Abstract
Background
Diagnostic testing has been pivotal in detecting SARS-CoV-2 infections and reducing transmission through the isolation of positive cases. We quantified the value of implementing frequent, rapid antigen (RA) testing in the workplace to identify screening programs that are cost-effective.
Methods
To project the number of cases, hospitalizations, and deaths under alternative screening programs, we adapted an agent-based model of COVID-19 transmission and parameterized it with the demographics of Ontario, Canada, incorporating vaccination and waning of immunity. Taking into account healthcare costs and productivity losses associated with each program, we calculated the incremental cost-effectiveness ratio (ICER) with quality-adjusted life year (QALY) as the measure of effect. Considering RT-PCR testing of only severe cases as the baseline scenario, we estimated the incremental net monetary benefits (iNMB) of the screening programs with varying durations and initiation times, as well as different booster coverages of working adults.
Results
Assuming a willingness-to-pay threshold of CDN$30,000 per QALY loss averted, twice weekly workplace screening was cost-effective only if the program started early during a surge. In most scenarios, the iNMB of RA screening without a confirmatory RT-PCR or RA test was comparable or higher than the iNMB for programs with a confirmatory test for RA-positive cases. When the program started early with a duration of at least 16 weeks and no confirmatory testing, the iNMB exceeded CDN$1.1 million per 100,000 population. Increasing booster coverage of working adults improved the iNMB of RA screening.
Conclusions
Our findings indicate that frequent RA testing starting very early in a surge, without a confirmatory test, is a preferred screening program for the detection of asymptomatic infections in workplaces.
Similar content being viewed by others
Background
Diagnostic testing has been instrumental to mitigating the COVID-19 pandemic, particularly for informing quarantine strategies, and evaluating spatiotemporal infection risk [1,2,3,4,5]. Prior to the widespread availability of rapid antigen (RA) tests, identifying SARS-CoV-2 infection relied predominantly on reverse transcription polymerase chain reaction (RT-PCR). The availability of RA tests has provided a viable alternative to RT-PCR methods by scaling up testing capacities and shortening test turnaround times from days to minutes [2, 6]. Despite their lower sensitivity compared to RT-PCR tests, low-cost self-administered RA tests are increasingly used outside clinical settings, especially for screening at home and workplaces [7, 8].
The effectiveness of RA tests in real-world settings has been demonstrated in several studies [8,9,10,11,12,13,14]. Relative to the detection of cases through RT-PCR screening for asymptomatic cases, RA tests have been able to identify 20–81% of these cases [2, 8, 12,13,14,15,16,17]. However, the fast turnaround time of RA tests can allow for increased frequency of testing compared to RT-PCR, thus improving case detection in the early stages of disease for screening programs and limiting the extent of onward transmission [17, 18].
A recent study provides a roadmap for the scalable implementation of frequent RA testing to detect asymptomatic infection in workplaces, suggesting that screening programs could interrupt chains of transmission, thereby reducing the burden of disease [10]. Although low rates of false positives in large-scale screening programs with frequent RA testing may not disrupt workplace operations [10], the scale of false-negative outcomes remains a concern, especially for the identification of breakthrough infections [8]. Commissioned by Health Canada, we evaluated the costs and benefits of frequent RA testing in workplaces post-Omicron BA.1 wave by performing cost-effectiveness analyses of screening programs with and without confirmatory RT-PCR testing.
Methods
General framework
To evaluate the cost-effectiveness of RA screening in workplaces, we used both direct and indirect costs associated with SARS-CoV-2 infection and outcomes derived from an agent-based model of COVID-19 transmission dynamics based on 500 Monte-Carlo replications (Additional file 1) [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. We have previously used this model for estimating the impact of non-pharmaceutical interventions and vaccination on reducing the COVID-19 burden [19,20,21, 47]. Taking the province of Ontario, Canada as the population study, we simulated incidence of infections and outcomes over a 1-year time horizon from the beginning of April 2022. We accounted for the population immunity generated by vaccination in different age groups. The model was initiated with a 10% naturally acquired population immunity against infection and calibrated to an effective reproduction number of 1.2 [25]. The 10% proportion of the population with immunity due to a prior infection is based on the reported incidence [48], but it may be conservative given the possibility of undocumented asymptomatic or mild symptomatic infections.
We considered the primary and booster vaccination coverage in different age groups as of April 1, 2022 (status quo scenario) [49], accounting for the temporal waning of immunity post-vaccination or infection. Primary vaccination is defined as the first two doses of approved vaccines in Canada (i.e., Moderna SpikeVax™, or Pfizer-Bio-NTech Comirnaty). Booster refers to an additional (third) vaccine dose. Under the status quo, 81.2% of the Ontario population was fully vaccinated of whom 59% had received a booster. Among working adults aged 18–65 years, the coverage of booster vaccination was ~48% [50]. We also considered additional scenarios in which the booster coverage of working adults was increased by 20% and 80% over the status quo. Simulations for each testing scenario were run by implementing the model in Julia Language, and statistical analyses were conducted using outputs in MATLAB.
