Abstract
Background
Multimorbidity (the presence of two or more chronic conditions) is common amongst people with chronic kidney disease, but it is unclear which conditions cluster together and if this changes as kidney function declines. We explored which clusters of conditions are associated with different estimated glomerular filtration rates (eGFRs) and studied associations between these clusters and adverse outcomes.
Methods
Two population-based cohort studies were used: the Stockholm Creatinine Measurements project (SCREAM, Sweden, 2006–2018) and the Secure Anonymised Information Linkage Databank (SAIL, Wales, 2006–2021). We studied participants in SCREAM (404,681 adults) and SAIL (533,362) whose eGFR declined lower than thresholds (90, 75, 60, 45, 30 and 15 mL/min/1.73m2). Clusters based on 27 chronic conditions were identified. We described the most common chronic condition(s) in each cluster and studied their association with adverse outcomes using Cox proportional hazards models (all-cause mortality (ACM) and major adverse cardiovascular events (MACE)).
Results
Chronic conditions became more common and clustered differently across lower eGFR categories. At eGFR 90, 75, and 60 mL/min/1.73m2, most participants were in large clusters with no prominent conditions. At eGFR 15 and 30 mL/min/1.73m2, clusters involving cardiovascular conditions were larger and were at the highest risk of adverse outcomes. At eGFR 30 mL/min/1.73m2, in the heart failure, peripheral vascular disease and diabetes cluster in SCREAM, ACM hazard ratio (HR) is 2.66 (95% confidence interval (CI) 2.31–3.07) and MACE HR is 4.18 (CI 3.65–4.78); in the heart failure and atrial fibrillation cluster in SAIL, ACM HR is 2.23 (CI 2.04 to 2.44) and MACE HR is 3.43 (CI 3.22–3.64). Chronic pain and depression were common and associated with adverse outcomes when combined with physical conditions. At eGFR 30 mL/min/1.73m2, in the chronic pain, heart failure and myocardial infarction cluster in SCREAM, ACM HR is 2.00 (CI 1.62–2.46) and MACE HR is 4.09 (CI 3.39–4.93); in the depression, chronic pain and stroke cluster in SAIL, ACM HR is 1.38 (CI 1.18–1.61) and MACE HR is 1.58 (CI 1.42–1.76).
Conclusions
Patterns of multimorbidity and corresponding risk of adverse outcomes varied with declining eGFR. While diabetes and cardiovascular disease are known high-risk conditions, chronic pain and depression emerged as important conditions and associated with adverse outcomes when combined with physical conditions.
Similar content being viewed by others
Background
As the world’s populationlives longer, an increasing number of people are living with multiple chronic conditions (multimorbidity) [1]. These people suffer from high treatment burden as they often must cope with numerous medications and attend multiple specialists [2]. Multimorbidity is a leading challenge facing twenty-first-century medicine, and the optimal management of people with several complex medical conditions is yet to be established [3, 4].
Chronic kidney disease (CKD), defined as a persistent and irreversible degradation of kidney function, affects around 10% of the world’s population [5, 6]. Its multifactorial nature, progressive trajectory which is often associated with complications, and the development of cardiometabolic conditions mean that CKD is usually linked to multimorbidity. The care of people with CKD has been reported to be more complex than that of patients attending any other specialist [7] and they are disproportionately susceptible to adverse outcomes such as hospitalisation [8] and cardiovascular events [9]. Research into people with multiple chronic conditions has primarily focused on the number of conditions, and there has been less focus on clusters of conditions, particularly amongst people with CKD. Identifying clusters of conditions may help to improve the management of these people by informing preventative strategies and targeting treatments [10]. Some conditions may cluster together in clinically meaningful ways, such as if cluster membership tells us about common risk factors or if it helps stratify the risk of subsequent adverse events [11].
How multimorbidity changes with declining kidney function and how this contributes to adverse outcomes are not known. Clustering techniques can be used to uncover unknown patterns within data and are used in this study to identify clusters of conditions in two geographically distinct population-based cohorts. We identified these clusters in people at different levels of kidney function (including estimated glomerular filtration rate (eGFR) >60 mL/min/1.73m2) and studied the associated risk of mortality and major adverse cardiovascular events (MACE).
