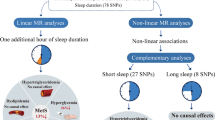
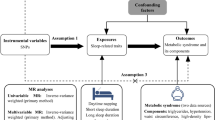
Abstract
Background
Few studies have investigated the joint effects of sleep traits on the risk of acute myocardial infarction (AMI). No previous study has used factorial Mendelian randomization (MR) which may reduce confounding, reverse causation, and measurement error. Thus, it is prudent to study joint effects using robust methods to propose sleep-targeted interventions which lower the risk of AMI.
Methods
The causal interplay between combinations of two sleep traits (including insomnia symptoms, sleep duration, or chronotype) on the risk of AMI was investigated using factorial MR. Genetic risk scores for each sleep trait were dichotomized at their median in UK Biobank (UKBB) and the second survey of the Trøndelag Health Study (HUNT2). A combination of two sleep traits constituting 4 groups were analyzed to estimate the risk of AMI in each group using a 2×2 factorial MR design.
Results
In UKBB, participants with high genetic risk for both insomnia symptoms and short sleep had the highest risk of AMI (hazard ratio (HR) 1.10; 95% confidence interval (CI) 1.03, 1.18), although there was no evidence of interaction (relative excess risk due to interaction (RERI) 0.03; 95% CI −0.07, 0.12). These estimates were less precise in HUNT2 (HR 1.02; 95% CI 0.93, 1.13), possibly due to weak instruments and/or small sample size. Participants with high genetic risk for both a morning chronotype and insomnia symptoms (HR 1.09; 95% CI 1.03, 1.17) and a morning chronotype and short sleep (HR 1.11; 95% CI 1.04, 1.19) had the highest risk of AMI in UKBB, although there was no evidence of interaction (RERI 0.03; 95% CI −0.06, 0.12; and RERI 0.05; 95% CI –0.05, 0.14, respectively). Chronotype was not available in HUNT2.
Conclusions
This study reveals no interaction effects between sleep traits on the risk of AMI, but all combinations of sleep traits increased the risk of AMI except those with long sleep. This indicates that the main effects of sleep traits on AMI are likely to be independent of each other.
Similar content being viewed by others
Background
Poor sleep is a major public health problem that has emerged as being associated with several health conditions [1, 2], including those related to cardiovascular health such as hypertension [2, 3], obesity [2, 4], and dyslipidemia [5]. Cardiovascular diseases (CVDs) account for a large part of global morbidity and are the leading cause of death [6]. Since sleep problems can be managed through cognitive-behavioral therapy and medication [7], understanding how sleep impacts cardiovascular health can have important implications for interventions that aim to target sleep with an objective to lower the risk of CVDs.
Sleep is a complex and multifaceted biological phenomenon which comprises several traits [8]. Previous observational studies have mainly focused on individual sleep traits as separate risk factors for CVDs [9,10,11,12,13]. Insomnia symptoms, short or long sleep duration, and evening chronotype have been identified as individual risk factors for acute myocardial infarction (AMI) [9, 11, 13, 14]. Sleep traits are often correlated and can together assert their influence on the disease risk. Few observational studies have investigated the joint effects of sleep traits and have found evidence that sleep traits interact to increase the risk of cardiovascular outcomes [14,15,16,17,18,19,20]. For instance, insomnia with short sleep considered the most biologically severe sleep disorder phenotype [21], has been found to be associated with increased cardiometabolic risk [14, 16, 18,19,20]. In our recent study, we observed that those reporting two sleep traits (including insomnia symptoms, short sleep, long sleep, and evening chronotype) had a higher incidence of AMI than those reporting only one sleep trait. Any relative excess risk due to interaction (RERI) was only observed among those reporting insomnia symptoms and long sleep duration [14]. However, the available evidence on the joint effects of sleep traits on the risk of AMI is based on conventional observational studies that are prone to bias due to residual confounding, reverse causation, and measurement error [22].
Mendelian randomization (MR) uses genetic variants as instruments that are robustly associated with a modifiable risk factor to investigate the causal effect on an outcome [23]. MR exploits the fact that genetic variants are randomly assigned to individuals and fixed at conception, making it less susceptible to the bias observed in conventional observational studies. Recent MR studies have evaluated individual effects of sleep traits on CVDs, providing evidence of an adverse effect of insomnia symptoms on prevalent coronary artery disease (CAD) [24,25,26,27] and AMI [28], and a protective effect per hour increase in sleep duration and an adverse effect of short sleep on CAD and AMI [11, 29] (see a summary in Additional file 1: Table S1) [11, 24,25,26,27,28,29,30,31,32,33]. MR investigation of chronotype is scarce and lacks compelling evidence [28]; thus, it remains unclear whether chronotype itself is causally associated with an increased risk of AMI or if the adverse effect of circadian preference can be explained by insomnia symptoms or sleep duration. More importantly, MR investigations exploring the joint causal effects of sleep traits on risk of AMI remain largely untapped, which could provide robust evidence on the risk of AMI from experiencing two sleep traits simultaneously.
In this study, we therefore used one-sample and factorial MR to investigate the causal effects of individual sleep traits (insomnia symptoms, sleep duration and chronotype) and their joint effects on incident AMI, in two large longitudinal studies (UK Biobank (UKBB) and the second survey of the Trøndelag Health Study (HUNT2)).
Methods
Study participants
UK Biobank
Out of 9.2 million eligible adults (ranging between 40 and 70 years) in the UK who were invited to participate, more than 500 000 participated in the study during March 2006–July 2010 (5.5% response rate). The participants visited one of the 22 study assessment centers located throughout England, Scotland, and Wales, where they signed an electronic consent and completed a touchscreen questionnaire along with a brief computer-assisted interview. They provided detailed information about their lifestyle and physical measures and had blood, urine, and saliva samples collected and stored for future analysis, as described elsewhere [34]. The UKBB received approval from the National Health Service (NHS) Research Ethics Service (reference number 11/NW/0382), and the database was created in compliance with the Declaration of Helsinki.
HUNT study
All inhabitants aged 20 years or older in the Nord-Trøndelag region of Norway were invited to participate in a four-phase population-based health survey (the HUNT study), first in 1984–1986 (HUNT1), then in 1995–1997 (HUNT2) and 2006–2008 (HUNT3), and last in 2017–2019 (HUNT4). This study is based on data from HUNT2, where 93 898 individuals were invited and 65 228 (69.5%) participated [35]. The invitation letter was sent by mail along with a self-administered questionnaire. The participants attended examination stations where clinical examination was performed, and blood samples were drawn by trained personnel. Detailed information regarding HUNT2 study has been published elsewhere [36]. The HUNT Study was approved by the Data Inspectorate of Norway and recommended by the Regional Committee for Ethics in Medical Research (REK; reference number 152/95/AH/JGE). Additionally, the ethical clearance for conducting this study was obtained from the Regional Committee for Ethics in Medical Research (REK nord; reference number 2020/47206).
Sleep traits
Insomnia symptoms
In both UKBB and HUNT2, insomnia symptoms were defined as two night-time insomnia symptoms (i.e., difficulty falling asleep, difficulty maintaining sleep or waking up too early) without information about daytime impairment. Thus, our definition for insomnia symptoms did not include all components used in the frameworks for diagnosing insomnia [37].
In UKBB, participants were asked: “Do you have trouble falling asleep at night or do you wake up in the middle of the night?” (Field ID: 1200) with response options “Never/rarely”, “Sometimes”, “Usually” or “Prefer not to answer”. Participants were classified as having insomnia symptoms if they answered “Usually”; and not having insomnia symptoms if they answered “Never/rarely” or “Sometimes”. Other responses were coded as missing.