Rapid antigen screening programs
For a given coverage of booster vaccination, we set the baseline scenario for the cost-effectiveness analysis to be “RT-PCR testing of only severe symptomatic cases” (TOSC) in the population. For the screening program, we used the distribution of workplace sizes in Ontario (Additional file 1: Fig. S2) [24]. Screening of asymptomatic infection was simulated as an incremental to the baseline for workplaces with at least 50 employees, and with testing every Monday and Thursday. We considered scenarios in which either 50% or 100% of workplaces participated in an RA screening program. As a requirement of the policy, we assumed that all individuals working in places with a screening program in effect adhere to the testing schedules. For the daily number of contacts inside and outside workplaces, we relied on recent empirical distributions from the CONNECT study on time trends in social contacts before and during the COVID-19 pandemic [23]. We assumed that the result of an RA test will be available within several minutes, but the result of an RT-PCR test will be available 1 day from sample collection.
To infer the temporal diagnostic sensitivity of the RT-PCR assay, we fitted a time-dependent log-Normal probability density function [17] to serial testing data [51], assuming that the maximum of this function coincides with the peak of infectiousness. The diagnostic sensitivity of the rapid antigen tests was then expressed as the product of the diagnostic sensitivity of the RT-PCR and the temporal percent positive agreement (PPA) of the rapid antigen tests with an RT-PCR test (Additional file 1) [2, 6, 51,52,53,54,55,56,57]. Although a number of RA tests have been used in Canada, we performed our analysis with temporal diagnostic sensitivity of Abbott-PanbioTM (described in the Results section), as well as BD Veritor and Sofia tests (Additional file 1) derived based on the temporal PPA with an RT-PCR test relative to the time of symptom onset [54, 55]. A specificity of 99.8% was used for the RA tests and 99.9% for the RT-PCR test.
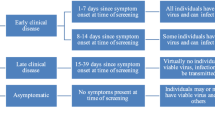
Screening programs were implemented with and without a confirmatory test for RA-positive cases (Table 1). For the screening program without a confirmatory test (SP1), if the RA test was positive, individuals would complete a 5-day isolation period before returning to work and normal activities (Fig. 1). For the RA screening program with either a confirmatory RT-PCR test (SP2) or a confirmatory RA test (SP3) 1 day after the initial RA positive [7, 58], the 5-day isolation period was reduced to 1 day if the confirmatory test was negative. The infectious period for an individual may be sampled to be longer than the isolation period (Additional file 1: Section 2). In this case, contacts will be associated with risk of disease transmission upon return to the workplace and normal activities.
The baseline of TOSC was implemented throughout the simulations. We then varied the initiation of RA screening at the workplace, considering programs with a duration of 16, 32, and 52 weeks. Comparison between RA screening programs and TOSC was done at the same coverage of primary and booster vaccination.
Cost-effectiveness analysis
We conducted a cost-effectiveness analysis of the RA screening programs (Table 1), with the benefits captured as QALYs. To estimate total costs and benefits, we used the number of mild and severe symptomatic infections, outpatient and emergency department visits, hospitalizations and ICU admissions, isolation days after a positive test and a false positive RA test, deaths, and the total number of different tests performed. Costs were captured from two perspectives of (i) healthcare, which included those associated with health outcomes and testing, and (ii) productivity loss due to illness (i.e., isolation for acute infection, hospitalization, and death), as well as time lost to testing (Table 2). All costs were converted and inflated to Canadian dollars in 2021 [72].
Cost-effectiveness results are presented by both the incremental cost-effectiveness ratio (ICER), and the incremental net monetary benefits (iNMB) for direct comparison of the testing scenarios. An ICER was calculated for each testing scenario, in comparison to the baseline of TOSC. NMB was calculated by subtracting the costs of a scenario from the monetary value of health gained using a willingness-to-pay (WTP) threshold of $CAD 30,000 [73].
Results
Effectiveness of testing programs
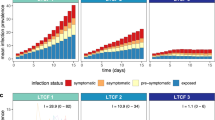
Compared to TOSC in the general population, the largest reduction of cumulative incidence was achieved when the workplace screening programs were implemented for the entire year without interruption (Fig. 2A3, B3, and C3). For screening programs with a shorter duration, an earlier start during a surge resulted in a lower cumulative incidence. For example, RA screening that initiated at the start of a surge for a duration of 16 weeks reduced the total incidence (compared with TOSC) by 9.84% (95% credible interval [CrI]: 6.78% to 13.04%) in SP1, 7.97% (95% CrI: 5.57% to 11.4%) in SP2, and 9.28 (95% CrI: 5.47 to 12.35) in SP3 (Fig. 2A1). With the same duration of RA screening but delaying the program until 16 weeks after the start of the surge (Fig. 2A4), the reduction of incidence was 2.81% (95% CrI: 0.65% to 5.59%) in SP1, 2.77% (95% CrI: 0.52% to 4.51%) in SP2, and 2.43 (95% CrI: 0.29 to 4.44) in SP3. These outcomes were qualitatively independent of the proportion of workplaces that participated in the RA screening programs, or the coverage of booster vaccination (Fig. 2; Additional file 1: Fig. S5). However, increasing booster coverage of working adults had a significant effect on both delaying and suppressing the surge.
