Abstract
Background
Both sleep quality and quantity are essential for normal brain development throughout childhood; however, the association between preterm birth and sleep problems in preschoolers is not yet clear, and the effects of gestational age across the full range from preterm to post-term have not been examined. Our study investigated the sleep outcomes of children born at very-preterm (<31 weeks), moderate-preterm (32–33 weeks), late-preterm (34–36 weeks), early-term (37–38 weeks), full-term (39–40 weeks), late-term (41 weeks) and post-term (>41 weeks).
Methods
A national retrospective cohort study was conducted with 114,311 children aged 3–5 years old in China. Children’s daily sleep hours and pediatric sleep disorders defined by the Children’s Sleep Habits Questionnaire (CSHQ) were reported by parents. Linear regressions and logistic regression models were applied to examine gestational age at birth with the sleep outcomes of children.
Results
Compared with full-term children, a significantly higher CSHQ score, and hence worse sleep, was observed in very-preterm (β = 1.827), moderate-preterm (β = 1.409), late-preterm (β = 0.832), early-term (β = 0.233) and post-term (β = 0.831) children, all p<0.001. The association of pediatric sleep disorder (i.e. CSHQ scores>41) was also seen in very-preterm (adjusted odds ratio [AOR] = 1.287 95% confidence interval [CI] (1.157, 1.433)), moderate-preterm (AOR = 1.249 95% CI (1.110, 1.405)), late-preterm (AOR = 1.111 95% CI (1.052, 1.174)) and post-term (AOR = 1.139 95% CI (1.061, 1.222)), all p<0.001. Shorter sleep duration was also found in very-preterm (β = −0.303), moderate-preterm (β = −0.282), late-preterm (β = −0.201), early-term (β = −0.068) and post-term (β = −0.110) compared with full-term children, all p<0.01. Preterm and post-term-born children had different sleep profiles as suggested by subscales of the CSHQ.
Conclusions
Every degree of premature, early-term and post-term birth, compared to full-term, has an association with sleep disorders and shortened daily sleep duration. Preterm, early-term, and post-term should therefore all be monitored with an increased threat of sleep disorder that requires long-term monitoring for adverse sleep outcomes in preschoolers.
Similar content being viewed by others
Background
It is well established that both sleep quality and quantity are essential for normal brain development throughout childhood, particularly for cognitive functions [1]. However, sleep problems are relatively common among young children, and according to parent reports, it has been suggested that approximately 20–30% of young children have various sleep problems [2]. In preterm infants younger than 1 year old, sleep problems are thought to be even more prevalent [3, 4].
However, the exact relationship between preterm birth and sleep problems beyond infancy is not yet clear. Studies have suggested that school-aged preterm children had different sleep patterns compared to full-term children, such as having earlier bedtimes and earlier wake times [5,6,7], but had no difference in overall sleep duration [6, 7]. Preterm children have been reported to have lower sleep quality, including more nocturnal awakenings [8, 9], and consistent with this more “shallow” and less “deep” non-rapid eye movement sleep [9]. It has been suggested that irreversible adverse factors related to preterm birth, such as brain injury, altered brain maturation, and respiratory problems may precipitate poor sleep. Furthermore, a range of parental factors related to preterm childcaring may also play a role. For example, increased parental concern about preterm children may be linked to earlier bedtimes, which may contribute to the different sleep outcomes of preterm children beyond infancy [10]. However, given the very limited studies available in the literature, variation in the degrees of prematurity and sample size makes it difficult to draw any clear conclusions about the sleep outcomes of preterm children beyond infancy.
Moreover, to our knowledge, no study has been conducted to date on the sleep outcomes of post-term-born children (>41 weeks). Studies have reported that post-term birth can negatively affect children’s short-term and long-term health outcomes [11,12,13,14,15]. Post-term birth can increase the risk of neonatal encephalopathy and death during the first year of life [16]. It has also been reported that, with respect to longer-term effects, post-term birth increases the risks of cognitive impairments, severe mental disorders, neuropsychological disorders, and other behavioural and emotional problems in early childhood [17,18,19,20]. Post-term delivery often has a higher risk for perinatal problems such as prolonged labour that can cause a perinatal lack of oxygen [21] and uteroplacental insufficiency [15]. These risk factors may predispose infants to abnormal brain development and respiratory problems [22] which may lead in turn to sleep problems in post-term children.