In HUNT2, insomnia symptoms were assessed by the following two questions: “Have you had difficulty falling asleep in the last month?”, and “During the last month, have you woken too early and not been able to get back to sleep?” with response options “Never”, “Sometimes”, “Often” or “Almost every night”. Participants who responded “Often” or “Almost every night” to at least one of these questions were classified as having insomnia symptoms. For participants who answered only one of these insomnia symptom questions, we did the following: (1) if they answered “Often” or “Almost every night” to one of the questions, but did not answer the other, they were classified as having insomnia symptoms, and (2) if they answered “Never” or “Sometimes” to one of the questions, but did not answer the other, they were excluded to avoid possible misclassification. The remaining participants were classified as not having insomnia symptoms.
Sleep duration
Sleep duration was assessed by the questions: “About how many hours sleep do you get in every 24 hours? (please include naps)” (Field ID: 1160) and “How many hours do you usually spend lying down (i.e., sleeping and/or napping) during a 24-hour period?” in UKBB and HUNT2, respectively. The answers could only contain integer values. Any influence of poor health on implausible short or long sleep durations was avoided by excluding extreme responses of less than 3 hours or more than 18 hours. Binary variables for short sleep (≤ 6 hours vs. 7–8 hours) and long sleep (≥ 9 hours vs. 7–8 hours) were also constructed.
Chronotype
Chronotype (morning or evening chronotype) in UKBB was assessed by the question: “Do you consider yourself to be?” (Field ID: 1180) with response options “Definitely a ‘morning’ person”, “More a ‘morning’ than ‘evening’ person”, “More an ‘evening’ than a ‘morning’ person”, “Definitely an ‘evening’ person”, “Do not know” or “Prefer not to answer”. Participants were classified as having a morning chronotype if they reported “Definitely a ‘morning’ person” or “More a ‘morning’ than ‘evening’ person” and as having an evening chronotype if they reported “More an ‘evening’ than a ‘morning’ person” or “Definitely an ‘evening’ person”. Other responses were coded as missing. Chronotype was not reported in any survey of the HUNT Study.
Acute myocardial infarction (AMI)
In UKBB, participants were followed through record linkage to the Hospital Episode Statistics (HES) for England, Scottish Morbidity Record (SMR), and Patient Episode Database for Wales (PEDW) where health-related outcomes had been defined by International Classification of Diseases (ICD)-9 and ICD-10 codes (Field IDs: 41270, 41271, 41280 and 41281). Also, mortality records were obtained from the NHS Digital for participants in England and Wales, and from the NHS Central Register (part of the National Records of Scotland) for participants in Scotland where cause of death had been defined by ICD-10 codes (Field IDs: 40001 and 40000).
In HUNT2, participants were followed via linkage to the medical records from the three hospitals (St. Olavs Hospital, Levanger Hospital and Namsos Hospital) of the Nord-Trøndelag region where health-related outcomes had been defined by ICD-9 and ICD-10 codes. Mortality records were identified by a linkage to the National Cause of Death Registry where cause of death had been defined by ICD-10 codes.
Any hospitalization or death due to AMI were identified using ICD-9 code 410 and ICD-10 codes I21 and I22. Each participant was followed until either first diagnosis/death due to AMI, death due to other cause, loss to follow-up, or end of follow-up (March 23, 2021 for UKBB and December 31, 2020 for HUNT2). Incident cases were defined as the first occurrence of either hospitalization or death due to AMI during follow-up. Participants with any previous AMI episode(s) before their date of participation in the study regarded as prevalent cases, were excluded in the study.
Covariates
Several factors to be potential confounders of the exposure-outcome relation were considered. The covariates selected a priori were age, gender, marital status (married, unmarried, or separated/divorced/widowed), frequency of alcohol intake (never, monthly, weekly, or daily), smoking history (never, ex-smoker, or current smoker), body mass index (BMI), level of physical activity (inactive/low, moderate, or high), Townsend deprivation index (TDI; for UKBB only), education attainment (≤ 10 years, 11–13 years, or ≥ 14 years), shift work (yes or no), employment status (employed or not employed), systolic blood pressure (SBP), blood cholesterol levels, blood glucose levels, depression (yes or no in UKBB; and Hospital Anxiety and Depression Scale (HADS) – Depression scores in HUNT2), anxiety (yes or no in UKBB; and HADS – Anxiety scores in HUNT2), use of sleep medication (yes or no), and chronic illness (yes or no). The details on how covariates were handled are described in the supplementary material (see Additional file 1) [36, 38,39,40,41,42,43,44,45,46,47].
Genetic variants
In UKBB, participants were genotyped using either one of the UK BiLEVE or the UK Biobank Axiom genotyping chips. The genetic variants used were extracted genotypes from the UK Biobank imputation dataset (imputed to the UK10K plus 1000 Genomes phase 3 and Haplotype Reference Consortium reference panels), that were quality controlled using a standard protocol [48, 49]. In HUNT, participants were genotyped with one of three different Illumina HumanCoreExome genotyping chips (HumanCoreExome 12 v.1.0, HumanCoreExome 12 v.1.1, and UM HUNT Biobank v.1.0), where genotypes from different chips were quality controlled separately and reduced to a common set of variants. The quality control measures used were similar to UKBB [50]. All genotyped samples included were of European decent.
A total of 248 single nucleotide polymorphisms (SNPs) were identified as robustly associated with insomnia symptoms [30], 78 SNPs associated with 24-hour sleep duration [31], and 351 SNPs associated with morning preference chronotype [32], at a genome-wide significance level (P < 5×10−8) from three large genome-wide association studies (GWASs). In addition, 27 and 8 SNPs were identified to associate with short and long sleep duration, respectively [31]. The detailed information about discovery GWASs from where genetic instruments were identified were listed in Table 1.
Statistical analysis
Genetic risk score (GRS) for each sleep trait were created as an instrument that could overcome the weak effect of most SNPs on their corresponding sleep trait [51]. Weighted GRS (wGRS) were calculated as the sum of the participants’ sleep trait increasing alleles (morning preference alleles for chronotype; thus evening chronotype as reference), weighted by the variant effect sizes from the external GWAS. wGRS were incorporated for our main analysis in HUNT2 only, whereas in UKBB, we used unweighted GRS (uwGRS) calculated as sum of the sleep trait increasing alleles. Since all included discovery GWASs used the UKBB cohort, the use of internal weights to calculate wGRS is not recommended [51].
Instrument strength was assessed by regressing each sleep trait on their respective GRS and reporting R2 and F-statistics. The causal effects of individual sleep traits (insomnia symptoms, 24-hour sleep duration, short sleep, long sleep and chronotype) on the risk of incident AMI were tested using a one-sample MR analysis. A factorial MR analysis was used to investigate the joint causal effects of any two sleep traits (i.e., insomnia symptoms and short sleep, or insomnia symptoms and long sleep, or insomnia symptoms and chronotype, or short sleep and chronotype, or long sleep and chronotype) on the risk of incident AMI. All analyses were conducted using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
One-sample MR analysis
One-sample MR analysis was performed for each sleep trait using individual-level data separately in UKBB and HUNT2. A two-stage predictor substitution (TSPS) regression estimator method was used to calculate average causal hazard ratios (HRs). The first stage involved regression of each sleep trait (linear regression for 24-hour sleep duration, and logistic regression for other sleep traits) on their GRS, and the second stage consisted of a Cox regression of AMI status on the fitted values from the first stage regression, with adjustment for age at recruitment, gender, assessment center (in UKBB), genetic principal components (40 in UKBB and 20 in HUNT2), and genotyping chip in both stages. As recommended for MR analysis with a binary outcome [52], the first stage regression was restricted to participants who did not experience AMI. To obtain corrected standard errors, a bootstrapping method was applied with 2000 iterations in UKBB and 5000 iterations in HUNT2 [52]. The causal estimates for insomnia symptoms, short sleep, long sleep, and chronotype were scaled to represent the risk increase in AMI per doubling in the odds of these exposures, by multiplying the obtained β values by 0.693 as previously described [53]. The causal estimate for 24-hour sleep duration represents the risk increase in AMI per additional hour of sleep.