Methods
Study populations
We used two databases with anonymised health and administrative data: the Stockholm Creatinine Measurements project (SCREAM) covers the entire region of Stockholm, Sweden (approximately 2.9 million people during the study period) [12], and the Secure Anonymised Information Linkage Databank (SAIL) covers 79% of the population of Wales (approximately 3.4 million people during the study period) [13]. In both cohorts, primary care, secondary care, prescribing and mortality data were linked. We included adults with outpatient serum/plasma creatinine values after 1 January 2006. Calibrated laboratory analysers for creatinine were used in SCREAM; in SAIL, non-calibrated analysers may have been used and so creatinine values were multiplied by 0.95 to account for possible lack of calibration [14]. Participants were lost to follow-up if they permanently left the region for SCREAM or if they left a participating GP practice or the country for SAIL. Participants were followed up until 31 December 2018 in SCREAM and 1 June 2021 in SAIL.
Selection of patients and kidney function thresholds
eGFR was calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine equation, but without considering the race coefficient [15]. We studied all adults with at least two eGFR values whose eGFR crossed one or more threshold during follow-up: 90, 75, 60, 45, 30 and 15 mL/min/1.73m2. All eGFR values were used to fit a linear mixed effects model, and this procedure is described in more detail in Additional file 1. By estimating the dates at which participants crossed these thresholds, we could define study covariates at these dates and outcomes thereafter. Participants could cross more than one eGFR threshold and could therefore be included in more than one eGFR category for subsequent analysis. Flow charts of included individuals are depicted in Additional file 1: Figs. S1A and S1B.
Chronic conditions
For each participant, and at each eGFR threshold, we evaluated the presence of 27 different chronic conditions. In SCREAM, ICD-10 codes recorded in primary and secondary care records were used. In SAIL, ICD-10 codes were used for secondary care records with separate primary care read codes used, as previously described [8]. These conditions were ascertained using a validated algorithm [16] with some modifications: we excluded CKD as it was our exposure, and we used a single cancer definition (excluding non-melanoma skin cancer), combining lymphoma, metastatic cancer and non-metastatic cancer. Conditions were defined for each participant at the estimated date of crossing eGFR thresholds and time windows were applied as per the algorithm in use [16]. The cause of CKD was not incorporated as it is rarely possible to determine this from population-level data. As depression and chronic pain are poorly recorded in healthcare records, we enriched the definitions of these conditions with prescription data, as previously described [1, 8]. In brief, a participant was assigned to have depression if they had four or more antidepressant prescriptions within a year and chronic pain if they had four or more prescriptions for painkillers within a year (including antiepileptic medications such as gabapentin, so long as the participant did not have epilepsy).
Outcomes
After identifying clusters of conditions, we studied associations between cluster membership and subsequent adverse outcomes. Outcomes were identified from death and secondary care records: all-cause mortality, MACE (myocardial infarction, stroke or cardiovascular death, denoted as MACE3) and MACE plus heart failure hospitalisation (denoted as MACE4). Relevant ICD-10 codes are available in Additional file 1: Table S1. To capture as many events as possible in both cohorts, the secondary care records used were from hospitals in Sweden and Wales that provide universal coverage.
Statistical analysis
Baseline characteristics including the prevalence of chronic conditions were compared between participants in each eGFR category. Categorical variables were expressed as frequencies with percentages and continuous variables as medians with interquartile intervals (IQI). We compared the participants with available eGFRs who were included in the analysis to those with available eGFRs not included in terms of their birth dates, sex and number of eGFR measurements.
We applied a k-modes algorithm within each eGFR category to identify clusters of conditions [17]. This clustering technique identifies clusters of participants with similar combinations of covariates, in our case the 27 chronic conditions, maximising homogeneity within clusters and heterogeneity between clusters. We chose to use this algorithm as it can perform clustering with categorical data and is computationally efficient (given our large sample sizes). We ran the algorithm for two to 10 possible clusters, as we deemed a larger number of clusters not clinically useful. We allowed for a maximum of 20 iterations of the algorithm. The optimal number of clusters was selected using the elbow method, to minimise the within-cluster distance while selecting a parsimonious number of clusters [18]. We plotted gradients of the elbow plots to ease the choice, as gradients approach zero when the elbow plots flatten. The k-modes algorithm was repeated with participants stratified by age (< and ≥65 years).