Therefore, in the current study, we used a retrospective cohort study design to systematically examine the effect of gestational age on sleep outcomes with a large sample of urban Chinese children. We hypothesized that compared with full-term children (39–40 weeks), that born very-preterm (<31 weeks), moderate-preterm (32–33 weeks), late-preterm (34–36 weeks), early-term (37–38 weeks) and post-term (>41 weeks) all had a higher incidence of sleep disorders and altered sleep outcomes.
Methods
Study design and participants
The present study was part of the Chinese National Cohort of Motor Development (CNCMD), which was designed to explore the neurobehavioral development and other health outcomes (including sleep health, cognition and language development) in Chinese preschool children [23]. A stratified cluster sampling plan was used to ensure that the participants included in the current study were representative of the Chinese population. The China 2018–2019 National Census provided the basis for the stratification by geographic region, age, sex, and socioeconomic status (SES). Ethnic information was not collected because more than 99% of the population in the targeted regions were Han according to the National Census. The government-supported maternity and children’s healthcare centre in each city were selected to invite their local kindergartens to participate in the study. Class teachers were responsible for distributing the notification to parents to complete an online questionnaire. Names and phone numbers of the researchers were provided in case the parents or teachers had queries about the study or about how to respond to the questionnaires. We used an electronic online questionnaire system to enhance the quality of the data by allowing the inclusion of pop-up instructions, error messages, links to further information and to set conditions to ensure participants could not skip questions. A data coordination centre was established to take charge of establishing, managing and maintaining the database, coordinating among health centres.
It is a normal practice for parents to keep in touch with their children’s nursery via smart devices in China, including all of the kindergartens involved in the current study. It was therefore assumed that all of the parents in the current study had relatively high proficiency in online questionnaire completion. All parents gave consent before starting to take part in the study.
From April 1, 2018, to December 31, 2019, a total of 155,377 children aged 3–5 years old from 2403 nurseries in 551 cities in China were recruited for the study. Children were excluded from the study if they had severe visual, hearing, intellectual impairments, cerebral palsy or other severe developmental disorders including autism spectrum disorder (ASD) who were required to receive special education needs and to attend special education schools/nurseries according to the local regulations. Only mainstream schools/nurseries were included in the study; this is the regular provision which excludes special education schools/nurseries. Children with any of the following conditions that may affect the accuracy of the information collected with the questionnaire were excluded from the study: (1) death of the mother; (2) illiterate parents; (3) children taking certain medications with known effects on sleep (such as aspirin, ritalin, amphetamine, caffeine, diazepam, phenobarbital) longer than 1 week at the time of the survey completion date [24,25,26,27]. Children who were twins or had missing covariates were also excluded. Parents of 25,939 children chose not to participate or left the questionnaire before fully completing it. In all, 114,311 children were included in the final analysis (Fig. 1).
The study was approved by the Ethics Committee of Shanghai First Maternity and Infant Hospital (KS18156). All information acquired was kept confidential and was only accessible by the researchers.
Measures
Outcome variables
Sleep duration and sleep disorders of the children were measured with the Children’s Sleep Habits Questionnaire (CSHQ) [28]. There are 33 parent-rated items in the questionnaire that assesses the frequency of behaviours associated with common pediatric sleep difficulties. The CSHQ instructs parents to rate the frequency with which their child has displayed various sleep behaviours in a typical week during the previous 4 weeks. Ratings are combined to create eight subscales that relate to common sleep problems in children: Bedtime Resistance, Sleep Onset Delay, Sleep Duration, Sleep Anxiety, Night Wakings, Parasomnias, Sleep Disordered Breathing, and Daytime Sleepiness. Finally, all ratings are summed to create a total sleep disturbance index. A higher CSHQ score indicates more sleep difficulties, and a score of over 41 indicates a pediatric sleep disorder [28]. The CSHQ has been shown to be a valid and reliable measurement when it is used with Chinese children [29].