Factorial MR analysis
A 2×2 factorial MR was applied where each of the sleep traits (except 24-hour sleep duration) was dichotomized at their median GRS (uwGRS for UKBB and wGRS for HUNT2), with values equal to or below the median represented low genetic risk for the sleep trait, and values above the median represented high genetic risk for the sleep trait. Thus, for any combination of two sleep traits, participants were categorized into 4 groups according to their genetic predisposition. For instance, when combining insomnia symptoms and short sleep, participants were categorized into: “Both GRS ≤ median” (reference; representing low genetic risk for both insomnia symptoms and short sleep), “Insomnia GRS > median” (representing high genetic risk for insomnia symptoms only), “Short sleep GRS > median” (representing high genetic risk for short sleep only), and “Both GRS > median” (representing high genetic risk for both insomnia symptoms and short sleep). Cox regression was then used to investigate the association between these groups and incident AMI, with adjustment for age at recruitment, gender, assessment center (in UKBB), genetic principal components (40 in UKBB and 20 in HUNT2), and genotyping chip. Furthermore, interaction between any two sleep traits on risk of AMI was assessed by calculating relative excess risk due to interaction (RERI) using the risk estimates obtained for each sleep trait combination when none of the HRs were less than 1 (i.e., preventive) [54, 55]. RERI equals 0 implies exact additivity (no interaction), RERI > 0 implies more than additivity (positive interaction or synergism), and RERI < 0 implies less than additivity (negative interaction or antagonism).
Sensitivity analyses
To check the proportionality of hazards, the Pearson’s correlations were used to test Schoenfeld residuals from one-sample MR and 2×2 factorial MR Cox regression models for an association with follow-up time.
To check the robustness of the findings, the one-sample MR and 2×2 factorial MR analyses were repeated using uwGRS in HUNT2.
To assess the second MR assumption that the genetic instruments used are independent of confounders, associations of the GRS and potential confounders were investigated in UKBB and HUNT2. Furthermore, one-sample MR analysis adjusted for any potential confounders found strongly associated with the sleep trait GRS in two cohorts (beyond a Bonferroni significance threshold of P < 5.88×10−4 in UKBB and P < 7.81×10−4 in HUNT2) were performed.
To investigate potential directional pleiotropy, the estimates of the SNP-exposure and SNP-outcome associations from the same participants were obtained, and two-sample MR methods, such as MR-Egger, weighted median, and weighted mode-based methods, were applied. Each of these methods makes different assumptions about the genetic instruments used, where the MR-Egger regression method gives a valid causal estimate under the InSIDE (instrument strength independent of direct effect) assumption and its intercept allows the size of any unbalanced pleiotropic effect to be determined [56], weighted median method assumes at least 50% of genetic variants are valid [57], and weighted mode-based estimation method assumes a plurality of genetic variants are valid [58]. These methods can be applied in a one-sample setting [59], and consistent estimates across these methods strengthens causal evidence. To further investigate pleiotropy due to insomnia symptoms’ instruments, 57 SNPs found robustly associated with insomnia symptoms by Lane et al. [24] in another GWAS on UKBB (n = 345 022 cases and 108 357 controls) representing crucial variants with effect sizes for any insomnia symptoms (“sometimes”/ “usually” as cases versus “never/rarely” as controls), were used in a post hoc one-sample MR Cox regression analysis using different methods.
To evaluate potential impact of winner’s curse, one-sample MR analysis was repeated using genetic variants that replicated at a genome-wide significance level (P < 5×10−8) in a large independent dataset for insomnia symptoms (23andMe, n = 944 477; see Additional file 2: Table G1) [30] and chronotype (23andMe, n = 240 098; see Additional file 2: Table G5) [32].
As an additional analysis, continuous factorial MR analysis using two GRS (for any combination of two sleep traits) as quantitative traits and their product term was applied, to avoid potential bias due to arbitrary dichotomization and to maximize power [60]. Furthermore, RERI was calculated as test of interaction using the risk estimates for the quantitative GRS and their product term for each sleep trait combination when none of the HRs were less than 1 (i.e., preventive) for AMI [54, 61].
As use of sleep medication has been associated with CVDs [62], one-sample MR (without applying bootstrap method) and 2×2 factorial MR analyses were repeated excluding participants who reported use of sleep medication(s).
Results
Among the 336 262 participants in UKBB who passed the genetic quality control and had information available on the sleep traits, 11 399 (3.4%) had ever received the diagnosis of AMI. Of these, 3 586 (1.1%) prevalent cases with AMI diagnosis were excluded, and 7 813 (2.3%) had their first AMI diagnosis during a mean (standard deviation (SD)) follow-up of 11.7 (1.9) years (see Additional file 1: Figure S1). Among the 45 602 participants in HUNT2 who passed the genetic quality control and had information available for sleep traits of interest, 5 362 (11.7%) had ever received diagnosis of AMI. Of these, 874 (1.9%) prevalent cases with AMI diagnosis were excluded, and 4 488 (10.0%) had their first AMI diagnosis during a mean (SD) follow-up of 20.4 (6.9) years (see Additional file 1: Figure S1).
Table 2 represents the baseline characteristics of the study participants stratified by their AMI status in UKBB and HUNT2. Participants with an incidence of AMI during follow-up in the UKBB and HUNT2 were older and more likely to be males and current smokers. They were more likely to have used sleep medication(s), have a higher BMI, have higher systolic blood pressure, higher blood glucose levels, and were suffering more from depression and chronic illness. They were also less likely to consume alcohol, be physically active, have a tertiary education, and be employed compared to participants with no episodes of AMI. The HUNT2 participants with an AMI incidence during follow-up were more likely to have higher serum cholesterol levels, but less likely to be suffering from anxiety and working shifts in contrast to UKBB participants when compared to participants with no episode of AMI.
Among UKBB participants, the variance explained by the uwGRS in insomnia symptoms, 24-hour sleep duration (h), short sleep (≤ 6 h vs. 7–8 h), long sleep (≥ 9 h vs. 7–8 h), and morning chronotype were 0.41%, 0.59%, 0.18%, 0.11%, and 1.54%, respectively, and corresponding F-statistics were 1370.92, 1962.0, 558.68, 285.42, and 5202.20 (see Additional file 1: Table S2). The variance explained by the wGRS among HUNT2 participants in insomnia symptoms, 24-hour sleep duration, short sleep, and long sleep were 0.16%, 0.09%, 0.01%, and 0.01%, respectively, and the corresponding F-statistics were 71.17, 38.94, 4.97, and 4.07 (see Additional file 1: Table S2).
Causal effects of individual sleep traits on the risk of AMI
There was evidence for an adverse causal effect on AMI risk per doubling in odds of insomnia symptoms in UKBB (HR 1.18; 95% CI 1.07, 1.31) and HUNT2 (HR 1.23; 95% CI 1.00, 1.55) (Fig. 1). The estimates for 24-hour sleep duration suggested no causal effect on AMI per hour increase in sleep duration in UKBB (HR 0.97; 95% CI 0.75, 1.29) and HUNT2 (HR 0.76; 95% CI 0.31, 1.79). The sleep duration findings were further investigated using genetic variants specifically associated with short and long sleep duration. There was weak evidence for an adverse causal effect on AMI per doubling in odds of short sleep in UKBB (HR 1.14; 95% CI 0.97, 1.32) but not in HUNT2 (HR 0.87; 95% CI 0.15, 3.24). However, there was evidence for a protective causal effect on AMI per doubling in odds of long sleep in UKBB (HR 0.83; 95% CI 0.67, 0.99), which was underpowered in HUNT2 (HR 0.53; 95% CI 0.01, 8.28). Also, there was some evidence for an adverse causal effect on AMI per doubling in odds of morning chronotype in UKBB (HR 1.06; 95% CI 0.99, 1.11) (Fig. 1).