The prevalence of chronic conditions in each cluster was compared to their prevalence in the overall eGFR category. Observed/expected (O/E) ratios were calculated by dividing condition prevalence in a cluster by the prevalence in each eGFR category. Prominent conditions for each cluster were identified as conditions which were common (≥20% prevalence) and more common than the overall eGFR category (O/E ratio ≥2) [19]. To prevent cluster descriptions becoming protracted, a maximum of three prominent conditions were selected as the defining condition(s) for each cluster, with the most prevalent conditions used if more than three were identified. To help compare the prominent conditions, the clusters were further categorised using the single most prevalent condition in each cluster, using that condition’s body system: cancer, cardiovascular, dermatological, endocrine, gastrointestinal, mental health and pain, neurological, respiratory, rheumatological and non-specific. We then compared the proportion of participants in clusters in each eGFR category.
Cluster allocation for all participants was fully determined prior to analysing the outcome data. We calculated crude rates of incident adverse events per cluster and expressed them per 1000 person-years at risk. Then, relationships between cluster membership and outcomes were assessed using Cox proportional hazard models, adjusting for age and sex. For the MACE analyses, participants were censored on the date of death. The reference groups were participants in clusters with no prominent conditions (based on prevalence). If there was more than one cluster with no prominent condition, the cluster with the highest number of participants was selected as the reference group. For each model, we tested the statistical significance of the clustering variable using Wald tests and produced standardised survival curves (using regression standardisation [20]) to quantify absolute risks for each cluster at each eGFR level considered in the study. We assessed the prediction of outcomes via internal validation of our models using time-varying area under the receiver operating characteristic curve (AUC) and Brier scores over the duration of follow-up; non-parametric bootstrap with 100 resamples was used to calculate standard errors for each metric. Models with age and sex only were compared to models which added cluster membership and models which added the number of chronic conditions.
Statistical analyses were conducted using R version 4.0.5 or later [21] with the tidyverse, nephro, lme4, SCREAM, klaR, glue, formattable, survival, broom, aod, ggalluvial, matrixStats, ggrepel, stdReg, ggtext, hrbrthemes, knitr, patchwork, readxl, riskRegression and cowplot packages. Code is available on Github for others to replicate our analysis: https://github.com/ellessenne/multimorbidity-ckd-clustering.
Results
Baseline characteristics
The SCREAM cohort consisted of 404,681 unique participants (53.5% women). The median age was lowest in the eGFR 90 category (58.8 years, IQI: 49.3–66.2) and highest in the eGFR 30 category (82.5 years, IQI: 73.7–88.4) (Table 1). The SAIL cohort consisted of 533,362 unique participants (55.3% women). The median age was lowest in the eGFR 90 category (55.5 years, IQI: 46.6–63.5) and highest in the eGFR 30 category (80.8 years, IQI: 73.1–86.5) (Table 2). Comparing both tables shows that SAIL participants in this study have a higher multimorbidity count when compared to their Swedish counterparts.
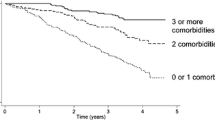
Participants in the low eGFR categories had the highest number of chronic conditions, particularly in those aged over 65 years (Fig. 1). Participants included in the analysis tended to be born at earlier dates and have more eGFR measurements than those excluded (Additional file 1: Figs. S2A and S2B). The proportions of females and males included were similar, except at eGFR 15mL/min/1.73m2, where proportionally fewer women were included.
Prevalence of chronic conditions by eGFR
The prevalence of most chronic conditions increased in lower eGFR categories (Additional file 1: Fig. S3). For example, in SAIL, the prevalence of cancer at eGFR 90 was 9.3% and at eGFR 15 25.7%.
The most frequently recorded chronic condition in SCREAM was hypertension, which ranged from 20.6% in the eGFR 90 group to 78.1% in the eGFR 15 group. Analogously, chronic pain ranged from 29.7% in the eGFR 90 group to 43.2% in the eGFR 15 group; diabetes ranged from 8.4 to 37.8% for eGFR 90 and 15; and heart failure ranged from 1.6 to 27.8% for eGFR 90 and 15.
The most frequently recorded chronic condition in SAIL was also hypertension, which ranged from 34.4 to 86.1% for eGFR 90 and 15, respectively. Analogously, chronic pain ranged from 21.5% for eGFR 90 to 38.4% for eGFR 15; diabetes ranged from 17.5 to 53.4% for eGFR 90 and 15. The proportion of participants with depression ranged from 35.0% for eGFR 90 to 28.7% for eGFR 15.