Projected average daily incidence of all (symptomatic and asymptomatic) infections for TOSC (black); SP1 in workplaces without a confirmatory test (blue); SP2 in workplaces with a confirmatory RT-PCR test (orange); and SP3 in workplaces with a confirmatory RA test (red). Screening programs were implemented for 50% of workplaces with a size of 50+ employees. The booster vaccination among adults aged 18–64 years was set to reported coverage as of April 1, 2022 [status quo] (A1–A5); an increase of 20% over status quo (B1–B5); and an increase of 80% over status quo (C1–C5). Shaded areas indicate the duration of SP1, SP2, and SP3; testing of severe cases with RT-PCR tests was implemented throughout the entire simulation. For SP2, RA-positive cases were isolated while awaiting the RT-PCR test result
Cost-effectiveness of testing programs (status quo scenario)
When RA screening was implemented by 50% of workplaces, SP1 with a 16-week duration from the start of a surge (Fig. 2A1) resulted in an average gain of 155 QALYs per 100,000 population with incremental costs of $−5,566,013, compared to the baseline of TOSC with status quo vaccine coverage. This produces the median ICER value of −35,739 (95% CrI: −83,556 to −5,375) per QALYs gained (Additional file 1: Table S5), suggesting that SP1 is a cost-saving (dominant) program (Fig. 3). We estimated $10.2 (95% CrI: 5.3, 15.7) million iNMB associated with this RA testing program (Table 3). For the same duration of the RA screening, SP2 generated an average gain of 111 QALYs per 100,000 population with incremental costs of $−3,001,675. The median ICER associated with SP2 was estimated at −27,828 (95% CrI: −93,085 to 16,388) per QALYs gained, with a 89% probability of being cost-saving; however, its iNMB was reduced, compared to SP1, to an estimated median of $6.4 (95% CrI: 0.9, 11.5) million. For RA testing with SP3, an average of 149 QALYs per 100,000 population was gained with incremental costs of $−6,215,098, resulting in the median ICER value of −42,166 (95% CrI: −95,002 to −7681) per QALYs gained. This suggests that SP3 is a cost-saving (dominant) program. The iNMB generated by SP3 was estimated to be $10.2 (95% CrI: 5.0 to 15.9) million.
Cost-effectiveness plane derived from 500 independent Monte-Carlo simulations for different testing scenarios with the associated 95% credible ellipse of the data point distributions. Colors correspond to testing only severe cases (black dot); SP1 in workplaces without a confirmatory test (blue); SP2 in workplaces with a confirmatory RT-PCR test (orange); and SP3 in workplaces with a confirmatory RA test (red). Screening programs were implemented for 50% of workplaces with a size of 50+ employees. The booster vaccination among adults aged 18–64 years was set to reported coverage as of April 1, 2022 [status quo] (A1−A5); an increase of 20% over status quo (B1–B5); and an increase of 80% over status quo (C1–C5). Comparison was done between the baseline for testing only severe cases (TOSC) and each of the screening programs with the same booster coverage. Costs are in 2021 Canadian dollars
As the duration of screening extended, the monetary benefits of RA testing reduced. For example, with a 32-week screening (Fig. 3A2), SP1 was cost-effective with a 62% probability (at the WTP threshold) with an estimated median ICER of 25,033 (95% CrI: -4,121 to 55,925). However, producing an estimated median ICER of 40,917 (95% CrI: 4,303 to 91,282), SP2 was deemed not cost-effective (cost-effective probability<29%) at the WTP threshold due to additional costs of confirmatory RT-PCR testing and lower QALYs gained. Similar to SP1, SP3 was cost-effective with a 82% probability (at the WTP threshold) and an estimated median ICER of 14,509 (95% CrI: −21,018 to 50,235) per QALY gained. The median iNMB associated with SP1 and SP3 were positive at $1.1 and 2 million, respectively, but with SP2 was negative at $−1.5 million per 100,000 population (Table 3). Similar outcomes were obtained when RA screening was implemented for the entire 1-year simulation timelines, resulting in SP1 and SP3 being cost-effective with probabilities of 72% and 90%, respectively, and SP2 not cost-effective (cost-effectiveness probability<38%) (Fig. 3, Additional file 1: Table S5). In this scenario with 1-year duration of screening, the iNMB obtained from all scenarios did not differ [Mann–Whitney U test, p>0.5]. When screening started late during a surge (Fig. 2A4, A5), neither RA testing programs were cost-effective at the WTP threshold (Fig. 3), generating negative iNMB compared to the baseline of TOSC (Table 3).