Joint causal effects of sleep traits on the risk of AMI
The distribution of the baseline characteristics across the factorial groups for any combinations of two sleep traits were equal (see Additional file 1: Tables S3 – S9), which indicates random allocation of the study participants into approximately equal-sized groups based on their genetic risk for any combinations of two sleep traits.
In UKBB, participants with high genetic risk for insomnia symptoms and high genetic risk for short sleep had slightly higher risk of AMI (HR 1.03; 95% CI 0.96, 1.10 and HR 1.05; 95% CI 0.98, 1.12, respectively), whereas participants with high genetic risk for both traits had the highest risk (HR 1.10; 95% CI 1.03, 1.12) (Fig. 2), but there was no evidence of interaction (RERI 0.03; 95% CI −0.07, 0.12). This pattern was however not consistent in HUNT2, with imprecise estimates and a lack of evidence of interaction (RERI −0.05; 95% CI −0.20, 0.09) (Fig. 2). The joint effects of insomnia symptoms and long sleep on risk of AMI were inconclusive in both UKBB and HUNT2 (Fig. 2).
In addition, UKBB participants with high genetic risk for insomnia symptoms and high genetic risk for a morning chronotype had slightly higher risk of AMI (HR 1.03; 95% CI 0.97, 1.10 and HR 1.03; 95% CI 0.97, 1.10, respectively) whereas participants with high genetic risk for both sleep traits had the highest risk (HR 1.09; 95% CI 1.03, 1.17) (Fig. 2). There was no evidence of interaction (RERI 0.03; 95% CI −0.06, 0.12). Similarly, the UKBB participants with high genetic risk for short sleep and high genetic risk for a morning chronotype had slightly higher risk of AMI (HR 1.04; 95% CI 0.98, 1.12 and HR 1.02; 95% CI 0.96, 1.10, respectively) whereas participants with high genetic risk for both had the highest risk (HR 1.11; 95% CI 1.04, 1.19) (Fig. 2), with no strong statistical evidence of interaction (RERI 0.05; 95% CI −0.05, 0.14). The joint effects of long sleep and morning chronotype were imprecise and not conclusive (Fig. 2).
Sensitivity analysis
The proportionality of hazards assumption was met for the one-sample and the 2×2 factorial MR Cox regression analyses (see Additional file 1: Tables S10 and S11).
The one-sample MR and 2×2 factorial MR estimates in HUNT2 using the uwGRS for the sleep traits remained unchanged (see Additional file 1: Table S12 and Figure S2).
After adjusting for multiple testing, several confounding factors were associated with the sleep trait uwGRS in UKBB, whereas only a few were associated with the sleep trait wGRS in HUNT2 (see Additional file 1: Tables S13 and S14). When the one-sample MR analysis adjusting for these potential confounding factors was carried out, evidence of adverse causal effects of insomnia symptoms was slightly weaker and less precise in UKBB (HR 1.04; 95% CI 0.92, 1.17) and HUNT2 (HR 1.13; 95% CI 0.87, 1.47) (see Additional file 1: Table S15).
The causal estimates obtained using MR-Egger, weighted median- and weighted mode-based methods attenuated slightly and were less precise (see Additional file 1: Figures S3-S7, Tables S16 and S17). The MR-Egger regression for insomnia symptoms in UKBB showed evidence of directional pleiotropy (HR 0.77; 95% CI 0.62, 0.95; and intercept 0.007; 95% CI 0.003, 0.012). Furthermore, the post hoc one-sample MR analysis using insomnia symptoms variants from Lane et al. [24] gave similar estimates (see Additional file 1: Figure S8 and Table S18), where the MR-Egger regression showed evidence of directional pleiotropy in UKBB (HR 0.69; 95% CI 0.50, 0.96; and intercept 0.013; 95% CI 0.005, 0.022) and in HUNT2 (HR 0.97; 95% CI 0.78, 1.19; and intercept 0.006; 95% CI 0.001, 0.012).
The causal estimates were consistent when using GRS comprising 116 insomnia SNPs (one missing in HUNT imputed dataset) and 72 chronotype SNPs which replicated at genome-wide significance level (P < 5×10−8) in the independent 23andMe dataset (see Additional file 1: Tables S19 and S20).
The estimates from the continuous factorial MR analysis using sleep trait GRS as quantitative traits (per SD increase) and their product term inferred similar effects (see Additional file 1: Figure S9). In UKBB, the GRS for insomnia symptoms and short sleep were independently linked to an increased risk of AMI (HR 1.03; 95% CI 1.01, 1.06 and HR 1.02; 95% CI 0.99, 1.04, respectively), with no evidence of interaction (RERI 0.02; 95% CI −0.01, 0.04). Similarly, the GRS for insomnia symptoms and morning chronotype were independently associated with an increased risk of AMI (HR 1.04; 95% CI 1.02, 1.06 and HR 1.03; 95% CI 1.00, 1.05, respectively), though there was no evidence of interaction (RERI 0.02; 95% CI −0.01, 0.04). Also, the GRS for short sleep and morning chronotype were both independently linked to an increased risk of AMI (HR 1.02; 95% CI 1.00, 1.04 and HR 1.03; 95% CI 1.00, 1.05, respectively) but suggested no evidence of interaction (RERI 0.01; 95% CI −0.02, 0.03).
On excluding the participants who self-reported the use of sleep medication(s), our one-sample and 2×2 factorial MR estimates remain unchanged (see Additional file 1: Figures S10 and S11).
Discussion
Using individual-level data from the UKBB and HUNT2 cohorts, we performed one-sample and factorial MR analyses to investigate the causal effects of individual sleep traits (insomnia symptoms, sleep duration and morning chronotype) and their joint effects on the risk of AMI. We found evidence of an adverse causal effect of insomnia symptoms and a weak causal effect of short sleep on the risk of incident AMI, while long sleep had a protective effect in UKBB. We found no statistical evidence of interaction effects between sleep traits on the risk of AMI, but those with a high genetic risk for two sleep traits in combination (including insomnia symptoms, short sleep, and a morning chronotype) had the highest risk of AMI in UKBB. Moreover, our results showed a protective effect of genetically predisposed long sleep that was not affected by additionally being genetically predisposed to insomnia symptoms or a morning chronotype on incident AMI in UKBB. However, these results were not replicated in HUNT2, where the estimates were imprecise. These findings indicate that the main effects of sleep traits on the risk of AMI are likely to be independent of each other.
Comparison with other studies
Direct comparison of MR results with observational findings is limited given that inherited genetic variation influences sleep behaviors over the life course, whereas observational estimates represent sleep behaviors measured at one time-point. Additionally, caution should be made when comparing our findings with other studies, due to variation in the definitions used for sleep traits.
Causal effects of individual sleep traits on the risk of AMI
Nonetheless, our finding showing evidence of an adverse causal effect of insomnia symptoms and a weak adverse causal effect of short sleep on the risk of AMI is consistent with prior observational [9, 11, 14] and MR research [11, 28, 29]. Our causal estimate of short sleep on the risk of AMI in UKBB was weaker compared to Daghlas et al. [11] (odds ratio (OR) 1.21; 95% CI 1.08, 1.37) and Ai et al. [29] (OR 1.21; 95% CI 1.09, 1.34), which might be due to different methodological approaches. Our analyses relied on survival data and reported HR considering incident cases of AMI on follow-up after recruitment in the cohorts, rather than OR. Our finding suggests a protective causal effect of long sleep on the risk of AMI contradicts with prior observational studies [11, 14] but aligns with a weak concordant effect shown by another MR study [29]. Long sleep may be an indicator of poor health status, being closely associated with depression, poor sleep quality, sedentary lifestyles, and underlying comorbid conditions [63, 64], and so residual confounding or reverse causation may have biased previous observational findings. Moreover, our finding suggesting a weak causal effect of morning chronotype on the risk of AMI is inconsistent with our prior study that identified evening chronotype as detrimental [14]. It is likely that the previously reported protective association of morning chronotype is confounded.
