Abstract
Background
Rezivertinib (BPI-7711) is a novel third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). This phase IIa study was part of a phase I/IIa study (NCT03386955), aimed to evaluate the efficacy and safety of rezivertinib as the first-line treatment for patients with locally advanced or metastatic/recurrent EGFR mutated non-small cell lung cancer (NSCLC).
Methods
Patients received the first-line treatment of 180 mg rezivertinib orally once daily until disease progression, unacceptable toxicity, or withdrawal of consent. The primary endpoint was the objective response rate (ORR) assessed by blinded independent central review (BICR). Secondary endpoints included disease control rate (DCR), duration of response (DoR), progression-free survival (PFS), overall survival (OS), and safety.
Results
From Jun 12, 2019, to Oct 17, 2019, 43 patients were enrolled. At the data cutoff date on Dec 23, 2021, the ORR by BICR was 83.7% (95% CI: 69.3–93.2%). The median DoR was 19.3 (95% CI: 15.8–25.0) months. The median PFS by BICR was 20.7 (95% CI: 13.8–24.8) months and 22.0 (95% CI: 16.8–26.3) months by investigators. Data on OS was immature. Totally, 40 (93.0%) patients had at least one treatment-related adverse event while 4 (9.3%) of them were grade ≥ 3.
Conclusions
Rezivertinib (BPI-7711) showed promising efficacy and a favorable safety profile for the treatment among the locally advanced or metastatic/recurrent NSCLC patients with EGFR mutation in the first-line setting.
Trial registration
ClinicalTrials.gov, NCT03386955.
Similar content being viewed by others
Background
Lung cancer is the second most commonly diagnosed cancer worldwide but leading the cause of cancer death [1]. Non-small cell lung cancer (NSCLC) comprises about 80 to 85% of all lung cancers, with the majority of patients presenting with locally advanced or metastatic disease [2, 3]. Epidermal growth factor receptor (EGFR) mutations are found in up to 10% of Caucasians, and more than 40% of East Asian adenocarcinoma patients [4,5,6]. According to the latest National Comprehensive Cancer Network (Version 3.0, 2022) and the Chinese guideline, EGFR tyrosine kinase inhibitors (TKIs) are the current first-line treatments for NSCLC with EGFR mutation [7, 8], including the first-generation EGFR TKIs with gefitinib, erlotinib, and icotinib; the second-generation EGFR TKIs with afatinib and dacomitinib; and the third-generation EGFR TKIs with osimertinib, almonertinib, and furmonertinib. As reported, after around 1-year treatment of the first- or second-generation EGFR TKIs, most patients acquired drug resistance [9, 10]. EGFR T790M mutation occurs in more than 50% of the patients showing acquired drug resistance [11]. Osimertinib, a third-generation EGFR TKI, was approved by the US Food and Drug Administration (FDA) on November 13, 2015, for advanced EGFR T790M mutated NSCLC [12]. Almonertinib and furmonertinib were first approved by the National Medical Products Administration (NMPA) in the People’s Republic of China on Mar 17, 2020, and Mar 3, 2021, respectively [13,14,15,16]. Meanwhile, the clinical development for novel third-generation EGFR TKIs is ongoing widely due to the high proportion of EGFR-mutant patients and diversified features of different third-generation EGFR TKIs [12].
Rezivertinib (BPI-7711) is a novel third-generation EGFR TKI jointly developed by Beta Pharma (Shanghai) Co., Ltd., Shanghai, People’s Republic of China, and Beta Pharma Inc., Princeton, NJ, USA, which can selectively target specific mutated EGFR and form irreversible covalent binding at the active binding site, showing the highly selective inhibitory effect on EGFR mutation such as exon 19 deletion, L858R point mutation, T790M mutation, and the weak inhibitory effect on EGFR wild-type. In a previous phase I study, rezivertinib resulted in an objective response rate (ORR) of 59.3%, a disease control rate (DCR) of 91.3%, and a median progression-free survival (PFS) of 9.7 months for advanced NSCLC patients with EGFR T790M mutation, and the recommended phase II dose (RP2D) was identified as 180 mg once daily [17]. The phase IIb study results further revealed the promising efficacy with a manageable safety profile of rezivertinib for patients with locally advanced or metastatic/recurrent EGFR T790M-mutated NSCLC [18]. This phase IIa study was part of the phase I/IIa study. In this study, we evaluate the efficacy and safety of rezivertinib in the first-line treatment of locally advanced or metastatic/recurrent NSCLC patients with EGFR mutation.