Cost-effectiveness of testing programs (increased booster coverage)
Increasing booster vaccination coverage among working adults aged 18 to 65 years improved the iNMB of screening programs (Table 3). For a 16-week duration of screening initiated early in a surge within the exponential growth of cases, the per capita iNMB generated by SP1 and SP2 were statistically not different [Kruskal-Wallis test, p>0.17], but both were different from SP3 [Kruskal-Wallis test, p<0.001]. However, when the screening programs were extended to 32 weeks or 1 year, SP2 generated a greater per capita iNMB [Kruskal-Wallis test, p<0.001] than SP1 or SP3. Compared to TOSC, the RA screening programs started late during a surge (Fig. 2B4, B5) were not cost-effective and generated negative iNMB (Table 3).
Increasing booster coverage of working adults by 80% over the status quo resulted in qualitatively similar outcomes (Table 3). However, SP1 and SP2 performed equivalently with similar iNMB [Kruskal-Wallis test, p<0.001], but higher than iNMB generated by SP3 for all programs that started early in the surge (Table 3). Neither screening programs were cost-effective and generated negative iNMB when started late during the outbreak. Our results remained qualitatively intact when 100% of workplaces with 50+ employees participated in the screening programs (Additional file 1).
Simulating scenarios with BD Veritor and Sofia RA tests, we found qualitatively similar trends in the cost-effectiveness of the screening program and the iNMB generated by SP1, SP2, and SP3 (Additional file 1: Tables S8-S11). Specifically, screening programs with early start during the exponential growth of a surge were cost-effective, and increasing booster coverage of vaccination improved their iNMB. However, iNMB achieved in each specific screening program varied by the type of RA test, indicating the influence of the test sensitivity on monetary benefits. Cost-effectiveness analyses of the RA screening programs using only direct costs of healthcare and testing (excluding indirect costs) revealed similar outcomes, with a greater iNMB obtained by SP1 than SP2 or SP3 in all simulated scenarios under the same booster vaccination coverage (Additional file 1: Tables S12-S17).
Discussion
In this study, we evaluated the cost-effectiveness of workplace screening post-Omicron wave of BA.1 variant. We found that increasing booster coverage of working adults improved outcomes and therefore higher net monetary benefits of the screening program would be expected under a higher coverage of booster doses. In addition to booster vaccination, the timing for the start of RA screening during a surge and the duration of the program can have a large impact on the cost-effectiveness of the testing strategy. Delaying the start of RA screening until after the exponential growth or around the peak of a surge would not be a cost-effective strategy. Overall, an RA screening program without a confirmatory test may be a preferred strategy.
Although cost-effectiveness analysis is vital to policy decision-making regarding testing strategies, determining an optimal RA screening program is a challenging task [74, 75]. Previous research on RA testing within schools has highlighted how the optimal testing strategy is dependent on the specific objectives and their associated tradeoffs [76]. These tradeoffs arise from the interplay between testing and incidence: more frequent testing reduces the extent of transmission in the community from the identified cases, which may then justify less frequent testing. Furthermore, the evolving nature of the pandemic may change the cost-effectiveness of workplace-screening programs, including the immune-evasiveness and transmissibility of the virus, levels of vaccine-elicited protection, and durability of immunity against reinfection [77, 78].
Our findings rest on a number of simplifying assumptions in the model. First, we assumed that all individuals who test positive self-isolate for 5 days (SP1) or at least 1 day (SP2, SP3) if the confirmatory RT-PCR or RA test was negative. The implication of this assumption is that their daily contacts, both in and outside the workplace, are substantially reduced. For the screening program with a confirmatory RT-PCR test (SP2), we assumed only a 1-day turnaround time for the results without delay in sample collection from the time of the first positive RA test. However, additional delay in sample collection and results could alter our results further in favor of only RA tests for asymptomatic screening. For the scenarios evaluated here, we considered a frequency of two RA tests per week, as recommended by the stakeholders and consensus among participants in the Health Canada workshop held on January 31, 2022. However, the frequency of testing may vary among different workplaces [79] and could affect the cost-effectiveness results.
Our analysis is based on temporal sensitivity of Abbot-Panbio, BD Veritor, and Sofia rapid antigen tests; however, there are several RA tests currently being used with similar sensitivity and specificity estimates [2]. We calibrated the transmission parameter in the model to the estimated reproduction number in April 2022 [25], which implicitly accounted for the effect of non-pharmaceutical interventions. This transmissibility could change with time (e.g., seasonal effects) and other virus-specific characteristics. For example, with the same transmissibility, the iNMB achieved by implementing a screening program would be expected to decrease for a more severe variant that causes higher rates of hospitalization and/or death. Our analysis was restricted to the size and the proportion of workplaces participating in the screening program without consideration of their type or other attributes [79, 80] such demographics of the workforce, risk of exposure, and contact patterns (e.g., essential workplaces, healthcare facilities or other congregated settings) [7, 81,82,83,84,85]. In settings like hospitals or long-term care facilities, a screening program may consider additional components such as different frequency of RA testing for employees and patients, or screening of visitors. We considered a 10% naturally-acquired immunity at the initiation of the model based on incidence of disease as of April 2022 in Ontario; however, this level is unlikely to alter the qualitative aspect of the results due to a significantly higher level of immunity generated by the two-dose vaccination in primary series.