Joint causal effects of sleep traits on the risk of AMI
Our finding that UKBB participants with high genetic risk for both insomnia symptoms and short sleep had the highest risk of AMI is consistent with evidence from our previous observational study where we found that insomnia symptoms and short sleep together increased the risk of AMI in UKBB more than the risk attributed to either insomnia symptoms or short sleep alone [14], and is supported by finding from another prospective study [16]. Moreover, our finding suggesting no interaction between insomnia symptoms and short sleep on risk of AMI is also in line with prior research [14, 16]. However, our finding of no positive interaction between insomnia symptoms and long sleep on the risk of AMI in UKBB contrasts with our previous observational study [14], where insomnia symptoms and long sleep together were found to increase the risk of AMI beyond their mere additive effects. This observed interaction could be due to confounding apparent in conventional observational studies, where poor health could be a confounder that would lead to false indications of harmful consequences of prolonged sleep. As previously mentioned, our finding in UKBB suggests a protective effect of genetic predisposition to long sleep on incident AMI, which was not affected by additionally being genetically predisposed to insomnia symptoms.
Our findings that UKBB participants with high genetic risk for both insomnia symptoms and a morning chronotype; and those with high genetic risk for both short sleep and a morning chronotype had the highest risk of AMI are in contrast with our observational study where we found evening chronotype to be more deleterious than morning chronotype in combination with insomnia symptoms or short sleep [14]. Although there was no interaction, these findings may suggest that the weak adverse effect of morning chronotype on AMI might partly be explained by concomitant genetic predisposition to insomnia symptoms or short sleep. Our finding that UKBB participants with high genetic risk for both long sleep and a morning chronotype likely decreased the risk of AMI is incongruous to our previous observational study, where long sleep together with morning chronotype was associated with an increased risk [14]. Again, there was no interaction and — if anything — our finding suggests a protective effect of genetic predisposition to long sleep on incident AMI, which was not affected by additionally being genetically predisposed to morning chronotype.
Potential mechanisms
The underlying mechanisms by which insomnia symptoms or short sleep increase in the risk of AMI are multifactorial [65]. Insomnia and short sleep independently increase the risk of autonomic dysfunction, by increasing sympathetic tone (stress response) consequently accompanied by increased metabolic rate, increased heart rate, and decreased heart rate variability [66,67,68,69]. Furthermore, experimentally induced sleep restriction has been shown to cause hormonal imbalance which stimulate proinflammatory pathways [70], increase appetite [71, 72], and increase insulin resistance [73]. These autonomic and hormonal disturbances lead to hypertension [74, 75], diabetes [73], dyslipidemia, and obesity [71, 72], thus constituting a set of interrelated metabolic disorders that are pathophysiological in the development of cardiac dysfunction by accelerating endothelial dysfunction and atherosclerosis [76].
Our findings and these potential mechanisms might raise a concern that insomnia symptoms and short sleep could be regarded as similar traits. However, insomnia symptoms and sleep duration were found only moderately phenotypically (r = −0.25; P < 0.001) and genetically (rg = −0.50; P < 6×10−17) correlated to each other [77]. It is also important to highlight that our findings on the joint causal effects of insomnia symptoms and short sleep on the risk of AMI do not employ that concomitant presence of insomnia symptoms and short sleep causes higher increase in risk of AMI through overstimulation of the suggested underlying mechanisms, or involve any supplementary mechanisms yet to be determined.
The underlying mechanism by which chronotype may influence AMI is not yet established. Studies have found evening chronotypes have more susceptibility for cardiometabolic risk behaviors and risk factors [12, 78, 79]. On the contrary, our causal findings suggesting that having a morning chronotype may be detrimental for incident AMI compared to having an evening chronotype might be explained by the concomitant genetic predisposition to insomnia symptoms or short sleep.
Strengths and limitations
This MR study leverages genetic information to assess the causal relationships between sleep traits and AMI, reducing the potential bias due to residual confounding, reverse causation, and measurement error in conventional observational studies [22]. The novelty of this study is our application of factorial MR to explore the causal interplay between sleep traits on the risk of AMI, where participants were grouped based on their genetic predisposition for multiple sleep traits [60]. We are not aware of another study that has investigated the joint effects of sleep traits in the MR context. Another major novelty is that the study benefitted from the use of results from three large GWASs for insomnia symptoms [30], sleep duration [31], and chronotype [32] and used two large cohorts (UKBB and HUNT2) to replicate the findings. Moreover, this study draws on the principle of triangulation [80], where findings were compared from different methodological approaches, which further strengthened evidence supporting causation.
Nonetheless, there are a number of limitations of this study. Factorial MR analysis is usually underpowered to detect interaction which may raise the concerns of false negative results [60]. However, this study included the UKBB cohort with 332 676 participants constituting the largest factorial MR study on sleep traits to date. The strong instrument strength observed in UKBB cohort partially overcomes concerns due to underpowered factorial MR findings [81]. Another limitation is that although factorial MR can identify whether two independent exposures interact and have a joint effect of public health importance [81], it assumes exposures remain stable throughout the life course. Thus, the magnitude of effects should be cautiously interpreted.
Also, the validity of MR findings can be weakened by pleiotropy [82]. We used several sensitivity analyses to investigate possible sources of bias in MR. We found that the genetic risk for insomnia symptoms was strongly associated with BMI, smoking status, depression, and education among other covariates [30], which may be indicative of confounding, mediation, or horizontal pleiotropy. Further to this, our results remained consistent across various MR methods, except for insomnia symptoms which showed evidence of an unbalanced pleiotropy in MR-Egger analysis. Additionally, previous studies have shown only mild attenuation of causal effects of insomnia symptoms on CAD risk when adjusted for BMI, smoking, depression, and education using multivariable Mendelian randomization (MVMR) [25, 26]. Moreover, simulations have shown that MR-Egger may be unreliable when applied to a single dataset [59], and this is a limitation of our study.
The sleep traits were based on self-report. It remains unclear if self-reported sleep duration represents time in bed or actual sleep time. Also, the insomnia questions in UKBB or HUNT2 did not cover all aspects of insomnia (difficulty falling asleep, night awakenings, waking up early and daytime impairments) [83]. Chronotype in this study was assessed from a single question in UKBB, whereas validated instruments such as the Morningness-Eveningness Questionnaire and the Munich Chronotype Questionnaire use diverse questions to better estimate chronotype [84, 85]. Other sleep traits (e.g., sleep apnea, snoring, daytime napping) were not included, and we do not know whether these interact with insomnia symptoms or sleep duration. Moreover, the sleep traits we used are binary exposures (except for 24-hour sleep duration), which are likely coarsened approximations of the true latent exposure [86]. This opens up alternate pathways from the genetic instruments to the outcome, which may violate the exclusion restriction assumption, resulting in biased effect estimates [86]. In addition, causal estimates from MR of binary exposures on a binary outcome are difficult to interpret [87].
Due to the small sample size in HUNT2, we might have missed weak causal effects due to insufficient power. In addition, the genetic instrument explained little variance in short sleep and long sleep within HUNT2, implying possible weak instrument bias [88] and leading to wide CIs as shown in the bootstrap simulations [89]. Furthermore, SNPs for short and long sleep were not replicated in other independent cohorts [31], meaning that the GRS used is not validated in any other population.
The inclusion of UKBB in all exposure GWASs could lead to winner’s curse that might bias the causal estimates in UKBB [90]. We therefore used unweighted GRS for our exposures in UKBB as recommended [51]. Also, we derived GRS for insomnia symptoms and chronotype composed of SNPs that replicated in an independent study (23andMe) [30, 32], which showed similar estimates, indicating winner’s curse is unlikely to have substantially biased effect estimates. However, we could not apply the same approach to explore the impact of winner’s curse on the sleep duration due to the limited sample size of the replication datasets in those studies [31], meaning that genetic associations might be imprecise.