The optimal number of clusters varied at each eGFR level. Elbow plots (Additional file 1: Fig. S4) and gradient plots (Additional file 1: Fig. S5) suggested that the model fitness stabilised in each eGFR level at between five and nine clusters, i.e. using more clusters did not significantly improve the goodness of fit. Overall, the optimal number of clusters was highest at eGFRs 15 and 30 mL/min/1.73m2.
Prevalence of conditions by cluster
Table S2 in Additional file 1 shows the prevalence of chronic conditions in each cluster, simplified graphically in heatmaps (Fig. 2). Hypertension, diabetes and chronic pain were common in many clusters.
Prominent conditions (based on prevalence)
Figure S6 in Additional file 1 depicts how prominent conditions were identified within each cluster. Although hypertension was the commonest condition in each eGFR category, it could not be a prominent condition in most of the clusters because the background prevalence was >50% and the O/E ratio therefore could not be ≥2. Tables 3 and 4 summarise the number of participants and the prominent condition(s) in each cluster. Some clusters in the same eGFR category share the same description, but are distinct because there are differences between the conditions separate to the “prominent” conditions. For example, at eGFR 15 in SAIL, there were two “Cancer” clusters, but in one cluster all participants had diabetes and in the other no participants had diabetes. Figure 3 shows the proportion of participants in each cluster by eGFR category. In both cohorts, most participants were included in one or two clusters with no system-specific prominent conditions, i.e. there was either no prominent condition or hypertension was the most prominent condition. The cluster-wise proportion of participants with no prominent condition, however, decreased as kidney function declined. As expected, diabetes and cardiovascular conditions featured more prominently as eGFR worsened. Chronic pain and, in SAIL, depression featured in clusters across the spectrum of kidney function.
When clustering was stratified by age, proportionally more participants aged over 65 years were in clusters with prominent conditions (based on prevalence) compared to those under 65 years (Additional file 1: Figs. S7A and S7B). Clusters which featured heart failure and myocardial infarction existed at eGFRs 30 and 45 in both cohorts and in all age groups, but these were proportionally larger in those over the age of 65 than under 65. In SAIL, cancer featured in more clusters in those over the age of 65 than under 65.
Outcomes
In SCREAM, the median follow-up time ranged from 1.94 years (IQI 1.87–2.03) at eGFR 15 to 6.32 years (IQI 6.30–6.34) at eGFR 90 (Additional file 1: Table S3A). In SAIL, the median follow-up time ranged from 5.51 years (IQI 5.32–5.68) at eGFR 15 to 7.45 years (IQI 7.42–7.47) at eGFR 90 (Additional file 1: Table S3B). In both cohorts, crude event rates were higher at lower eGFR categories compared to higher eGFR (Additional file 1: Fig. S8). Event rates were lowest in clusters with no prominent condition.
Clustering membership was significantly associated with event rates for each outcome (Wald test p-values < 0.001 in all eGFR categories, adjusted for age and sex). This was reflected in the standardised survival curves at every eGFR level (Additional file 1: Fig. S9). Finally, the predictive performance (of predicting adverse outcomes) of cluster membership information was, overall, similar to that of using the number of conditions (AUCs displayed in Additional file 1: Fig. S10 and Brier scores in Additional file 1: Fig. S11).
The relative rates of all-cause mortality and MACE were highest in the clusters with cardiometabolic prominent conditions (Additional file 1: Fig. S12, Additional file 1: Table S4). Figure 4 features results from low (30) and high (90) eGFR categories. In SAIL at eGFR 30, cluster 5 (heart failure and atrial fibrillation) showed a hazard ratio (HR) for all-cause mortality of 2.23 (95% confidence interval (CI) 2.04–2.44) and for MACE HR 3.43 (CI 3.22–3.64).
Hazard ratios tended to be higher when cardiometabolic conditions were combined with chronic pain or depression. In SCREAM at eGFR 90, cluster 1 (hypertension and diabetes) showed an HR for all-cause mortality of 1.24 (CI 1.19–1.29) and for MACE HR 1.54 (CI 1.49–1.60). Also, at eGFR 90 in SCREAM, cluster 2 (in which chronic pain was prominent in addition to hypertension and diabetes), the HR for all-cause mortality was 3.87 (CI 3.51–4.27) and MACE 4.08 (CI 3.72–4.48).