Two extra questions were also included in the parent questionnaire to collect information on the exact daily sleep hours of the children: “How many hours does your child sleep during the weekdays?” and “How many hours does your child sleep at the weekends (including daytime nap)?” Daily sleep hours were then calculated as the value of 5/7×Sleep hours during weekdays+2/7×Sleep hours at the weekends [30]. Daytime nap during the weekdays was not asked for because all of the recruited children attended nursery full-time and a standard daytime nap for the same duration was part of the daily routine in all nurseries.
Independent variables
Gestational age at birth was obtained from the mother’s medical records, which was based on ultrasound examination and date of last menstrual period (LMP). Following the literature [31], seven categories of gestational ages were decided: very-preterm (<31 weeks), moderate-preterm (32–33 weeks), late-preterm (34–36 weeks), early-term (37–38 weeks), completely full-term (39–40 weeks), late-term (41 weeks) and post-term (>41 weeks).
Covariates
We included a range of family, child and maternal characteristics as covariates according to the literature: (1). Child characteristics included the child’s age, sex, child body mass index (BMI), right-handedness, eyesight, birth weight, delivery mode, newborn intensive care unit (NICU) admission and developmental disorders (attention deficit syndrome, attention deficit and hyperactivity disorder, learning disabilities). Body mass index (BMI) is an indicator of obesity that is based on height and weight (BMI = weight(kg)/height(m)). Eyesight was grouped into normal and abnormal (including myopia, hyperopia, astigmatism). Co-sleeping was identified with a particular question: “How often does your child sleep in the parent(s)/caregiver(s) bed at night?” The answers were (1) usually, 5 to 7 nights per week; (2) sometimes, 2 to 4 nights per week; (3) rarely, 0 to 1 night per week. In this study, we defined co-sleeping as bed-sharing that occurred 5 to 7 nights per week [32]. (2) Family characteristics included the following variables: higher education of mother and father (yes vs. no); mother and father’s employment status (employed vs. unemployed); family annual per capita income; the number of children in the family (one vs. two or more); and family structure (single-family, nuclear family and extended family). The “single-family” means the child lives with one of his/her parents; the “nuclear family” refers to the child living only with his/her parents; and the “extended family” refers to the child living with his/her parents and grandparents, which is a traditional family structure in China. (3) Maternal health characteristics included the following variables: maternal age at delivery (<30, 30–34 and ≥35 years); smoking or passive smoking during pregnancy; and maternal complications during pregnancy. Maternal complications were defined according to the International Classification of Diseases, Revision 10 (ICD 10) [33]. The classification is defined as having one of the following maternal complications during pregnancy: gestational diabetes, hypertensive disorders, vaginal bleeding during pregnancy, risk of miscarriage, use of antibiotics, use of fertility drugs, intrauterine distress and foetal asphyxia.
Statistical analysis
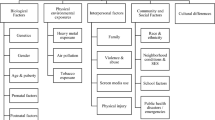
Differences in the child, family and maternal health characteristics by sex and gestational age categories were analysed using independent t-tests, one-way analysis of variance (ANOVA) and chi-squared tests. Based on the previous literature [34,35,36,37,38] and our exploratory analysis, the summarized directed acyclic graph (DAG) between gestational age, daily sleep hours and CSHQ scores (Additional file 1: Figure S1) was generated by using a web-based tool DAGitty (www.dagitty.net). As shown in the DAGs, maternal characteristics and family characteristics as confounders; age, gender, eyesight and co-sleep were considered as competing exposures. Birth weight, BMI, NICU admission, other developmental disorder, handedness and delivery mode were considered as mediators. The distribution of sleep hours and CSHQ scores were relatively symmetric, and multivariable linear regression analysis was conducted to examine the associations of gestational age with daily sleep hours and the CSHQ scores when adjusting for all confounders and competing exposures, while the mediators as the variables on the causal pathway were not included in the adjusted model [39]. Logistic regression analyses were then conducted to examine the associations between different gestational groups and sleep disorders when adjusting for all confounders and competing exposures, while mediators were not included in the adjusted model.