Methods
Study design and patients
This was a multicenter, single-arm, open-label, phase IIa study, which was part of the phase I/IIa study (NCT03386955), conducted across 20 hospitals in the People’s Republic of China. Key inclusion criteria included patients aged 18–75 years; with a histologically or cytologically confirmed locally advanced or metastatic NSCLC harboring EGFR-sensitive mutations, including exon 19 deletion, L858R, G719X, and L861Q which were detected through tissue or/and plasma biopsies by central laboratory testing using the Cobas® EGFR Mutation Test, Version 2, Roche Diagnostics, South Branchburg, NJ, USA; who have at least one measurable lesion; with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 to 1; who have no disease deterioration over the previous 2 weeks; and with at least a 12-week life expectancy who were not suitable for operation or radiotherapy. Patients with previous neoadjuvant or adjuvant therapies including chemotherapy, radiotherapy, and investigational drug were acceptable, except EGFR TKIs. Adequate organ function was required as defined by platelet (PLT) count ≥ 100 × 109/L, absolute neutrophil count (ANC) ≥ 1.5 × 109/L, hemoglobin ≥ 90 g/L, total bilirubin ≤ 1.5 × the upper limit of normal (ULN), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤ 3 × ULN (total bilirubin ≤ 3 × ULN, ALT ≤ 5 × ULN, and AST ≤ 5 × ULN were allowed if liver metastases existed), serum creatinine ≤ 1.5 × ULN, or creatinine clearance ≥ 50 mL/min according to the Cockcroft-Gault equation, QT interval corrected for heart rate using Fridericia’s formula (QTcF) prolongation ≤ 470 ms at rest, international normalized ratio (INR) and activated partial thromboplastin time (APTT) ≤ 1.5 × ULN without taking an anticoagulant. Before the first dose of rezivertinib, all prior treatment-related adverse events (TRAEs) had to be at grade ≤ 1 except for hair loss and peripheral nerve toxic reaction. The ability to swallow capsules was required.
Key exclusion criteria included patients who received previous anticancer treatment for advanced NSCLC including EGFR TKIs, cytotoxic chemotherapy, and investigational agent; any clinically significant electrocardiogram (ECG) abnormality (such as QTcF prolongation > 470 ms at rest, complete left bundle branch block); any factor that increased the risk of QTcF prolongation (such as New York Heart Association II-IV, hypokalemia, long QT syndrome); any condition that possibly affected drug absorption such as severe or uncontrolled inflammatory gastrointestinal disease, abdominal colostomy, gastrointestinal perforation within 6 months, extensive bowel resection, or tube feeding patients; medical history of interstitial lung disease (ILD), radiation pneumonitis that required steroid treatment, and acute or progressive lung disease that could lead to ILD; active infection disease such as hepatitis B, hepatitis C, and human immunodeficiency virus, inactive hepatitis B was acceptable; major surgery within 4 weeks, minor operation within 2 weeks; radiotherapy with a wide field within 4 weeks, or radiotherapy within a limited field within 1 week before the first dose of rezivertinib; patients with any other concomitant cancer or recurrent cancer within 5 years, except radical operation of carcinoma in situ of cervix, non-melanoma skin cancer, noninvasive superficial bladder cancer, or radical operation of carcinoma in situ with no recurrence within 3 years; patients with spinal cord compression or meningeal metastases, symptomatic brain metastases, except asymptomatic brain metastases not requiring steroids and/or local therapy before this study, asymptomatic brain metastases after local therapy such as radiotherapy and steroids and/or antiepileptic therapy at least 7 days before the first dose of rezivertinib.
Informed consent was obtained from every patient before enrollment. The study was done in accordance with the Declaration of Helsinki and approved by the institutional review board or independent ethics committee associated with each participating hospital.
Procedures
Eligible patients received rezivertinib 180 mg orally once daily until disease progression, unacceptable toxicity, or withdrawal of consent. Treatment beyond progression was permitted if clinical benefits could be obtained in the judgment of the investigators.
Dose adjustment was allowed according to such principles. If a patient had a grade ≥ 3 TRAE, the administration of rezivertinib should be suspended, and supportive care should be given accordingly. After the grade ≥ 3 TRAE was relieved or recovered to grade ≤ 1 within 2 weeks after dose interruption, the investigators would restart the treatment at the initial dose (180 mg) or a lower dose (120 mg → 60 mg) according to the patient’s condition, and close medical monitoring was necessary.
Efficacy was assessed by blinded independent central review (BICR) and by investigators with enhanced computed tomography scans for the chest and abdomen or magnetic resonance imaging scans for the brain at baseline and every 2 treatment cycles (6 weeks) from the first dose of rezivertinib. In the period between the time when the informed consent was signed and 30 days after the last dose of rezivertinib, adverse events (AEs) were monitored continuously. During the treatment period, physical examinations, vital signs, ECOG PS scores, hematology, serum chemistry, urinalysis, 12-lead ECGs, and echocardiography were documented and assessed at protocol-specified time points.