However, when chronic pain or depression was the sole prominent condition, these clusters were either not at increased risk of adverse outcomes or the increased risk was minimal. For example, in SCREAM at eGFR 60, cluster 1 (chronic pain) all-cause mortality HR was 1.11 (CI 1.07–1.14) and MACE HR 1.14 (CI 1.11–1.17). In SAIL at eGFR 60, cluster 4 (depression) all-cause mortality HR was 0.97 (CI 0.89–1.05) and MACE HR 1.02 (CI 0.97–1.07).
Discussion
In two geographically distinct health systems, we report the following findings: (1) low eGFR is accompanied by increasing age and increasing prevalence of chronic conditions; (2) these chronic conditions often cluster, with differential patterns across the eGFR spectrum, and show strong associations with the risk of adverse outcomes; (3) clusters with cardiovascular conditions were more prominent at low eGFR; (4) chronic pain and depression were common and, when combined with physical conditions, were associated with adverse outcomes; (5) clustering information could predict the risk of adverse outcomes in a similar way to the number of chronic conditions, with the advantage of being more clinically relevant. Collectively, these findings illustrate the complexity of medical conditions for people with CKD and have practical implications for service delivery, by supporting a move away from healthcare for individual diseases towards the development of clinical guidelines for common clusters of conditions.
In both cohorts, there was a dichotomy between low-risk clusters with low rates of chronic conditions and high-risk clusters featuring cardiovascular conditions. This agrees with an analysis of people with CKD in the Chronic Renal Insufficiency Cohort Study which found one large cluster with relatively healthy individuals [22] and a longitudinal study which found that as clusters were compared over follow-up, cardiovascular conditions became prominent as the participants aged [23]. Our finding that cardiovascular clusters became more dominant at eGFR 30 and 15 mL/min/1.73m2 was not surprising, as people with CKD, diabetes and heart disease are a well-recognised group with consistently poor outcomes[24]. This group of patients may benefit from integrated clinics, where multiple specialties see patients together. For example, clinics with cardiology, nephrology and endocrinology have been found to be effective at optimising treatment (e.g. improving glycated haemoglobin levels and commencing sodium-glucose co-transporter-2 inhibitors) [25]. There is evidence that integrated clinics may help address some of the problems associated with attending hospital clinics, such as by reducing the number of appointments patients must attend and by improved continuity of care [26]. However, the impact on quality of life is less clear [27] and further work is required to determine if these models of care work well. As these clusters in our study were at heightened risk of adverse events, they may benefit from targeted evidence-based interventions such as statins [28], renin-angiotensin system inhibitors [29,30,31,32], sodium-glucose co-transporter-2-inhibitors [33, 34] and smoking cessation support [35].
Chronic pain was common in both cohorts, particularly at low eGFR, and identified in many clusters. This agrees with a recent systematic review reporting that chronic pain was common in people with CKD[36]. The systematic review reported a higher prevalence of chronic pain (48%) [36] compared to our cohorts, perhaps because estimates were based on clinical studies with assessment of pain scales, and our estimates may be affected by poor recognition of pain by health professionals. We in part used prescribing data to identify chronic pain and depression, and a reluctance amongst clinicians to prescribe nephrotoxic medication may have led some patients with chronic pain to go undetected. It also agrees with a clustering analysis of people with multimorbidity in England that found chronic pain to feature in 13 of 20 clusters and to be associated with frequent health service use [37]. We similarly found that adverse outcome rates were higher when chronic pain featured in clusters alongside physical conditions, but not on its own. Management of chronic pain is challenging, especially in people with CKD. Prescribers often avoid non-steroidal anti-inflammatory drugs because of nephrotoxic effects, and both these medications and opioids are associated with significant harm [38]. Previous studies of multimorbidity in people with CKD have not explored the importance of chronic pain [39, 40]. More research must therefore be done to understand why its prevalence in CKD is so high and what can be done to improve its management.
Depression featured in clusters in SAIL, often alongside physical conditions. Mental and physical conditions are known to occur together frequently, but treatment in these people can be challenging. Clusters in our study which featured depression in combination with physical conditions were associated with an increased risk of adverse outcomes, which is consistent with previous studies [41]. Depression in people with CKD is currently under-recognised and under-treated, and antidepressant medications do not work as well as when kidney function is normal [42]. In a systematic review of interventions for people with multimorbidity, those targeting depression were the most effective, particularly alterations to care delivery, such as nurses and psychologists setting goals with patients [43]. Interventions like these therefore warrant investigation in people with CKD and multimorbidity.