A statistical significance level was set at a p-value <0.05 (two-tailed). To correct for multiple testing, the Benjamini-Hochberg false discovery rate method was used to decrease the probability of false positives [40]. All analyses were performed with the Statistical Package for the Social Sciences (SPSS) (IBM-SPSS Statistics v24.0, Inc Chicago, IL) and R version 3.5.3.
Results
Sample demographic characteristics
A total of 114,311 children aged 4.40±0.79 years old were enrolled in the final analysis. Among all the children, 54,617 (47.8%) were girls and 59,694 (52.2%) were boys. A total of 2379 (2.08%) were very-preterm births; 1893 (1.66%) were moderate-preterm births; 10,238 (9.96%) were late-preterm births; 29,179 (25.53%) were early-term births; 58,043 (50.78%) were full-term births; 7016 (6.14%) were late-term births; 5563 (4.87%) were post-term births (Table 1). Co-sleeping was common, and 82.02% of the children shared a bed with their parents (Table 2). The average daily sleep hours is 10.715±2.693 (Table 1), and 87,773 children (76.78%) were reported to have sleep disorders (Table 3). The prevalence of sleep disorders was 81.21%, 80.67%, 78.62.%, 76.58%, 76.02%, 76.82% and 79.17%, in very-preterm, moderate-preterm, late-preterm, early-term, full-term, late-term and post-term births, respectively (Table 3). The child, family and maternal health during pregnancy characteristics in the study population are shown in Table 2.
Association of gestational age with childhood daily sleep hours and sleep disorder
Compared with completely full-term-born children, higher CSHQ scores were observed in very-preterm, moderate-preterm, late-preterm, early-term and post-term categories. Very-preterm, moderate-preterm, late-preterm and post-term birth were associated with higher CSHQ scores (β = 1.827, 1.409, 0.832, and 0.831 respectively, each p<0.001, p correction<0.001, Table 4), and very-preterm, moderate-preterm, late-preterm, early-term and post-term categories were associated with shorter daily sleep hours (β = −0.303, −0.282, −0.201, −0.068 and −0.110 respectively, each p<0.01, p correction <0.01, Table 4) after controlling for confounders and competing exposures. These positive associations of gestational age with the CSHQ scores were also found within all three age groups (Table 4). The significance of post-term with daily sleep hours was only shown in the 3-year-old group (Table 4).
The associations between very-preterm, moderate-preterm, late-preterm and post-term categories with the CSHQ subscale scores were found in six subscales but not bedtime resistance or sleep onset delay (Table 5).
Gestational age predicted pediatric sleep disorder (i.e. CHSQ>41; adjusted odds ratio (AOR) = 1.287 95% CI (1.157, 1.433), 1.249 95% CI (1.110, 1.405), 1.111 95%CI (1.052, 1·174), and 1.139 95%CI (1.061, 1.222), for the very-preterm, moderate-preterm, late-preterm, and post-term birth, respectively, each p<0.001, p correction <0.001, Table 3), after controlling for all the confounders and competing exposures. The associations were also evident in different age groups, mainly in the preterm and post-term categories (Table 3).
Based on these results in linear and logistic regression models, the associations between gestational ages and childhood daily sleep hours and childhood sleep disorders have been established, especially in preterm (including very-preterm, moderate-preterm and late-preterm) and post-term when compared with completely full-term birth (Figs. 2 and 3).