Endpoints and assessments
The primary endpoint was ORR in full analysis set (FAS) evaluated by BICR per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [19]. The efficacy for patients with central nervous system (CNS) metastases was measured by BICR according to the Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria [20]. The secondary endpoints included DCR, duration of response (DoR), and PFS assessed by both BICR and investigators; overall survival (OS); safety assessed by investigators. Safety referred to treatment-emergent adverse events (TEAEs) and TRAEs which were assessed in the safety set (SS) according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
FAS referred to all patients enrolled who received at least one dose of rezivertinib. SS included all patients who received at least one dose of rezivertinib and had safety data available. ORR was defined as the proportion of patients with confirmed complete response (CR) or partial response (PR). DCR was defined as the proportion of patients who had confirmed CR, PR, or stable disease (SD). DoR was defined as the time from the first CR or PR to disease progression or death which applied only to patients with confirmed CR or PR. PFS was defined as the time from the first dose of rezivertinib until the earliest date of documented disease progression or death due to any cause. OS was defined as the time from the date of the first dose of rezivertinib until death due to any cause. CNS-ORR was defined as the proportion of confirmed CR or PR in brain metastatic lesions, as evaluated by BICR according to the RANO-BM criteria. CNS-DCR was defined as the proportion of confirmed CR, PR, or SD in brain metastatic lesions evaluated by BICR according to the RANO-BM criteria.
Statistical analysis
The 95% confidence interval (CI) for ORR and DCR was determined by the Clopper-Pearson method. The 95% CI for median values of PFS, DoR, and OS was calculated by the Kaplan–Meier method. All statistical analyses were performed using SAS® Version 9.3 or higher.
Results
Patients
From Jun 12, 2019, to Oct 17, 2019, 68 patients were screened. Finally, a total of 43 eligible patients were enrolled, and all patients received rezivertinib treatment (Fig. 1). Patient baseline characteristics are presented in Table 1.
Patient disposition. *Two patients accompanied with T790M mutation were mistakenly enrolled, one patient was with T790M positive and exon 19 deletion mutation and the other was with T790M positive and L858R mutation. Abbreviations: EGFR, epidermal growth factor receptor; QTcF, QT interval corrected for heart rate using Fridericia’s formula; PD, progressive disease; TEAEs, treatment-emergent adverse events
At the data cutoff date on Dec 23, 2021, all patients had terminated rezivertinib treatment, while 22 (51.2%) patients had progressive disease (PD), 15 (34.9%) patients terminated the study by sponsor due to the data cutoff, 4 (9.3%) patients discontinued due to TEAEs, 1 patient terminated due to investigator decision, and 1 patient withdrew of consent (Fig. 1). The median duration of follow-up was 25.3 (95% CI: 25.0–26.2) months.
Efficacy
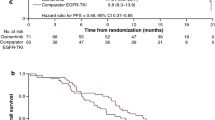
All 43 patients were included in FAS. The BICR-assessed and investigator-assessed ORRs were 83.7% (95% CI: 69.3–93.2%) and 69.8% (95% CI: 53.8–83.0%), respectively. The BICR-assessed and investigator-assessed DCRs were 97.7% (95% CI: 87.7–99.9%) and 95.3% (95% CI: 84.2–99.4%), respectively. The tumor shrinkage was observed in 95.3% (41/43) of patients (Fig. 2A). The forest plot for subgroups of patients having objective responses assessed by BICR in FAS was presented in Fig. 2B. The BICR-assessed and investigator-assessed median DoR were 19.3 (95% CI: 15.8–25.0) months and 19.3 (95% CI: 8.3–25.0) months, respectively. The median duration of rezivertinib exposure was 20.6 (range: 1.1–27.5) months (Fig. 3A), and the BICR-assessed percentage change of tumor size from baseline at different time points was presented in Fig. 3B. At the data cutoff date on Dec 23, 2021, 26 (60.5%) of 43 patients were with PFS events, while 22 (51.2%) had developed PD and 4 (9.3%) patients died before PD. The BICR-assessed and investigator-assessed median PFS were 20.7 (95% CI: 13.8–24.8) months and 22.0 (95% CI: 16.8–26.3) months, respectively (Fig. 4A). Among the 43 patients, 2 patients harbored uncommon EGFR mutations. For these 2 patients, one patient was with G719X and S768I mutations, and the BICR-assessed PFS was 5.6 months with the best objective response (BOR) of PR (the Cobas® EGFR Mutation Test, Version 2, Roche