The variation in the occurrence of AMI between UKBB (2.35%) and HUNT2 (10.03%) may be attributed to several factors related to the composition of the cohorts: (a) the HUNT2 cohort followed up relative older participants, aged 20 years or above, with a mean baseline age of 48 years, while UKBB consisted of participants aged 40 to 69 years, with a mean baseline age of 56 years; (b) the duration of follow-up was longer in HUNT2, spanning 20.4 years, compared to UKBB’s follow-up period of 11.7 years; (c) UKBB (5.5% response rate) may represent a healthier sample [91], whereas HUNT2 (69.5% response rate) may be a more representative sample [36]; and (d) baseline differences in the two underlying populations or differences due to time trend (for example, more current smokers in HUNT2 which was conducted about a decade earlier than UKBB). Moreover, competing risk from death among participants would potentially hinder the occurrence of AMI, that might overestimate the risks [92]. This is another limitation of our study.
Finally, our findings rely on analyses in UKBB due to its large sample. However, the generalizability of these findings may be limited due to a selected sample (5.5% response rate) in the UKBB cohort, which can bias both observational and MR estimates [93, 94]. Selection bias may artificially induce associations between genetic variants and confounders leading to the instrumental variable becoming invalid [95]. This might partly explain differences in UKBB and HUNT2 estimates observed in this study, where HUNT2 sample (69.5% response rate) more closely represents target population. The difference in demographics of the two cohorts might also cause inconsistent estimates. Moreover, the inclusion of cohorts from the European ancestry may further restrict generalizability of our findings.
Conclusions
This study reveals no interaction effects between sleep traits on the risk of AMI, but found that two sleep traits in combination (including insomnia symptoms, short sleep, and a morning chronotype) had the highest risk of AMI. The role of chronotype in AMI risk remains uncertain, as the adverse causal effect of morning chronotype could partly be explained by genetic predisposition to insomnia symptoms or short sleep. This indicates that the main effects of insomnia symptoms and short sleep are likely to be independent of each other, i.e., the magnitude of the effect of insomnia symptoms on AMI does not depend on whether there is accompanying genetic predisposition to short sleep, and vice-versa. Thus, interventions targeting both insomnia symptoms and short sleep could be relevant for preventive initiatives to reduce the risk of AMI. Moreover, this study also suggests a potential protective effect of genetically predisposed long sleep that was not affected by additionally being genetically predisposed to insomnia symptoms and a morning chronotype.
Availability of data and materials
The data supporting the findings are available in the supplementary material and upon request. The UK Biobank data is available to researchers, subject to successful registration and application process via their website (https://www.ukbiobank.ac.uk/). The data from the HUNT Study are available from the HUNT Research Centre, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. However, the data are available for export given approval of application to the HUNT Research Centre (http://www.ntnu.edu/hunt/data). The data on hospital records linkages to the HUNT Study participants are available from Nord-Trøndelag Hospital Trust and require permission. All other data used are publicly available and referenced according in the main text.
Abbreviations
- AMI:
-
Acute myocardial infarction
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- GRS:
-
Genetic risk score
- GWAS:
-
Genome-wide association study
- HADS:
-
Hospital Anxiety and Depression Scale
- HES:
-
Hospital Episode Statistics
- HR:
-
Hazard ratio
- HUNT:
-
The Trøndelag Health Study
- ICD:
-
International Classification of Diseases
- MR:
-
Mendelian randomization
- MVMR:
-
Multivariable Mendelian randomization
- NHS:
-
National Health Service
- OR:
-
Odds ratio
- PEDW:
-
Patient Episode Database for Wales
- RERI:
-
Relative excess risk due to interaction
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- SMR:
-
Scottish Morbidity Record
- SNP:
-
Single nucleotide polymorphism
- TDI:
-
Townsend deprivation index
- TSPS:
-
Two-stage predictor substitution
- UKBB:
-
UK Biobank
References
Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7-10.
St-Onge M-P, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–86.
Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7.
Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26.
Wong PM, Hasler BP, Kamarck TW, Muldoon MF, Manuck SB. Social Jetlag, Chronotype, and cardiometabolic risk. J Clin Endocrinol Metab. 2015;100:4612–20.
Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 27 Mar 2023.
Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125–33.
Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37:9–17.
Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction. Circulation. 2011;124:2073–81.
Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: a population study. Eur Heart J. 2014;35:1382–93.
Daghlas I, Dashti HS, Lane J, Aragam KG, Rutter MK, Saxena R, et al. Sleep duration and myocardial infarction. J Am Coll Cardiol. 2019;74:1304–14.
Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int. 2013;30:470–7.
Fan Y, Wu Y, Peng Y, Zhao B, Yang J, Bai L, et al. Sleeping late increases the risk of myocardial infarction in the middle-aged and older populations. Front Cardiovasc Med. 2021;8:709468.
Arora N, Richmond RC, Brumpton BM, Åsvold BO, Dalen H, Skarpsno ES, et al. Self-reported insomnia symptoms, sleep duration, chronotype and the risk of acute myocardial infarction (AMI): a prospective study in the UK Biobank and the HUNT Study. Eur J Epidemiol. 2023;38(6):643–56.
Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J. 2020;41:1182–9.
Bertisch SM, Pollock BD, Mittleman MA, Buysse DJ, Bazzano LA, Gottlieb DJ, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Stud. Sleep. 2018;41(6):zsy047.
Sands-Lincoln M, Loucks EB, Lu B, Carskadon MA, Sharkey K, Stefanick ML, et al. Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the women’s health initiative. J Womens Health (Larchmt). 2013;22:477–86.
Westerlund A, Bellocco R, Sundström J, Adami H-O, Åkerstedt T, Trolle LY. Sleep characteristics and cardiovascular events in a large Swedish cohort. Eur J Epidemiol. 2013;28:463–73.
Chien K-L, Chen P-C, Hsu H-C, Su T-C, Sung F-C, Chen M-F, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–84.
Chandola T, Ferrie JE, Perski A, Akbaraly T, Marmot MG. The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep. 2010;33:739–44.
Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–54.
Davies NM, Holmes MV, Smith GD. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362: k601.
Burgess S, Thompson SG. Mendelian randomization: methods for causal inference using genetic variants. 2nd ed. New York: Chapman and Hall/CRC; 2021.
Lane JM, Jones SE, Dashti HS, Wood AR, Aragam KG, van Hees VT, et al. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet. 2019;51:387–93. https://www.nature.com/articles/s41588-019-0361-7.
Larsson SC, Markus HS. Genetic liability to insomnia and cardiovascular disease risk. Circulation. 2019;140:796–8.
Yuan S, Mason AM, Burgess S, Larsson SC. Genetic liability to insomnia in relation to cardiovascular diseases: a Mendelian randomisation study. Eur J Epidemiol. 2021;36:393–400.
Liu X, Li C, Sun X, Yu Y, Si S, Hou L, et al. Genetically predicted insomnia in relation to 14 cardiovascular conditions and 17 cardiometabolic risk factors: a Mendelian randomization study. J Am Heart Assoc. 2021;10: e020187.
Yang Y, Fan J, Shi X, Wang Y, Yang C, Lian J, et al. Causal associations between sleep traits and four cardiac diseases: a Mendelian randomization study. ESC Heart Failure. 2022;9:3160–6.
Ai S, Zhang J, Zhao G, Wang N, Li G, So H-C, et al. Causal associations of short and long sleep durations with 12 cardiovascular diseases: linear and nonlinear Mendelian randomization analyses in UK Biobank. Eur Heart J. 2021;42:3349–57.
Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, Hammerschlag AR, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51:394–403. https://www.nature.com/articles/s41588-018-0333-3.
Dashti HS, Jones SE, Wood AR, Lane JM, van Hees VT, Wang H, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. 2019;10:1100. https://www.nature.com/articles/s41467-019-08917-4.
Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019;10:343. https://www.nature.com/articles/s41467-018-08259-7.