Associations of gestational age with daily sleep hours. Legend: Associations of gestational age with daily sleep hours: A Compared with full-term birth, preschool children born very-preterm, moderate-preterm, late-preterm, early-term and post-term were associated with lower daily sleep hours, all p < 0.01. B A fit spline described an inverse U-shape relationship of gestational age (weeks) with daily sleep hours (hours/day)
Associations of gestational age with CSHQ score and sleep disorder. Legend: Associations of gestational age with CSHQ scores and sleep disorder. A Compared with full-term birth, preschool children born very-preterm, moderate-preterm, late-preterm, early-term and post-term were associated with higher CSHQ scores, all p < 0.001. B A fit spline described a U-shape relationship of gestational age (weeks) with CSHQ scores. C Compared with full-term birth, preschool children born very-preterm, moderate-preterm, late-preterm and post-term were associated with a higher prevalence of pediatric sleep disorder, all p < 0.001
Discussion
The current population-based prospective cohort study was the first to investigate the association between gestational age across the full range of sleep outcomes in preschoolers. Our study demonstrated that 3–5-year-old children born very-preterm (<31 weeks), moderate-preterm (32–33 weeks), late-preterm (34–36 weeks), early-term (37–38 weeks) and post-term (>41 weeks) were more likely to have sleep problems including having shorter daily sleep hours and a higher odds of sleep disorders, compared to their full-term-born peers, as reported by their parents. Moreover, preterm and post-term children have different sleep profiles as suggested by the different subscales of the CSHQ.
In the present study, a prevalence of sleep disorders was reported with a nationally representative sample in China. Sleep disorder defined with a CSHQ score higher than 41 was found to be prevalent in up to 81.27% of the very-preterm group, 80.67% of the moderate-preterm group, 78.62% of the late-preterm group, 76.58% of the early-term group, 76.02% of the full-term group and 79.17% of the post-term group. The remarkably high incidence of CHSQ-indicated sleep disorders is consistent with previous studies which also reported a very high prevalence of sleep disorders measured by the CSHQ in Chinese [41,42,43,44] and Japanese preschoolers [41], compared to a prevalence of sleep disorders reported in other populations 20–30% in the western population [2, 45]. Our results suggest the cultural features in sleep behaviours of children, and a local standard is required when using the CSHQ to define sleep disorders in children in East Asian countries.
In the present study, we found that sleep disorder as defined by CSHQ scores was significantly higher in all preterm groups compared with the full-term group. Our findings were consistent with previous work which also found that preterm children were more likely to have adverse sleep outcomes compared with full-term children even beyond infancy. The association between preterm birth and sleep problems in children beyond infancy have been reported by studies in both preschool ages [46,47,48] and school ages [10], and our results further suggest that preterm-born preschoolers, especially very-preterm (<31 weeks), were more likely to have sleep disorder consistently across our age range from 3 to 5 years old. Moreover, when we compared individual sleep subscales in the CSHQ, including Bedtime Resistance, Sleep Onset Delay, Sleep Duration, Sleep Anxiety, Night Wakings, Parasomnias, Sleep Disordered Breathing and Daytime Sleepiness, a significant difference between the very-preterm/moderate-preterm and full-term groups was found in all subscales. The preterm groups also showed significantly shorter daily sleep hours compared to full-term children. These results suggest that preterm children may have a global sleep problem. The results are different from a previous study that found preterm preschoolers had higher total scores in the CSHQ but not on any of the subscales [47], which may be associated with their small sample sizes (137 preterms vs. 145 full-term children aged 4–6 years old). As demonstrated by previous studies, circadian rhythms of the foetus are developing in the second trimester and mature in the third trimester [49, 50]. The cycles of foetal activity were reported to show a significant increase in cycle length with advancing gestation [51]. The last few weeks of gestation (37–41 weeks) could therefore be considered as the critical period for sleep patterns as circadian rhythms are established. On the other hand, the shorter daily sleep duration and more sleep problems in bedtime resistance and sleep onset delay of preterm children may also reflect other factors associated with preterm birth. A growing body of evidence indicates that unmodulated parental care and noncircadian environmental conditions may be detrimental to the establishment of circadian rhythms [52,53,54,55]. More behaviour problems and more social segregation have also been observed in children born preterm compared to full-term [56, 57]. As a consequence, these factors may also contribute to the shorter sleep duration and sleep problems of preterm children. Extrinsic and behavioural aspects of the sleep problems of preterm children might be worth investigating in future research.