Diagnostics, South Branchburg, NJ, USA, was used for EGFR mutations detection, which was not able to further confirm this patient’s specific G719X type); the other patient was with only L861Q mutation, and the BICR-assessed PFS was 3.6 months with the BOR of SD. Additionally, there were 2 patients accompanied with T790M mutation mistakenly enrolled in this study, one patient was a 67-year-old male with T790M positive and exon 19 deletion mutation who had SD and a PFS of 19.0 months and the other patient was a 65-year-old male with T790M positive and L858R mutation who had PR and a PFS of 21.0 months. At the data cutoff date on Dec 23, 2021, the OS was immature, and 14 (37.2%) patients died, while 27 (62.8%) patients were still alive, one patient was lost to follow-up, and one withdrew of consent. Among 12 (27.9%) of 43 patients with CNS metastases at baseline, the CNS-ORR and CNS-DCR were 50.0% (95%CI: 21.1–78.9%) and 58.3% (95%CI: 27.7–84.8%), respectively. The probability of CNS progression at 12 months was 33.3%. Efficacy results are listed in Table 2. The BICR-assessed median PFS for the patients without and with CNS metastases at baseline were 22.0 (95% CI: 13.8–not calculable [NC]) months and 15.2 (95% CI: 6.4–NC) months, respectively (p = 0.3991, Fig. 4B); while the BICR-assessed median PFS for patients with EGFR exon 19 deletion mutation and EGFR L858R mutation were 20.7 (95% CI: 13.8–NC) and 17.7 (95% CI: 6.4–NC) months, respectively (p = 0.1835, Fig. 4C).
A Waterfall plot for BICR-assessed tumor best percentage change from baseline in FAS. The dashed line at 20% represents the boundary for the determination of PD, and the dashed line at − 30% represents the boundary for the determination of PR. Color code: orange for PR; brown for SD; blue for PD. B Forest plot for subgroups of patients having BICR-assessed objective responses in FAS. *Other includes one patient with G719X and S768I mutations, and one patient with only L861Q mutation. The Cobas® EGFR Mutation Test, Version 2, Roche Diagnostics, South Branchburg, NJ, USA, was used for EGFR mutations detection, which was not able to further confirm this patient’s specific G719X type. Abbreviation: FAS, full analysis set; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; PS, performance status; BICR, blinded independent central review; ORR, objective response rate; DCR, disease control rate; CI, confidence interval; PR, partial response; SD, stable disease; PD, progressive disease
A Swimmer plot for the exposure and response duration of rezivertinib in FAS. As per the protocol and the RECIST version 1.1, after the patient’s disease progression, the patient may continue the treatment if investigators considered the patient would still benefit from the study treatment. In patients with a BICR-assessed confirmed objective response, the time when the objective response was first observed is indicated by an × , and the time when the objective response was terminated is indicated by a circle. B Spider plot for BICR-assessed percentage change of tumor size from baseline at different time points. Abbreviation: BICR, blinded independent central review; PR, partial response; SD, stable disease; PD, progressive disease; FAS, full analysis set; RECIST: Response Evaluation Criteria in Solid Tumors
A Kaplan–Meier curve for BICR-assessed PFS and investigator-assessed PFS in FAS. B Kaplan–Meier curve for BICR-assessed PFS in patients with and without CNS metastases. C Kaplan–Meier curve for BICR-assessed PFS in patients with EGFR Exon 19 deletion and L858R mutations. Abbreviation: BICR, blinded independent central review; CI, confidence interval; PFS, progression-free survival, FAS, full analysis set; CNS, central nervous system; NC, not calculable
Safety
All 43 patients were included in SS. TEAEs occurred in 42 patients (97.7%), while TRAEs occurred in 40 patients (93.0%) (Additional file 1: Table S1). The most common TRAEs were white blood cell (WBC) decreased (19 of 43, 44.2%), PLT decreased (17 of 43, 39.5%), ANC decreased (13 of 43, 30.2%), anemia (11 of 43, 25.6%), ALT increased (8 of 43, 18.6%), lymphocyte count decreased (6 of 43, 14.0%), AST increased (5 of 43, 11.6%), etc. (Table 3). No ILD was reported. Dose interruption occurred in 2 (4.7%) patients due to TRAEs, and no dose reduction or discontinuation due to TRAEs was recorded.
Discussion
In this phase IIa study, rezivertinib showed promising efficacy and a manageable safety profile in the first-line treatment of locally advanced or metastatic/recurrent NSCLC patients with EGFR mutation, including those with CNS metastases.