Conclusions
Our findings provide important insights which can inform testing strategies. The modeling outcomes suggest that RA testing of asymptomatic infection could provide substantial economic benefits, especially when the capacity for RT-PCR testing with rapid turnaround times is limited. Coverage of booster vaccination among working adults remains an important consideration in the implementation of RA screening. As the booster coverage increases, greater monetary benefits may be achieved by an RA workplace-screening program; however, depending on the starting time of screening during a surge, such benefits may be comparable or even lower than those accrued by testing only severe cases.
Availability of data and materials
Details of the model are provided in Additional file 1. Computational model for this simulation study is freely available at: https://github.com/thomasvilches/testing_COVID.
Abbreviations
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- RA:
-
Rapid antigen
- SP:
-
Screening program
- TOSC:
-
Testing of only severe symptomatic cases
- ICER:
-
Incremental cost-effectiveness ratio
- QALY:
-
Quality-adjusted life year
- iNMB:
-
Incremental net monetary benefits
References
Wells CR, Pandey A, Fitzpatrick MC, Crystal WS, Singer BH, Moghadas SM, et al. Quarantine and testing strategies to ameliorate transmission due to travel during the COVID-19 pandemic: a modelling study. Lancet Reg Health - Eur. 2022;14:100304.
Wells CR, Pandey A, Moghadas SM, Singer BH, Krieger G, Heron RJL, et al. Comparative analyses of FDA EUA-approved rapid antigen tests and RT-PCR for COVID-19 quarantine and surveillance-based isolation. preprint. Public and Global Health; 2021.
Wells CR, Townsend JP, Pandey A, Moghadas SM, Krieger G, Singer B, et al. Optimal COVID-19 quarantine and testing strategies. Nat Commun. 2021;12:356.
Rosenberg ES, Holtgrave DR. Widespread and Frequent Testing is Essential to Controlling Coronavirus Disease 2019 (COVID-19) in the United States. Clin Infect Dis. 2021;73:e2918–20.
Neilan AM, Losina E, Bangs AC, Flanagan C, Panella C, Eskibozkurt GE, et al. Clinical Impact, Costs, and Cost-effectiveness of Expanded Severe Acute Respiratory Syndrome Coronavirus 2 Testing in Massachusetts. Clin Infect Dis. 2021;73:e2908–17.
Wells CR, Pandey A, Gokcebel S, Krieger G, Donoghue AM, Singer BH, et al. Quarantine and serial testing for variants of SARS-CoV-2 with benefits of vaccination and boosting on consequent control of COVID-19. PNAS Nexus. 2022;1(3):pgac100. https://doi.org/10.1093/pnasnexus/pgac100.
Connor BA, Rogova M, Garcia J, Patel D, Couto-Rodriguez M, Nagy-Szakal D, et al. Comparative Effectiveness of Single vs Repeated Rapid SARS-CoV-2 Antigen Testing Among Asymptomatic Individuals in a Workplace Setting. JAMA Netw Open. 2022;5:e223073.
Chu VT, Schwartz NG, Donnelly MAP, Chuey MR, Soto R, Yousaf AR, et al. Comparison of Home Antigen Testing With RT-PCR and Viral Culture During the Course of SARS-CoV-2 Infection. JAMA Intern Med. 2022. https://doi.org/10.1001/jamainternmed.2022.1827.
Pavelka M, Van-Zandvoort K, Abbott S, Sherratt K, Majdan M, CMMID COVID-19 working group, et al. The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia. Science. 2021;372:635–41.
Rosella LC, Agrawal A, Gans J, Goldfarb A, Sennik S, Stein J. Large-scale implementation of rapid antigen testing system for COVID-19 in workplaces. Sci Adv. 2022;8:eabm3608.
Humphreys DP, Gavin KM, Olds KM, Bonaca MP, Bauer TA. At-home sample collection is an effective strategy for diagnosis and management of symptomatic and asymptomatic SARS-CoV-2 carriers. BMC Infect Dis. 2022;22:443.
Homza M, Zelena H, Janosek J, Tomaskova H, Jezo E, Kloudova A, et al. Performance of Seven SARS-CoV-2 Self-Tests Based on Saliva, Anterior Nasal and Nasopharyngeal Swabs Corrected for Infectiousness in Real-Life Conditions: A Cross-Sectional Test Accuracy Study. Diagnostics. 2021;11:1567.