Nikpay M, Goel A, Won H-H, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–30.
UK Biobank: Protocol for a large-scale prospective epidemiological resource. 2006. https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us. Accessed 25 Oct 2021.
Åsvold BO, Langhammer A, Rehn TA, Kjelvik G, Grøntvedt TV, Sørgjerd EP, et al. Cohort Profile Update: The HUNT Study, Norway. Int J Epidemiol. 2023;52:e80-91.
Holmen J, Midthjell K, Krüger Ø, Langhammer A, Holmen TL, Bratberg GH, et al. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): objectives, contents, methods and participation. Norsk epidemiologi. 2003;13:19–32.
Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675–700.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
International Physical Activity Questionnaire (IPAQ): Guidelines for data processing analysis of the International Physical Activity Questionnaire (IPAQ) - Short and long forms. 2005. https://biobank.ndph.ox.ac.uk/showcase/refer.cgi?id=540. Accessed 25 Oct 2021.
International Physical Activity Questionnaire (IPAQ): IPAQ scoring protocol. 2005. https://sites.google.com/site/theipaq/scoring-protocol. Accessed 25 Oct 2021.
Brumpton BM, Langhammer A, Ferreira MAR, Chen Y, Mai X-M. Physical activity and incident asthma in adults: the HUNT Study Norway. BMJ Open. 2016;6: e013856.
Kurtze N, Rangul V, Hustvedt BE, Flanders WD. Reliability and validity of self-reported physical activity in the Nord-Trøndelag Health Study (HUNT 2). Eur J Epidemiol. 2007;22:379–87.
Townsend P. Poverty in the United Kingdom: a survey of household resources and standards of living. London: Allen Lane and Penguin Books; 1979.
Elliott P, Peakman TC, UK Biobank. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–44.
Skapinakis P. Hospital Anxiety and Depression Scale (HADS). In: Michalos AC, editor. Encyclopedia of quality of life and well-being research. Dordrecht: Springer, Netherlands; 2014. p. 2930–3.
Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77.
Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–4.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9.
Mitchell RE, Hemani G, Dudding T, Corbin L, Harrison S, Paternoster L. UK Biobank Genetic Data: MRC-IEU Quality Control, version 2. 2019. https://data.bris.ac.uk/data/dataset/1ovaau5sxunp2cv8rcy88688v.
Brumpton BM, Graham S, Surakka I, Skogholt AH, Løset M, Fritsche LG, et al. The HUNT study: a population-based cohort for genetic research. Cell Genomics. 2022;2: 100193.
Burgess S, Thompson SG. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol. 2013;42:1134–44.
Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2017;26:2333–55.
Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33:947–52.
Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452.
Knol MJ, VanderWeele TJ, Groenwold RHH, Klungel OH, Rovers MM, Grobbee DE. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol. 2011;26:433–8.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–98.
Minelli C, Del Greco MF, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. 2021;50:1651–9.
Rees JMB, Foley CN, Burgess S. Factorial Mendelian randomization: using genetic variants to assess interactions. Int J Epidemiol. 2020;49:1147–58.
Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36:1111–8.
Kim Y-H, Kim H-B, Kim D-H, Kim J-Y, Shin H-Y. Use of hypnotics and the risk of or mortality from heart disease: a meta-analysis of observational studies. Korean J Intern Med. 2017;33:727–36.
Grandner MA, Drummond SPA. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11:341–60.
Stamatakis KA, Punjabi NM. Long sleep duration: a risk to health or a marker of risk? Sleep Med Rev. 2007;11:337.
Hsieh CG, Martin JL. Short sleep, insomnia, and cardiovascular disease. Curr Sleep Med Rep. 2019;5:234–42.
D’Aurea C, Poyares D, Piovezan RD, Passos GS, Tufik S, de Mello MT. Objective short sleep duration is associated with the activity of the hypothalamic-pituitary-adrenal axis in insomnia. Arq Neuro-Psiquiatr. 2015;73:516–9.
Grimaldi D, Carter JR, Van Cauter E, Leproult R. Adverse impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults. Hypertension. 2016;68:243–50.
Castro-Diehl C, Diez Roux AV, Redline S, Seeman T, McKinley P, Sloan R, et al. Sleep duration and quality in relation to autonomic nervous system measures: the multi-ethnic study of atherosclerosis (MESA). Sleep. 2016;39:1927–40.
Jarrin DC, Ivers H, Lamy M, Chen IY, Harvey AG, Morin CM. Cardiovascular autonomic dysfunction in insomnia patients with objective short sleep duration. J Sleep Res. 2018;27: e12663.
Vgontzas AN, Zoumakis E, Bixler EO, Lin H-M, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26.
Kjeldsen JS, Hjorth MF, Andersen R, Michaelsen KF, Tetens I, Astrup A, et al. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int J Obes (Lond). 2014;38:32–9.
Broussard JL, Kilkus JM, Delebecque F, Abraham V, Day A, Whitmore HR, et al. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity (Silver Spring). 2016;24:132–8.
Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–57.
Lanfranchi PA, Pennestri M-H, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep. 2009;32:760–6.
Fernandez-Mendoza J, He F, Vgontzas AN, Liao D, Bixler EO. Objective short sleep duration modifies the relationship between hypertension and all-cause mortality. J Hypertens. 2017;35:830–6.
Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016;77:1–7.
Lane JM, Liang J, Vlasac I, Anderson SG, Bechtold DA, Bowden J, et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat Genet. 2017;49:274–81.
Makarem N, Paul J, Giardina E-GV, Liao M, Aggarwal B. Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiol Int. 2020;37:673–85.
Maukonen M, Kanerva N, Partonen T, Kronholm E, Konttinen H, Wennman H, et al. The associations between chronotype, a healthy diet and obesity. Chronobiol Int. 2016;33:972–81.
Lawlor DA, Tilling K, Davey SG. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45:1866–86.
North T-L, Davies NM, Harrison S, Carter AR, Hemani G, Sanderson E, et al. Using genetic instruments to estimate interactions in Mendelian randomization studies. Epidemiology. 2019;30:e33–5.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89-98.
Littner M, Hirshkowitz M, Kramer M, Kapen S, Anderson WM, Bailey D, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26:754–60.
Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110.
Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90.
Tudball MJ, Bowden J, Hughes RA, Ly A, Munafò MR, Tilling K, et al. Mendelian randomisation with coarsened exposures. Genet Epidemiol. 2021;45:338–50.
Palmer TM, Sterne JAC, Harbord RM, Lawlor DA, Sheehan NA, Meng S, et al. Instrumental Variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am J Epidemiol. 2011;173:1392–403.
Burgess S, Thompson SG, CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–64.
Moreira MJ, Porter JR, Suarez GA. Bootstrap validity for the score test when instruments may be weak. J Econometr. 2009;149:52–64.
Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40:597–608.
Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank Participants with those of the general population. Am J Epidemiol. 2017;186:1026–34.
Puddu PE, Amaduzzi PL, Ricci B. Coronary heart disease incidence and competing risks: an important issue. J Geriatr Cardiol. 2017;14:425–9.
Hernán MA, Hernández-Díaz S, Robins JM. A Structural approach to selection bias. Epidemiology. 2004;15:615.
Canan C, Lesko C, Lau B. Instrumental variable analyses and selection bias. Epidemiology. 2017;28:396.
Gkatzionis A, Burgess S. Contextualizing selection bias in Mendelian randomization: how bad is it likely to be? Int J Epidemiol. 2019;48:691–701.
Acknowledgements
This research has been conducted using data from UK Biobank, a major biomedical database (www.ukbiobank.ac.uk), under application number 40135.
The Trøndelag Health Study (HUNT) is a collaboration between HUNT Research Centre (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU)), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. We want to thank clinicians and other employees at Nord-Trøndelag Hospital Trust for their support and for contributing to data collection in this research project.