There are three third-generation EGFR TKIs available in the People’s Republic of China for advanced NSCLC. Osimertinib, the first third-generation irreversible EGFR TKI, significantly improved the PFS and OS versus first-generation EGFR TKIs gefitinib or erlotinib in patients with EGFR mutation-positive NSCLC and had been sequentially approved by both FDA on April 18, 2018, and NMPA on August 31, 2019, as the first-line treatment [21,22,23]. Another two China innovative third-generation EGFR TKIs, almonertinib and furmonertinib, were approved for the first-line treatment by NMPA on Dec 16, 2021, and Jun 28, 2022, respectively. In the AENEAS study, almonertinib significantly prolonged median PFS (19.3 vs 9.9 months; hazard ratio [HR]: 0.46; p < 0.0001) and median DoR (18.1 vs 8.3 months; HR: 0.38; p < 0.0001) over gefitinib, with immature OS and acceptable safety [24]. In the furmonertinib FURLONG study, the median PFS (21.0 vs 11.1 months; HR: 0.44; p < 0.0001) and median DoR (19.7 vs 10.5 months; HR: 0.39; p < 0.0001) were significantly prolonged while compared with gefitinib. The OS data was not yet mature, and the safety was acceptable [25].
The third-generation EGFR TKIs revealed the optimal subgroup efficacy based on patients’ EGFR mutation types. In the FLAURA study, the median PFS was 21.4 (95% CI: 16.5–24.3) months and 11.0 (95% CI: 9.7–12.6) months for patients with EGFR exon 19 deletion mutation in the osimertinib and first-generation EGFR TKI gefitinib/erlotinib groups, respectively (HR: 0.43 [95% CI: 0.32–0.56]; p < 0.001); 14.4 (95% CI: 11.1–18.9) months and 9.5 (95% CI: 8.1–11.0) months for patients with EGFR L858R mutation in the two groups, respectively (HR: 0.51 [95% CI: 0.36–0.71]; p < 0.001) [21]. In the AENEAS study, among patients with EGFR exon 19 deletion mutation, the median PFS for the almonertinib and gefitinib groups was 20.8 (95% CI: 18.1–20.9) months and 12.3 (95% CI: 9.6–13.8) months, respectively (HR: 0.39; p < 0.0001), while among patients with L858R mutation, the median PFS were 13.4 (95% CI: 7.3–18.0) months and 8.3 (95% CI: 6.8–9.9) months for the two groups, respectively (HR: 0.60; p = 0.0102) [24]. In the FURLONG study, furmonertinib significantly reduced the risk of progression or death while compared with gefitinib with HRs of 0.35 (95% CI: 0.23–0.53; p < 0.0001) and 0.54 (95% CI: 0.37–0.77; p = 0.0006) among patient with EGFR exon 19 deletion and L858R mutations, respectively [25]. Despite being a single-arm study, the rezivertinib phase IIa study was revealed to be associated with long median PFS for patients with EGFR exon 19 deletion and L858R mutations (20.7 [95% CI: 13.8–NC] months and 17.7 [95% CI: 6.4–NC] months; p = 0.1835), which were consistent with the overall efficacy. Apart from clinical trial results, real-world data of osimertinib from the FLOWER and the ASTRIS global study had demonstrated similar efficacy and safety consistent with its previous clinical studies [26, 27]. More real-world data of other third-generation EGFR TKIs are awaited.
Compared with the first- or second-generation EGFR TKIs, the third-generation EGFR TKIs have improved CNS efficacy. In the FLAURA study, osimertinib reduced the risk of CNS progression or death while compared with the first-generation EGFR TKI gefitinib/erlotinib with an HR of 0.48 (95% CI: 0.26–0.86; p = 0.014) in the CNS full analysis set (cFAS) [28]. In the AENEAS study, almonertinib achieved longer median CNS-PFS over gefitinib in cFAS [29.0 vs 8.3 months; HR: 0.323 (95% CI: 0.181–0.576); p < 0.0001] [29]. Furmonertinib achieved a median CNS-PFS of 11.6 (95% CI: 8.3–13.8) months for EGFR T790M mutated patients in its phase IIb study for the cFAS and further prolonged the median CNS-PFS while compared with gefitinib in cFAS (20.8 vs 9.8 months; HR: 0.40 [95% CI: 0.23–0.71]; p = 0.0011) in the FURLONG study [15, 30].