We found that clustering conditions did not significantly improve the prediction of outcomes over counting conditions. This is consistent with a study of over 8 million English people, which could not identify any clusters which could be targeted to reduce emergency hospitalisations [44]. However, our study was not aimed at developing a prediction model for the risk of adverse outcomes and metrics were only internally validated, thus limiting our conclusions with regard to the predictive ability of clusters versus condition counts. Rather than being incorporated into risk stratification, clusters of conditions may be more helpful in informing preventative measures and clinical guidelines. For example, public health measures might encourage healthy lifestyles to reduce the numbers of people in high-risk clusters, e.g. those with CKD, diabetes and heart disease. Clinical guidelines could be developed to help clinicians treat chronic pain amongst people with CKD and cardiometabolic conditions.
The strengths of this study are its state-of-the art methods and its unrivalled sample size in researching multimorbidity and CKD. Observing similarities across two distinct cohorts does increase generalisability, but we did not expect results to be identical given differences in the frequency of blood tests, lifestyles, genetic backgrounds and variation of timely diagnoses of conditions such as pulmonary disease or heart failure, which can be challenging especially in inactive patients. For example, respiratory conditions were more common in SAIL than in SCREAM, which is consistent with the high rates of these conditions in Wales compared to Sweden [45]. Our analyses were restricted to participants whose eGFR crossed thresholds, and future work should consider clustering analyses in other populations, e.g. people with stable kidney function and people on dialysis. We openly provide the statistical code that we used for this work and encourage other researchers to replicate this analysis in their settings. Given age and kidney function are closely linked [46], the conditions prominent in each eGFR strata will have been largely influenced by age. It is unclear to what extent changes in clusters as eGFR declines are explained by advancing age rather than being specific to changes in kidney function. There are inherent limitations of health records research in that they rely on routine coding, a subjective process that if incomplete can lead to misclassification of clusters identified. We tried to improve the sensitivity of our ascertainment of chronic conditions by using previously validated algorithms [16], supplemented in some cases with medication data. We chose to enrich the definitions of depression and chronic pain with prescribing data, which will have increased the prevalence of these conditions and contributed to them featuring clusters. Other conditions may have featured more prominently in the clusters if we had used prescribing data to define them, also. We studied patients across the range of eGFR, without considering proteinuria data. Many of the patients in the eGFR categories 75 and 90 were therefore unlikely to have CKD, and instead, their inclusion allowed us to study clusters of conditions in people with good kidney function. Some of the follow-up period in SAIL was during the COVID-19 pandemic, when blood tests and recording of chronic conditions may have been inconsistent. However, this was a small proportion of the follow-up period and results were, overall, similar to SCREAM. We used k-modes as the clustering method, whereas other studies have used alternative techniques such as hierarchical clustering [47], latent class analysis [37] and consensus clustering [22]. This may limit our capacity to compare findings across studies. Finally, we did not account for the severity of chronic conditions. Such information may have been useful, but with a heterogenous list of conditions, this would have been challenging to ascertain for each condition or to include in the analysis.
Conclusions
In summary, our study shows that there are clinically meaningful clusters of conditions which vary with declining kidney function. Cardiovascular conditions are prominent at low eGFR and associated with adverse outcomes, and hence, cardiovascular risk assessment and management should be included in the management of these patients. Importantly, chronic pain and depression are also common across the spectrum of kidney function but these conditions currently receive less attention or have fewer available treatment options in CKD. These data illustrate that CKD is not simply a biochemical ‘diagnosis’ but exists as part of the complex interactions between multiple chronic conditions. Identification and awareness of clusters of conditions may inform public health initiatives and permit health professionals to provide targeted interventions for patients with CKD.
Availability of data and materials
The data that support the findings of this study are available from SCREAM and SAIL, for collaborative research projects subject to successful application processes and fulfilment of ethics and GDPR regulations. Further details can be found by contacting juan.jesus.carrero@ki.se for SCREAM and at www.saildatabank.com for SAIL.