Funding
Open access funding provided by Norwegian University of Science and Technology This study was made possible with the financial support from the National Association for Public Health in Norway (Nasjonalforeningen for folkehelsen; project number 19479), mobility grant funds from the Liaison Committee for Education, Research and Innovation in Central Norway (Helse Midt-Norge; project number 2023/34249), and top-up financing from the Department of Public Health and Nursing, Norwegian University of Science and Technology, Trondheim, Norway. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
N.A. interpreted and analyzed the data, interpreted the findings, and wrote the paper; L.B.S. and R.C.R. had the original idea for this study, interpreted the data, and critically revised the paper; E.S.S., B.M.B., and B.O.Å. had the original idea for this study and critically revised the paper; L.B. assisted with analysis and critically revised the paper; and H.D. assisted with interpreting data on acute myocardial infarction from medical records assessed through hospitals in the Trøndelag County and critically revised the paper. All authors read and approved the final manuscript.
Authors’ Twitter handles
Nikhil Arora: @dr_nikhil_arora
Laxmi Bhatta: @laxmibhatta001
Eivind Schjelderup Skarpsno: @E_Skarpsno
Bjørn Olav Åsvold: @BAsvold
Ben Michael Brumpton: @bmbrumpton
Rebecca Claire Richmond: @BeckyRichmond90
Linn Beate Strand: @strandlb
Corresponding author
Ethics declarations
Ethics approval and consent to participate
UK Biobank received ethical approval from the National Health Service (NHS) Research Ethics Service (reference number 11/NW/0382). The HUNT Study was approved by the Data Inspectorate of Norway and recommended by the Regional Committee for Ethics in Medical Research (REK; reference number 152/95/AH/JGE). The ethical approval for conducting this study was also obtained from the Regional Committee for Ethics in Medical Research (REK nord; reference number 2020/47206).
Informed consent was obtained from all individual participants of both the cohorts included in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Information on covariates; Supplementary figures. Figure S1. Flow chart of the participant selection process. Figure S2. 2×2 factorial Mendelian randomization Cox regression analysis assessing the joint effects of two sleep traits with risk of incident acute myocardial infarction in HUNT2 using weighted and unweighted genetic risk scores for sleep traits. Figure S3. Association of insomnia SNPs from Jansen et al., 2019 and acute myocardial infarction (AMI) within a) UK Biobank b) HUNT2. IVW, MR-Egger, simple median and weighted median estimates are indicated by the red, green, blue and purple lines respectively. Figure S4. Association of 24-hour sleep duration SNPs from Dashti et al., 2019 and acute myocardial infarction (AMI) within a) UK Biobank b) HUNT2. IVW, MR-Egger, simple median and weighted median estimates are indicated by the red, green, blue and purple lines respectively. Figure S5. Association of short sleep duration SNPs from Dashti et al., 2019 and acute myocardial infarction (AMI) within a) UK Biobank b) HUNT2. IVW, MR-Egger, simple median and weighted median estimates are indicated by the red, green, blue and purple lines respectively. Figure S6. Association of long sleep duration SNPs from Dashti et al., 2019 and acute myocardial infarction (AMI) within a) UK Biobank b) HUNT2. IVW, MR-Egger, simple median and weighted median estimates are indicated by the red, green, blue and purple lines respectively. Figure S7. Association of chronotype (morning preference) SNPs from Jones et al., 2019 and acute myocardial infarction (AMI) within UK Biobank. IVW, MR-Egger, simple median and weighted median estimates are indicated by the red, green, blue and purple lines respectively. Figure S8. Association of insomnia SNPs from Lane et al., 2019 and acute myocardial infarction (AMI) within a) UK Biobank b) HUNT2. IVW, MR-Egger, simple median and weighted median estimates are indicated by the red, green, blue and purple lines respectively. Figure S9. Continuous factorial Mendelian randomization analysis using genetic risk score as quantitative traits with their product term assessing the joint effects of two sleep traits with risk of incident acute myocardial infarction in UK Biobank and HUNT2. Figure S10. One-sample Mendelian randomization Cox regression analysis for risk of incident acute myocardial infarction associated with sleep traits in UK Biobank and HUNT2 after excluding participants who reported self-reported use of sleep medication. Figure S11. 2×2 factorial Mendelian randomization Cox regression analysis assessing the joint effects of two sleep traits with risk of incident acute myocardial infarction in UK Biobank and HUNT2 after excluding participants who reported self-reported use of sleep medication; and Supplementary tables. Table S1. Detailed summary of Mendelian randomization (MR) studies previously conducted on sleep traits and risk of coronary artery disease (CAD) or acute myocardial infarction (AMI). Table S2. Summary of genetic instruments showing their strength applying to UK Biobank and HUNT2. Table S3. Baseline characteristics of participants across groups categorized by dichotomizing to the median genetic risk scores for insomnia symptoms and short sleep in UK Biobank. Table S4. Baseline characteristics of participants across groups categorized by dichotomizing to the median genetic risk scores for insomnia symptoms and short sleep in HUNT2. Table S5. Baseline characteristics of participants across groups categorized by dichotomizing to the median genetic risk scores for insomnia symptoms and long sleep in UK Biobank. Table S6. Baseline characteristics of participants across groups categorized by dichotomizing to the median genetic risk scores for insomnia symptoms and long sleep in HUNT2. Table S7. Baseline characteristics of participants across groups categorized by dichotomizing to the median genetic risk scores for insomnia symptoms and chronotype (morning preference) in UK Biobank. Table S8. Baseline characteristics of participants across groups categorized by dichotomizing to the median genetic risk scores for short sleep and chronotype (morning preference) in UK Biobank. Table S9. Baseline characteristics of participants across groups categorized by dichotomizing to the median genetic risk scores for long sleep and chronotype (morning preference) in UK Biobank. Table S10. Statistical test of the proportional hazard assumption for one-sample Mendelian randomization (MR) Cox regression models. Table S11. Statistical test of the proportional hazard assumption for 2×2 factorial Mendelian randomization (MR) Cox regression models. Table S12. One-sample Mendelian randomization Cox regression analysis for risk of incident acute myocardial infarction associated with sleep traits in HUNT2 using weighted and unweighted genetic risk scores for sleep traits. Table S13. Associations between genetic risk scores and potential confounders in UK Biobank. Table S14. Associations between genetic risk scores and potential confounders in HUNT2. Table S15. One-sample Mendelian randomization analysis for risk of incident acute myocardial infarction associated with sleep traits with and without adjustment for potential confounders in UK Biobank and HUNT2. Table S16. Sensitivity analysis for risk of incident acute myocardial infarction associated with sleep traits in UK Biobank. Table S17. Sensitivity analysis for risk of incident acute myocardial infarction associated with sleep traits in HUNT2. Table S18. One-sample Mendelian randomization Cox regression analysis for risk of incident acute myocardial infarction associated with insomnia symptoms using instruments from Lane et al., 2019 in UK Biobank and HUNT2. Table S19. Sensitivity analysis for risk of incident acute myocardial infarction associated with insomnia symptoms and chronotype in UK Biobank using genetic variants genome-wide significant in 23andMe. Table S20. Sensitivity analysis for risk of incident acute myocardial infarction associated with insomnia symptoms in HUNT2 using genetic variants genome-wide significant in 23andMe. Table S21. List of medications used to define the sleep medication covariate in UK Biobank.
Additional file 2.
Genetic variants. Table G1. Summary information of genetic variants identified for insomnia symptoms. Table G2. Summary information of genetic variants identified for sleep duration. Table G3. Summary information of genetic variants identified for short sleep. Table G4. Summary information of genetic variants identified for long sleep. Table G5. Summary information of genetic variants identified for chronotype.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Arora, N., Bhatta, L., Skarpsno, E.S. et al. Investigating the causal interplay between sleep traits and risk of acute myocardial infarction: a Mendelian randomization study. BMC Med 21, 385 (2023). https://doi.org/10.1186/s12916-023-03078-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-03078-0