Rezivertinib is one of the novel third-generation EGFR TKIs; the phase IIa study results were consistent with the previous phase I study [17] and further verified by the results of the phase IIb study (n = 226) which investigated the efficacy and safety of rezivertinib in patients with locally advanced or metastatic/recurrent EGFR T790M mutated NSCLC, including treatment-naïve patients and EGFR TKI previously treated patients [18]. The results of the phase IIb study showed that the ORR was 64.6% (95% CI: 58.0–70.8%), and the median PFS was 12.2 (95% CI: 9.6–13.9) months by BICR. The subgroup efficacy was consistent with the overall efficacy, with the ORR of 70% (95% CI: 61.0–78.0%) and median PFS of 13.9 (95% CI: 11.3–17.9) months for the tissue sample T790M positive group. The median OS was 23.9 (95% CI: 20.0–NC) months in FAS. Furthermore, the median CNS-PFS was 16.6 (95% CI: 11.1–NC) months for the 91 (40.3%) patients with CNS metastases at baseline. The efficacy of this phase IIa study was consistent with previous reported results.
The safety profile of rezivertinib was favorable without new safety signals. Here we would like to discuss three aspects of safety profile. Firstly, dermatological and gastrointestinal toxicities were common for the first-/second-generation EGFR TKIs and osimertinib in the global FLAURA study [21, 31], while rezivertinib mainly presented hematological toxicity which was partially similar to osimertinib among Chinese patients [23]. The top five most common TEAEs were WBC decreased (41%), anemia (38%), rash or acne (37%), PLT decreased (28%), and diarrhea (24%) in the FLAURA China study [23], while that in this phase IIa study of rezivertinib were WBC decreased (44.2%), PLT decreased (41.9%), ANC decreased (32.6%), anemia (30.2%), and ALT increased (20.9%). However, the mechanism of hematological toxicity has not been elucidated. Fortunately, the hematological toxicity was all well-tolerated with a limited effect on the study dose adjustment. Secondly, ALT elevated related to rezivertinib was observed in 8 (18.6%) patients and none of them was grade ≥ 3, while AST elevated related to rezivertinib was observed in 5 (11.6%) patients and none of them was grade ≥ 3. Also, there was no sign indicating that ALT or AST elevated was related to viral hepatitis since two patients with hepatitis B disease at baseline experienced no ALT or AST elevation by the data cutoff date. Thirdly, drug-induced ILD has been considered as an infrequent but non-negligible serious fatal TRAEs that occurred in all generations of EGFR TKIs, and the molecular mechanisms have not been clarified [32]. Among the clinical studies of third-generation EGFR TKIs in the first-line setting, 6 patients with ILD were recorded as osimertinib-related in the global FLAURA study while grade 3 ILD occurred in one patient as severe TRAE in the FLAURA China study [21,22,23]. For almonertinib and furmonertinib, the ILD was also observed in two and one patient in the AENEAS and the FURLONG studies, respectively [24, 25]. However, in this phase IIa study, no ILD was observed after the median follow-up duration of 23.3 (95% CI: 22.8–23.9) months. Furthermore, throughout the phase I and phase IIb studies of rezivertinib, no ILD has been observed either [17, 18].
There are limitations to this phase IIa study. Firstly, the sample size was limited to only 43 patients and a strict randomized trial with a larger sample size is needed to further confirm the potential efficacy of rezivertinib. Encouragingly, a phase III REZOR study comparing rezivertinib with gefitinib in the first-line setting is ongoing, and the patient enrollment has been completed (NCT03866499). Secondly, there might be a potential bias while comparing with other ethnic patients since this phase IIa study was conducted among Chinese patients only.
Conclusions
In conclusion, in this study, rezivertinib (BPI-7711) showed promising efficacy and a favorable safety profile for the treatment among the locally advanced or metastatic/recurrent NSCLC patients with EGFR mutation in the first-line setting.
Availability of data and materials
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AE:
-
Adverse event
- ALT:
-
Alanine aminotransferase
- ANC:
-
Absolute neutrophil count
- APTT:
-
Activated partial thromboplastin time
- AST:
-
Aspartate aminotransferase
- BICR:
-
Blinded independent central review
- BOR:
-
Best objective response
- cFAS:
-
CNS full analysis set
- CI:
-
Confidence interval
- CNS:
-
Central nervous system
- CR:
-
Complete response
- CTCAE:
-
National Cancer Institute Common Terminology Criteria for Adverse Events
- DCR:
-
Disease control rate
- DoR:
-
Duration of response
- ECG:
-
Electrocardiogram
- ECOG:
-
Eastern Cooperative Oncology Group
- EGFR:
-
Epidermal growth factor receptor
- FAS:
-
Full analysis set
- FDA:
-
Food and Drug Administration
- HR:
-
Hazard ratio
- ILD:
-
Interstitial lung disease
- INR:
-
International normalized ratio
- NC:
-
Not calculable
- NMPA:
-
National Medical Products Administration
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PLT:
-
Platelet
- PR:
-
Partial response
- PS:
-
Performance status
- QTcF:
-
QT interval corrected for heart rate using Fridericia's formula
- RANO-BM:
-
Response Assessment in Neuro-Oncology Brain Metastases
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- RP2D:
-
Recommended phase II dose
- SD:
-
Stable disease
- SS:
-
Safety set
- TEAE:
-
Treatment-emergent adverse event
- TKI:
-
Tyrosine kinase inhibitor
- TRAE:
-
Treatment-related adverse event
- ULN:
-
Upper limit of normal
- WBC:
-
White blood cell
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. https://doi.org/10.3322/caac.21565.