The current study also shows that some sleep outcomes of early-term children (37–38 weeks) were more likely to be affected compared with those of full-term children. Not only preterm birth but also early-term birth can cause disruption at specific periods during the development of neural connections for specific brain areas which can affect sleep patterns [58]. A systematic review has suggested that early-term infants had poorer outcomes in school performance, neurodevelopment, behaviour and emotional status and long-term social outcomes [58]. Sleep disturbance might be associated with a loss of active cortical and cerebellar development between 34 and 40 weeks of gestation [59]. A cohort study also found that early-term deliveries were associated with a higher rate of pediatric obstructive sleep apnea (OSA), which decreases gradually as gestational age advances [60]. The sleep problems of early-term-born children may be mild and the differences are only revealed with large samples, as in the current study. In the present study, the altered sleep outcomes in early-term-born children faded in the 5-year age group. The results further suggested that the mild sleep problems observed in 3- and 4-year-old early-term-born children can be moderated by biological maturation or relevant social factors as children mature. To our knowledge, our study is the first to report sleep problems in early-term birth, which suggests that children born early-term should also be monitored more carefully due to the higher odds of experiencing sleep problems.
One important finding of the current study is that an association between post-term birth (>41 weeks) and altered sleep outcomes were observed. Previous studies have suggested that post-term birth may be associated with a range of adverse neurological, developmental, behavioural and emotional outcomes in early childhood [14, 19, 61]. Mechanisms concerning post-term birth with a higher risk of sleep disorder and shorter daily sleep hours might involve placental deterioration or insufficiency causing foetal hypoxia or nutritional deficiencies, which in turn could result in injury to the foetal brain [62]. Meconium aspiration, which is more common in post-term birth, may result in neonatal asphyxia thereby increasing the risk for brain injury and later neurodevelopmental problems [62, 63]. Lower melatonin concentration in post-term children might also contribute to a higher risk of sleep problems in post-term-born children [64].
Moreover, it should also be noted that the post-term children had different sleep profiles from preterm children as shown by subscales of the CSHQ. Preterm children showed more difficulties getting to bed (i.e. Bedtime Resistance) and longer periods of awake before sleep (i.e. Sleep Onset Delay) compared to post-term children. There were similar rates of co-sleeping in our preterm and post-term groups, and differences in these sleep behaviour problems between children born preterm and post-term might reflect other parental styles of managing bedtime routines. For example, increased parental concerns with preterm-born children could result in earlier bedtimes [65]. Previous studies also found that increased time was spent in bed in preterm children, irrespective of sleep duration [7], thus arbitrarily reducing sleep efficiency. These findings suggested that parental concern related to preterm does not automatically lead to longer sleep durations, but may lead to a longer sleep onset delay of preterm children. Similarly, the bedtime resistance of preterm children may also be associated with the altered parent-child relationship and the parenting style [66]. Previous research has shown that children with difficulties falling asleep/bedtime resistance are more likely to have parents with a higher level of parental stress [67]. Extinction for bedtime resistance involves requiring children to go to bed and stay in bed and minimizing parental attention thereafter. However, if parents have increased concerns about preterm children, this can lead to the development of substantial bedtime resistance behaviours [68]. On the other hand, parents of post-term-born children may overlook the long-term effect of the prolonged gestation of their child [69], which protects these children from the possible influences of social factors that could cause the delay in sleep onset times and bedtime resistance. Moreover, the shorter daily sleep duration of post-term children compared to full-term births was only observed in the 3- and 4-year-old group, but not in the 5-year-old group, which also suggested the sleep duration of post-term children can be improved by biological maturation or relevant social factors such as a more structured daily sleep routine in nursery. These environmental and behavioural aspects of the sleep problems of preterm and post-term children will need to be further examined in future research.
Strengths and limitations
The main strength of our study was that we included and controlled for a wide range of possible confounding variables including prenatal, antenatal, and postnatal characteristics, and child and family characteristics. Another strength was that we used a nationwide large population sample, and we examined the gestational ages across the full range from preterm to post-term. Our study also had several limitations. First, we did not include all the covariates when analysing the relationship of gestational age with sleep outcomes. For example, some covariates such as maternal sleep of the mothers during pregnancy were not included in the current study but have been shown to affect childhood sleep [30]. Second, the reliance on parent-report information may raise the possibility of a differential misclassification. For example, parents of preterm children, especially very-preterm, may exaggerate the sleep problems of their preterm-born children. Thus large-scale studies using objective measures of sleep such as polysomnography PSG or actigraphy instead of parent reports would add substantially to the theoretical and practical utility of future findings. Fourthly, multiple testing exists in our analysis which may increase the probability of false positives. However, we used the Benjamini-Hochberg false discovery rate method for correction. Finally, it should be noted that some of the reported effect sizes were relatively small. However, the results still have clinical and public health relevance as preterm, early-term and post-term birth can affect a large segment of the population, and the results of the current study can also provide evidence for determining a composite risk.