Cocchio S, Nicoletti M, De Siena FP, Lattavo G, Furlan P, Fonzo M, et al. Prevalence of Asymptomatic SARS-CoV-2 Infection in the General Population of the Veneto Region: Results of a Screening Campaign with Third-Generation Rapid Antigen Tests in the Pre-Vaccine Era. Int J Environ Res Public Health. 2021;18:10838.
Allan-Blitz L-T, Klausner JD. A Real-World Comparison of SARS-CoV-2 Rapid Antigen Testing versus PCR Testing in Florida. J Clin Microbiol. 2021;59:e01107–21.
Fernandez-Montero A, Argemi J, Rodríguez JA, Ariño AH, Moreno-Galarraga L. Validation of a rapid antigen test as a screening tool for SARS-CoV-2 infection in asymptomatic populations. Sensitivity, specificity and predictive values. eClinicalMedicine. 2021;37:100954.
McKay SL, Tobolowsky FA, Moritz ED, Hatfield KM, Bhatnagar A, LaVoie SP, et al. Performance Evaluation of Serial SARS-CoV-2 Rapid Antigen Testing During a Nursing Home Outbreak. Ann Intern Med. 2021;174:945–51.
Wells CR, Pandey A, Moghadas SM, Singer BH, Krieger G, Heron RJL, et al. Comparative analyses of eighteen rapid antigen tests and RT-PCR for COVID-19 quarantine and surveillance-based isolation. Commun Med. 2022;2:84.
Larremore DB, Wilder B, Lester E, Shehata S, Burke JM, Hay JA, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7:eabd5393.
Vilches TN, Abdollahi E, Cipriano LE, Haworth-Brockman M, Keynan Y, Sheffield H, et al. Impact of non-pharmaceutical interventions and vaccination on COVID-19 outbreaks in Nunavut, Canada: a Canadian Immunization Research Network (CIRN) study. BMC Public Health. 2022;22:1042.
Shoukat A, Vilches TN, Moghadas SM, Sah P, Schneider EC, Shaff J, et al. Lives saved and hospitalizations averted by COVID-19 vaccination in New York City: a modeling study. Lancet Reg Health - Am. 2022;5:100085.
Vilches TN, Moghadas SM, Sah P, Fitzpatrick MC, Shoukat A, Pandey A, et al. Estimating COVID-19 Infections, Hospitalizations, and Deaths Following the US Vaccination Campaigns During the Pandemic. JAMA Netw Open. 2022;5:e2142725.
Statistics Canada. Population estimates on July 1st, by age and sex. https://doi.org/10.25318/1710000501-eng. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501. Accessed 5 June 2022.
Drolet M, Godbout A, Mondor M, Béraud G, Drolet-Roy L, Lemieux-Mellouki P, et al. Time trends in social contacts before and during the COVID-19 pandemic: the CONNECT study. BMC Public Health. 2022;22:1032.
Statistics Canada. Canadian Business Counts, with employees, June 2021. 2021.
National and Subnational estimates for Canada. 2022. https://epiforecasts.io/covid/posts/national/canada/. Accessed 5 June 2022.
Backer JA, Eggink D, Andeweg SP, Veldhuijzen IK, van Maarseveen N, Vermaas K, et al. Shorter serial intervals in SARS-CoV-2 cases with Omicron BA.1 variant compared with Delta variant, the Netherlands, 13 to 26 December 2021. Eurosurveillance. 2022;27(6):2200042.
Jansen L, Tegomoh B, Lange K, Showalter K, Figliomeni J, Abdalhamid B, et al. Investigation of a SARS-CoV-2 B.1.1.529 (Omicron) Variant Cluster — Nebraska, November–December 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1782–4.
Sah P, Fitzpatrick MC, Zimmer CF, Abdollahi E, Juden-Kelly L, Moghadas SM, et al. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc Natl Acad Sci. 2021;118:e2109229118.
Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020;368:489–93.
Moghadas SM, Fitzpatrick MC, Sah P, Pandey A, Shoukat A, Singer BH, et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci. 2020;117:17513–5.
Gatto M, Bertuzzo E, Mari L, Miccoli S, Carraro L, Casagrandi R, et al. Spread and dynamics of the COVID-19 epidemic in Italy: Effects of emergency containment measures. Proc Natl Acad Sci. 2020;117:10484–91.
He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Author Correction: Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:1491–3.
Ferretti L, Wymant C, Kendall M, Zhao L, Nurtay A, Abeler-Dörner L, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368:eabb6936.
Sayampanathan AA, Heng CS, Pin PH, Pang J, Leong TY, Lee VJ. Infectivity of asymptomatic versus symptomatic COVID-19. Lancet. 2021;397:93–4.
Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared With Previous Waves. JAMA. 2022;327:583.
UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England, Technical briefing: Update on hospitalisation and vaccine effectiveness for Omicron VOC-21NOV-01 (B.1.1.529). 2021.