Shi JF, Wang L, Wu N, et al. Clinical characteristics and medical service utilization of lung cancer in China, 2005–2014: Overall design and results from a multicenter retrospective epidemiologic survey. Lung Cancer. 2019;128:91–100. https://doi.org/10.1016/j.lungcan.2018.11.031.
Yamaoka T, Ohba M, Ohmori T. Molecular-targeted therapies for epidermal growth factor receptor and its resistance mechanisms. Int J Mol Sci. 2017;18(11):2420. https://doi.org/10.3390/ijms18112420.
Shi Y, Au JSK, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–62. https://doi.org/10.1097/JTO.0000000000000033.
Shi Y, Li J, Zhang S, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology - mainland China subset analysis of the PIONEER study. PLoS One. 2015;10(11). https://doi.org/10.1371/JOURNAL.PONE.0143515
NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung. Version 3.2022.
Chinese Association for Clinical Oncologists, Medical Oncology Branch of Chinese International Exchange, Promotion Association for Medical Healthcare. [Clinical practice guideline for stage IV primary lung cancer in China (2021 version)]. Zhonghua Zhong Liu Za Zhi. 2021;43(1):39–59. https://doi.org/10.3760/cma.j.cn112152-20201009-00884
Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014;11(8):473–81. https://doi.org/10.1038/nrclinonc.2014.104.
Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17(1):1–14. https://doi.org/10.1186/s12943-018-0777-1.
Nagano T, Tachihara M, Nishimura Y. Mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and a potential treatment strategy. Cells. 2018;7(11):1–16. https://doi.org/10.3390/cells7110212.
Nagasaka M, Zhu VW, Lim SM, Greco M, Wu F, Ou SHI. Beyond osimertinib: the development of third-generation EGFR tyrosine kinase inhibitors for advanced EGFR+ NSCLC. J Thorac Oncol. 2021;16(5):740–63. https://doi.org/10.1016/j.jtho.2020.11.028.
Yang JCH, Camidge DR, Yang CT, et al. Safety, efficacy, and pharmacokinetics of almonertinib (HS-10296) in pretreated patients with EGFR-mutated advanced NSCLC: a multicenter, open-label, phase 1 trial. J Thorac Oncol. 2020;15(12):1907–18. https://doi.org/10.1016/j.jtho.2020.09.001.
Shi Y, Zhang S, Hu X, et al. Safety, clinical activity, and pharmacokinetics of alflutinib (AST2818) in patients with advanced NSCLC with EGFR T790M mutation. J Thorac Oncol. 2020;15(6):1015–26. https://doi.org/10.1016/j.jtho.2020.01.010.
Shi Y, Hu X, Zhang S, et al. Efficacy, safety, and genetic analysis of furmonertinib (AST2818) in patients with EGFR T790M mutated non-small-cell lung cancer: a phase 2b, multicentre, single-arm, open-label study. Lancet Respir Med. 2021;9(8):829–39. https://doi.org/10.1016/S2213-2600(20)30455-0.
Lu S, Wang Q, Zhang G, et al. Efficacy of aumolertinib (HS-10296) in patients with advanced EGFR T790M+ NSCLC: updated post-national medical products administration approval results from the APOLLO Registrational Trial. J Thorac Oncol. 2022;17(3):411–22. https://doi.org/10.1016/j.jtho.2021.10.024.
Shi Y, Zhao Y, Yang S, et al. Safety, efficacy, and pharmacokinetics of rezivertinib (BPI-7711) in patients with advanced NSCLC with EGFR T790M mutation: a phase 1 dose-escalation and dose-expansion study. J Thorac Oncol. 2022;17(5):708–17. https://doi.org/10.1016/j.jtho.2022.01.015.