Conclusions
This cohort study demonstrated that every degree of preterm, early-term and post-term birth negatively affected sleep outcomes, including an increased tendency of parent-reported pediatric sleep disorders and shorter daily sleep hours compared to full-term children. Preterm and post-term births also showed different sleep profiles with preterm children having more sleep behaviour problems. The findings address a much-needed gap in the literature to date and provide important new evidence to support the associations between gestational age and childhood sleep outcomes. The findings suggest that children born preterm, early-term and post-term should be monitored more carefully concerning their sleep health. The potential biological mechanisms between gestational age and childhood sleep should be further studied.
Availability of data and materials
The datasets analysed in the current study are available from the corresponding authors on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- AOR:
-
Adjusted odds ratio
- ASD:
-
Autism spectrum disorder
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CNCMD:
-
The Chinese National Cohort of Motor Development
- CSHQ:
-
The Children’s Sleep Habits Questionnaire
- DAG:
-
Directed acyclic graph
- ICD 10:
-
The International Classification of Diseases, Revision 10
- NICU:
-
Newborn intensive care unit
- OSA:
-
Obstructive sleep apnea
- SES:
-
Socioeconomic status
- SPSS:
-
Statistical Package for the Social Sciences
References
Mason GM, Lokhandwala S, Riggins T, Spencer RMC. Sleep and human cognitive development. Sleep Med Rev. 2021;57: 101472.
Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. 3rd ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2015.
de Groot ER, Knoop MS, van den Hoogen A, Wang X, Long X, Pillen S, Benders M, Dudink J. The value of cardiorespiratory parameters for sleep state classification in preterm infants: a systematic review. Sleep Med Rev. 2021;58: 101462.
Werth J, Atallah L, Andriessen P, Long X, Zwartkruis-Pelgrim E, Aarts RM. Unobtrusive sleep state measurements in preterm infants - a review. Sleep Med Rev. 2017;32:109–22.
Hibbs AM, Storfer-Isser A, Rosen C, Ievers-Landis CE, Taveras EM, Redline S. Advanced sleep phase in adolescents born preterm. Behav Sleep Med. 2014;12(5):412–24.
Maurer N, Perkinson-Gloor N, Stalder T, Hagmann-von Arx P, Brand S, Holsboer-Trachsler E, Wellmann S, Grob A, Weber P, Lemola S. Salivary and hair glucocorticoids and sleep in very preterm children during school age. Psychoneuroendocrinology. 2016;72:166–74.
Stangenes KM, Fevang SK, Grundt J, Donkor HM, Markestad T, Hysing M, Elgen IB, Bjorvatn B. Children born extremely preterm had different sleeping habits at 11 years of age and more childhood sleep problems than term-born children. Acta Paediatr. 2017;106(12):1966–72.
Yiallourou SR, Arena BC, Wallace EM, Odoi A, Hollis S, Weichard A, Horne RSC. Being born too small and too early may alter sleep in childhood. Sleep. 2018;41(2):1–11.
Perkinson-Gloor N, Hagmann-von Arx P, Brand S, Holsboer-Trachsler E, Grob A, Weber P, Lemola S. The role of sleep and the hypothalamic-pituitary-adrenal axis for behavioral and emotional problems in very preterm children during middle childhood. J Psychiatr Res. 2015;60:141–7.
Visser SSM, van Diemen WJM, Kervezee L, van den Hoogen A, Verschuren O, Pillen S, Benders M, Dudink J. The relationship between preterm birth and sleep in children at school age: a systematic review. Sleep Med Rev. 2021;57: 101447.