León TM, Dorabawila V, Nelson L, Lutterloh E, Bauer UE, Backenson B, et al. COVID-19 Cases and Hospitalizations by COVID-19 Vaccination Status and Previous COVID-19 Diagnosis — California and New York, May–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:125–31.
Andeweg SP, de Gier B, Eggink D, van den Ende C, van Maarseveen N, Ali L, et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1 and Delta SARS-CoV-2 infections, the Netherlands, 22 November 2021- 19 January 2022. preprint. Epidemiology; 2022.
Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance — VISION Network, 10 States, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255–63.
Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022;28:1063–71.
Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, et al. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance — VISION Network, 10 States, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:139–45.
Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375:e068848.
Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375:331–6.
Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–16.
Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N Engl J Med. 2021;385:e83.
Wright BJ, Tideman S, Diaz GA, French T, Parsons GT, Robicsek A. Comparative vaccine effectiveness against severe COVID-19 over time in US hospital administrative data: a case-control study. Lancet Respir Med. 2022;10:557–65.
Vilches TN, Sah P, Moghadas SM, Shoukat A, Fitzpatrick MC, Hotez PJ, et al. COVID-19 hospitalizations and deaths averted under an accelerated vaccination program in northeastern and southern regions of the USA. Lancet Reg Health - Am. 2022;6:100147.
Government of Canada. COVID-19 epidemiology update. 2022.
Government of Canada. COVID-19 vaccination in Canada. 2022. https://health-infobase.canada.ca/covid-19/vaccination-coverage/.
Government of Ontario. COVID-19 vaccinations data. 2022. https://covid-19.ontario.ca/data.
The SAFER Investigators and Field Study Team, The Crick COVID-19 Consortium, CMMID COVID-19 working group, Hellewell J, Russell TW, Beale R, et al. Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections. BMC Med. 2021;19:106.
Ferretti L, Ledda A, Wymant C, Zhao L, Ledda V, Abeler-Dörner L, et al. The timing of COVID-19 transmission. preprint. Epidemiology; 2020.
Ashcroft P, Lehtinen S, Angst DC, Low N, Bonhoeffer S. Quantifying the impact of quarantine duration on COVID-19 transmission. eLife. 2021;10:e63704.
Gremmels H, Winkel BMF, Schuurman R, Rosingh A, Rigter NAM, Rodriguez O, et al. Real-life validation of the PanbioTM COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine. 2021;31:100677.
Linares M, Pérez-Tanoira R, Carrero A, Romanyk J, Pérez-García F, Gómez-Herruz P, et al. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J Clin Virol. 2020;133:104659.
Young S, Taylor SN, Cammarata CL, Varnado KG, Roger-Dalbert C, Montano A, et al. Clinical Evaluation of BD Veritor SARS-CoV-2 Point-of-Care Test Performance Compared to PCR-Based Testing and versus the Sofia 2 SARS Antigen Point-of-Care Test. J Clin Microbiol. 2020;59:e02338–20.
Pray IW, Ford L, Cole D, Lee C, Bigouette JP, Abedi GR, et al. Performance of an Antigen-Based Test for Asymptomatic and Symptomatic SARS-CoV-2 Testing at Two University Campuses — Wisconsin, September–October 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1642–7.
Papenburg J, Campbell JR, Caya C, Dion C, Corsini R, Cheng MP, et al. Adequacy of Serial Self-performed SARS-CoV-2 Rapid Antigen Detection Testing for Longitudinal Mass Screening in the Workplace. JAMA Netw Open. 2022;5:e2210559.
Campbell JR, Uppal A, Oxlade O, Fregonese F, Bastos ML, Lan Z, et al. Active testing of groups at increased risk of acquiring SARS-CoV-2 in Canada: costs and human resource needs. Can Med Assoc J. 2020;192:E1146–55.
Canadian Institute for Health Information. Hospital spending: Focus on the emergency department. 2020.
Thommes EW, Kruse M, Kohli M, Sharma R, Noorduyn SG. Review of seasonal influenza in Canada: Burden of disease and the cost-effectiveness of quadrivalent inactivated influenza vaccines. Hum Vaccin Immunother. 2017;13:867–76.
Ontario Ministry of Health. Schedule of Benefits: Physician Services Under the Health Insurance Act. 2022.
Canadian Institute for Health Information. COVID-19 hospitalization and emergency department statistics. 2022. https://www.cihi.ca/en/covid-19-hospitalization-and-emergency-department-statistics. Accessed 15 May 2022.
Mulberry N, Tupper P, Kirwin E, McCabe C, Colijn C. Vaccine rollout strategies: The case for vaccinating essential workers early. PLOS Glob Public Health. 2021;1:e0000020.
Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med. 2021;174:576–8.
Government of Canada. Testing for COVID-19: When to get tested and testing results. 2022. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/symptoms/testing/diagnosing.html.