Shi Y, Wu S, Wang K, et al. Efficacy and safety of rezivertinib (BPI-7711) in patients with locally advanced or metastatic/recurrent EGFR T790M-mutated NSCLC: a phase 2b study. J Thorac Oncol. 2022;S1556-0864(22):01557–X. https://doi.org/10.1016/j.jtho.2022.08.015.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/J.EJCA.2008.10.026.
Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–8. https://doi.org/10.1016/S1470-2045(15)70057-4.
Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113–25. https://doi.org/10.1056/NEJMOA1713137.
Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. https://doi.org/10.1056/NEJMOA1913662.
Cheng Y, He Y, Li W, et al. Osimertinib versus comparator EGFR TKI as first-line treatment for EGFR-mutated advanced NSCLC: FLAURA China, a randomized study. Target Oncol. 2021;16(2):165–76. https://doi.org/10.1007/S11523-021-00794-6.
Lu S, Dong X, Jian H, et al. AENEAS: a randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or metastatic non-small-cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. 2022;40(27):3162–71. https://doi.org/10.1200/JCO.21.02641.
Shi Y, Chen G, Wang X, et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. 2022;S2213–2600(22):00168. https://doi.org/10.1016/S2213-2600(22)00168-0.
Lorenzi M, Ferro A, Cecere F, et al. First-line osimertinib in patients with EGFR-mutant advanced non-small cell lung cancer: outcome and safety in the real world: FLOWER Study. Oncologist. 2022;27(2):87-e115. https://doi.org/10.1002/onco.13951.
de Marinis F, Wu YL, de Castro G, et al. ASTRIS: a global real-world study of osimertinib in ˃3000 patients with EGFR T790M positive non-small-cell lung cancer. Future Oncol. 2019;15(26):3003–14. https://doi.org/10.2217/fon-2019-0324.
Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non–small-cell lung cancer. J Clin Oncol. 2018;36(33):3290–7. https://doi.org/10.1200/JCO.2018.78.3118.
Lu S, Dong X, Jian H, et al. Aumolertinib activity in patients with CNS metastases and EGFR-mutated NSCLC treated in the randomized double-blind phase III trial (AENEAS). J Clin Oncol. 2022;40(16):9096–9096. https://doi.org/10.1200/JCO.2022.40.16_SUPPL.9096.
Shi Y, Chen G, Wang X, et al. Central nervous system efficacy of furmonertinib (AST2818) versus gefitinib as first-line treatment for EGFR-mutated NSCLC: results from the FURLONG study. J Thorac Oncol. 2022;S1556–0864(22):01496–504. https://doi.org/10.1016/j.jtho.2022.07.1143.
Lucchini E, Pilotto S, Spada E, Melisi D, Bria E, Tortora G. Targeting the epidermal growth factor receptor in solid tumors: focus on safety. Expert Opin Drug Saf. 2014;13(5):535–49. https://doi.org/10.1517/14740338.2014.904283.
Ohmori T, Yamaoka T, Ando K, et al. Molecular and clinical features of EGFR-TKI-associated lung injury. Int J Mol Sci. 2021;22(2):1–18. https://doi.org/10.3390/IJMS22020792.
Acknowledgements
The authors thank all the participating patients, their families, and the participating study teams. The authors would also like to thank Dr. Liling Huang and Dr. Haizhu Chen (National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, China) for providing medical editing assistance with this article.
Funding
This study (NCT03386955) was funded by Beta Pharma (Shanghai) Co., Ltd, Shanghai, People’s Republic of China, partly supported by the New National Natural Science Foundation of China (82172856, 81972805) and the China National Major Project for New Drug Innovation (2017ZX09304015).
Author information
Authors and Affiliations
Contributions
Conception and design: YKS. Administrative support: YKS and TTW. Provision of study material or patients: All authors. Collection and assembly of data: All authors. Data analysis and interpretation: YKS and TTW. Manuscript writing and revision: YKS and TTW. Accountable for all aspects of the work: All authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was first approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, and the approval number was 17–056/1311. This study was carried out in accordance with the protocol and complied with ICH-GCP and GCP (the People’s Republic of China), the Declaration of Helsinki, and other relevant laws and regulations. Written informed consent signed and dated by the patients has been obtained.
Consent for publication
Not applicable.
Competing interests
MG and TTW are employees of Beta Pharma, and all other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Safety Summary of rezivertinib in SS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, Y., Zhou, J., Zhao, Y. et al. Results of the phase IIa study to evaluate the efficacy and safety of rezivertinib (BPI-7711) for the first-line treatment of locally advanced or metastatic/recurrent NSCLC patients with EGFR mutation from a phase I/IIa study. BMC Med 21, 11 (2023). https://doi.org/10.1186/s12916-022-02692-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02692-8