Abbreviations
- ACM:
-
All-cause mortality
- AUC:
-
Area under the receiver operating characteristic curve
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- eGFR:
-
Estimated glomerular filtration rate
- HR:
-
Hazard ratio
- IQI:
-
Interquartile interval
- MACE:
-
Major adverse cardiovascular events
- O/E:
-
Observed/expected
- SAIL:
-
Secure Anonymised Information Linkage Databank
- SCREAM:
-
Stockholm Creatinine Measurements project
References
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. The Lancet. 2012;380:37–43.
Mair FS, May CR. Thinking about the burden of treatment. BMJ : British Medical Journal. 2014;349: g6680.
Whitty CJM, MacEwen C, Goddard A, et al. Rising to the challenge of multimorbidity. BMJ. 2020;368: l6964.
Multimorbidity: a priority for global health research. Academy of Medical Sciences 2018. https://acmedsci.ac.uk/file-download/82222577.
Coresh J. Update on the burden of CKD. Journal of the American Society of Nephrology. 2017;28:1020.
Stevens PE, Levin A, Members* for the KDIGOCKDGDWG. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline Ann Intern Med 2013 158 825 30
Tonelli M, Wiebe N, Manns BJ, et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. 2018;1:e184852–e184852.
Sullivan MK, Jani BD, McConnachie A, et al. Hospitalisation events in people with chronic kidney disease as a component of multimorbidity: parallel cohort studies in research and routine care settings. BMC Med. 2021;19:278.
van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. a collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–52.
Research NI for H. NIHR Collection: Multiple long-term conditions (multimorbidity): making sense of the evidence. 2021 https://doi.org/10.3310/collection_45881.
Bretos-Azcona PE, Sánchez-Iriso E, Cabasés Hita JM. Tailoring integrated care services for high-risk patients with multiple chronic conditions: a risk stratification approach using cluster analysis. BMC Health Serv Res. 2020;20:806.
Carrero JJ, Elinder CG. The Stockholm CREAtinine Measurements (SCREAM) project: fostering improvements in chronic kidney disease care. J Intern Med 2022; n/a. https://doi.org/10.1111/joim.13418.
Lyons RA, Jones KH, John G, et al. The SAIL databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak. 2009;9:3.
Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–72.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Tonelli M, Wiebe N, Fortin M, et al. Correction to: Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2019;19:177.
Huang Z. A fast clustering algorithm to cluster very large categorical data sets in data mining. In: Lu H, Motoda H, Luu H, editors. KDD: techniques and applications. Singapore: World Scientific; 1997. p. 21–34.
Kaufman L, Rousseeuw P. Finding groups in data: an introduction to cluster analysis. New York: Wiley; 1990.
Guisado-Clavero M, Roso-Llorach A, López-Jimenez T, et al. Multimorbidity patterns in the elderly: a prospective cohort study with cluster analysis. BMC Geriatr. 2018;18:16.
Sjölander A. Regression standardization with the R package stdReg. Eur J Epidemiol. 2016;31:563–74.
R Core Team. R : a language and environment for statistical computing. 2021.
Zheng Z, Waikar SS, Schmidt IM, et al. Subtyping CKD patients by consensus clustering: the Chronic Renal Insufficiency Cohort (CRIC) study. Journal of the American Society of Nephrology. 2021;32:639.
Vetrano DL, Roso-Llorach A, Fernández S, et al. Twelve-year clinical trajectories of multimorbidity in a population of older adults. Nat Commun. 2020;11:3223.
Lawson CA, Seidu S, Zaccardi F, et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClinicalMedicine. 2021;32: 100739.
Dubrofsky L, Lee JF, Hajimirzarahimshirazi P, et al. A unique multi- and interdisciplinary cardiology-renal-endocrine clinic: a description and assessment of outcomes: 2022; 9. https://doi.org/10.1177/20543581221081207.
Zimbudzi E, Lo C, Robinson T, et al. The impact of an integrated diabetes and kidney service on patients, primary and specialist health professionals in Australia: a qualitative study. PLoS One. 2019;14(7):e0219685.
Zimbudzi E, Lo C, Ranasinha S, et al. Health-related quality of life among patients with comorbid diabetes and kidney disease attending a codesigned integrated model of care: a longitudinal study. BMJ Open Diabetes Res Care. 2020;8:842.
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. The Lancet. 2011;377:2181–92.
Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. New England Journal of Medicine. 2001;345:851–60.