Statistics Canada. Income of individuals by age group, sex and income source, Canada, provinces and selected census metropolitan areas. 2022. https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1110023901.
Statistics Canada. Labour force characteristics by province, monthly, seasonally adjusted. 2022. https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1410028703.
Basu A, Gandhay VJ. Quality-Adjusted Life-Year Losses Averted With Every COVID-19 Infection Prevented in the United States. Value Health. 2021;24:632–40.
Kirwin E, Rafferty E, Harback K, Round J, McCabe C. A Net Benefit Approach for the Optimal Allocation of a COVID-19 Vaccine. PharmacoEconomics. 2021;39:1059–73.
Xie F, Pullenayegum E, Gaebel K, Bansback N, Bryan S, Ohinmaa A, et al. A Time Trade-off-derived Value Set of the EQ-5D-5L for Canada. Med Care. 2016;54:98–105.
Statistics Canada. Consumer Price Index, annual average, not seasonally adjusted. https://doi.org/10.25318/1810000501-eng. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501. Accessed 23 May 2022.
Ochalek J, Lomas J, Claxton K. Assessing health opportunity costs for the Canadian health care systems; 2018.
Du Z, Wang L, Bai Y, Wang X, Pandey A, Fitzpatrick MC, et al. Cost-effective proactive testing strategies during COVID-19 mass vaccination: A modelling study. Lancet Reg Health - Am. 2022;8:100182.
Du Z, Pandey A, Bai Y, Fitzpatrick MC, Chinazzi M, Pastore y Piontti A, et al. Comparative cost-effectiveness of SARS-CoV-2 testing strategies in the USA: a modelling study. Lancet. Public Health. 2021;6:e184–91.
Leng T, Hill EM, Thompson RN, Tildesley MJ, Keeling MJ, Dyson L. Assessing the impact of lateral flow testing strategies on within-school SARS-CoV-2 transmission and absences: A modelling study. PLoS Comput Biol. 2022;18:e1010158.
Amanatidou E, Gkiouliava A, Pella E, Serafidi M, Tsilingiris D, Vallianou NG, et al. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metab Open. 2022;14:100180.
Zheutlin A, Ott M, Sun R, Zemlianskaia N, Rubel M, Hayden J, et al. Durability of Protection against COVID-19 Breakthrough Infections and Severe Disease by Vaccines in the United States. preprint. Epidemiology; 2022.
Statistics Canada. Frequency on-site employees were tested using COVID-19 Rapid Test kits over the last month, third quarter of 2021. 2021.
Statistics Canada. Business or organization plans to start using COVID-19 Rapid Test kits to test on-site employees for COVID-19 infection in the next three months, third quarter of 2021. 2021.
Najafi M, Laskowski M, de Boer PT, Williams E, Chit A, Moghadas SM. The Effect of Individual Movements and Interventions on the Spread of Influenza in Long-Term Care Facilities. Med Decis Making. 2017;37:871–81.
Champredon D, Najafi M, Laskowski M, Chit A, Moghadas SM, 1 Agent-Based Modelling Laboratory, York University, Toronto, ON M3J 1P3, Canada, et al. Individual movements and contact patterns in a Canadian long-term care facility. AIMS Public Health. 2018;5:111–21.
Vilches TN, Nourbakhsh S, Zhang K, Juden-Kelly L, Cipriano LE, Langley JM, et al. Multifaceted strategies for the control of COVID-19 outbreaks in long-term care facilities in Ontario, Canada. Prev Med. 2021;148:106564.
Centers for Disease Control and Prevention. Interim Guidance for SARS-CoV-2 Testing in Non-Healthcare Workplaces. 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/testing-non-healthcare-workplaces.html#anchor_1615914276994.
Campbell JR, Dion C, Uppal A, Yansouni CP, Menzies D. Systematic on-site testing for SARS-CoV-2 infection among asymptomatic essential workers in Montréal, Canada: a prospective observational and cost-assessment study. CMAJ Open. 2022;10:E409–19.
Acknowledgements
Not applicable.
Funding
This work was commissioned and supported by Health Canada. SMM acknowledges the Canadian Institutes of Health Research [OV4 – 170643, COVID-19 Rapid Research] and the Natural Sciences and Engineering Research Council of Canada, Emerging Infectious Disease Modelling, MfPH grant.
Author information
Authors and Affiliations
Contributions
ER, APG, and SMM contributed to the conception and design of the work. TNV, ER, CRW, and SMM contributed to the development of the computational model, simulations, and acquisition, analysis, and interpretation of data. All authors contributed to drafting and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Details of the model with its parameterization and additional results.
Additional file 2.
Details of costs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vilches, T.N., Rafferty, E., Wells, C.R. et al. Economic evaluation of COVID-19 rapid antigen screening programs in the workplace. BMC Med 20, 452 (2022). https://doi.org/10.1186/s12916-022-02641-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02641-5