, CT, and AD analysed the data. WRJT and MM co-wrote the first draft of the paper. All authors have seen and approved the final submitted version and agreed to publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
These were obtained from the following: Ministry of Higher and University Education, the University of Kinshasa Public Health School Ethics Committee, and the city of Kinshasa Provincial Government Health Minister (135/MIN.SAN.AFFSOC and ACHUM/CM/JD/2017), Mbale Regional Hospital Institutional Review Committee (MRRH-REC OUT – COM 006/2017), National Drug Authority (CTA0028), and the Uganda National Council for Science and Technology (HS2205), and Oxford University Tropical Research Ethics Committee (reference 53–16). All parents/guardians gave fully informed consent to join the study and for the data to be published.
Consent for publication
This was obtained from parents/guardians at the signing of the consent form.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Changes in the mean haemoglobin concentrations showing the malaria attributable fractions in all treated children, irrespective of G6PD status and treatment.
Additional file 2: Table S1.
Factors associated with time to haemoglobin recovery by Cox Proportional Hazards model.
Additional file 3: Table S2.
Baseline characteristics of patients in the PK substudy. Tables S3-20. Risk factors, including PK parameters, for haematological outcomes in the PK patient subset.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Onyamboko, M.A., Olupot-Olupot, P., Were, W. et al. Factors affecting haemoglobin dynamics in African children with acute uncomplicated Plasmodium falciparum malaria treated with single low-dose primaquine or placebo. BMC Med 21, 397 (2023). https://doi.org/10.1186/s12916-023-03105-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-03105-0