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. New England Journal of Medicine. 1993;329:1456–62.
Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. The Lancet. 2005;365:939–46.
Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. New England Journal of Medicine. 2006;354:131–40.
Heerspink HJL, Stefánsson B v, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New England Journal of Medicine. 2019;380:2295–306.
Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30.
Lambourg E, Colvin L, Guthrie G, et al. The prevalence of pain among patients with chronic kidney disease using systematic review and meta-analysis. Kidney Int. 2021;100:636–49.
Zhu Y, Edwards D, Mant J, Payne RA, Kiddle S. Characteristics, service use and mortality of clusters of multimorbid patients in England: a population-based study. BMC Med. 2020;18:78.
Zhan M, Doerfler RM, Xie D, et al. Association of opioids and nonsteroidal anti-inflammatory drugs with outcomes in CKD: findings from the CRIC (Chronic Renal Insufficiency Cohort) study. American Journal of Kidney Diseases. 2020;76:184–93.
Corsonello A, Fabbietti P, Formiga F, et al. Chronic kidney disease in the context of multimorbidity patterns: the role of physical performance. BMC Geriatr. 2020;20:350.
Hirst JA, Ordóñez Mena JM, O’Callaghan CA, et al. Prevalence and factors associated with multimorbidity among primary care patients with decreased renal function. PLoS One. 2021;16:e0245131.
Mercer SW, Gunn J, Bower P, Wyke S, Guthrie B. Managing patients with mental and physical multimorbidity. BMJ : British Medical Journal. 2012;345: e5559.
Shirazian S, Grant CD, Aina O, Mattana J, Khorassani F, Ricardo AC. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep. 2016;2:94–107.
Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database of Systematic Reviews 2021; 2021. https://doi.org/10.1002/14651858.CD006560.pub4.
Stokes J, Guthrie B, Mercer SW, Rice N, Sutton M. Multimorbidity combinations, costs of hospital care and potentially preventable emergency admissions in England: a cohort study. PLoS Med. 2021;18:e1003514.
British Lung Foundation. 2022.
Stevens RJ, Evans J, Oke J, et al. Kidney age, not kidney disease. Can Med Assoc J. 2018;190:E389.
Zemedikun DT, Gray LJ, Khunti K, Davies MJ, Dhalwani NN. Patterns of multimorbidity in middle-aged and older adults: an analysis of the UK biobank data. Mayo Clin Proc. 2018;93:857–66.
Acknowledgements
Ewen Maclean (Patient Support and Advocacy Officer from the charity Kidney Care UK) contributed to the planning of this study and the interpretation of the results.
Funding
This work was supported by the UK Medical Research Council (Grant numbers MR/V001671/1 to MS and MR/S021949/1 to PH) and the Swedish Research Council (Grant number 2019-01059 to JC). The funders did not have any influence over the study design, analysis or decision to submit for publication.
Author information
Authors and Affiliations
Contributions
MKS, PBM, JJC, AG, FSM, BDJ, CA, DAM and AMC initiated the study. AG had access to the SCREAM data and MKS had access to the SAIL data and each of them did the data analysis. MKS drafted the manuscript. MKS, PBM, JJC, AG, FSM, BDJ, CA, DAM, PH, DN and AMC contributed to the design of the study, analysis and interpretation of the data and critical revision of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
For SCREAM, the Regional Ethics Review Board in Stockholm approved the study; informed participant consent was not deemed necessary since all data were de-identified at the Swedish Board of Health and Welfare. For SAIL, Swansea University’s Health Information Research Unit Information Governance Review Panel granted approval for this study as part of project 0830.
Consent for publication
Not applicable.
Competing interests
DN reports fees from GSK, outside the submitted work; PBM reports personal fees and non-financial support from Vifor; personal fees from Astrazeneca, Astellas, Novartis and Janssen; grants from Boehringer Ingelheim; personal fees and non-financial support from Pharmacosmos; and personal fees and non-financial support from Napp, outside the submitted work; MKS, AM, BDJ, JJC, FSM, PH, AG, CA and DAM have nothing to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sullivan, M.K., Carrero, JJ., Jani, B.D. et al. The presence and impact of multimorbidity clusters on adverse outcomes across the spectrum of kidney function. BMC Med 20, 420 (2022). https://doi.org/10.1186/s12916-022-02628-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02628-2