Persson M, Opdahl S, Risnes K, Gross R, Kajantie E, Reichenberg A, Gissler M, Sandin S. Gestational age and the risk of autism spectrum disorder in Sweden, Finland, and Norway: a cohort study. PLoS Med. 2020;17(9): e1003207.
Odd D, Glover Williams A, Winter C, Draycott T. Associations between early term and late/post term infants and development of epilepsy: a cohort study. PLoS ONE. 2018;13(12):e0210181.
Lahti M, Eriksson JG, Heinonen K, Kajantie E, Lahti J, Wahlbeck K, Tuovinen S, Pesonen AK, Mikkonen M, Osmond C, et al. Late preterm birth, post-term birth, and abnormal fetal growth as risk factors for severe mental disorders from early to late adulthood. Psychol Med. 2015;45(5):985–99.
El Marroun H, Zeegers M, Steegers EA, van der Ende J, Schenk JJ, Hofman A, Jaddoe VW, Verhulst FC, Tiemeier H. Post-term birth and the risk of behavioural and emotional problems in early childhood. Int J Epidemiol. 2012;41(3):773–81.
Caughey AB, Snegovskikh VV, Norwitz ER. Postterm pregnancy: how can we improve outcomes? Obstet Gynecol Surv. 2008;63(11):715–24.
Olesen AW, Basso O, Olsen J. Risk of recurrence of prolonged pregnancy. BMJ. 2003;326(7387):476.
Simon L, Nusinovici S, Flamant C, Cariou B, Rouger V, Gascoin G, Darmaun D, Roze JC, Hanf M. Post-term growth and cognitive development at 5 years of age in preterm children: evidence from a prospective population-based cohort. PLoS ONE. 2017;12(3):e0174645.
Movsas TZ, Paneth N. The effect of gestational age on symptom severity in children with autism spectrum disorder. J Autism Dev Disord. 2012;42(11):2431–9.
Glover Williams A, Odd D. Investigating the association between post-term birth and long term cognitive, developmental and educational impacts: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;33(7):1253–65.
Campbell MK, Ostbye T, Irgens LM. Post-term birth: risk factors and outcomes in a 10-year cohort of Norwegian births. Obstet Gynecol. 1997;89(4):543–8.
Norwitz ER, Snegovskikh VV, Caughey AB. Prolonged pregnancy: when should we intervene? Clin Obstet Gynecol. 2007;50(2):547–57.
Jetti A, Poovali S, Stanley KP. Prolonged pregnancy. Obstet Gynaecol Reprod Med. 2008;18(1):7–11.
Hua J, Barnett AL, Williams GJ, Dai X, Sun Y, Li H, Chen G, Wang L, Feng J, Liu Y, et al. Association of gestational age at birth with subsequent suspected developmental coordination disorder in early childhood in China. JAMA Netw Open. 2021;4(12):e2137581.
Weibel J, Lin YS, Landolt HP, Berthomier C, Brandewinder M, Kistler J, Rehm S, Rentsch KM, Meyer M, Borgwardt S, et al. Regular caffeine intake delays REM sleep promotion and attenuates sleep quality in healthy men. J Biol Rhythms. 2021;36(4):384–94.
Vigliano P, Galloni GB, Bagnasco I, Delia G, Moletto A, Mana M, Cortese S. Sleep in children with attention-deficit/hyperactivity disorder (ADHD) before and after 6-month treatment with methylphenidate: a pilot study. Eur J Pediatr. 2016;175(5):695–704.
Francois D, Roberts J, Hess S, Probst L, Eksioglu Y. Medical management with diazepam for electrical status epilepticus during slow wave sleep in children. Pediatr Neurol. 2014;50(3):238–42.
Tyagi P, Dixit U, Tyagi S, Jain A. Sedative effects of oral midazolam, intravenous midazolam and oral diazepam. J Clin Pediatr Dent. 2012;36(4):383–8.
Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–51.
Liu Z, Wang G, Tang H, Wen F, Li N: Reliability and validity of the Children's Sleep Habits Questionnaire in preschool-aged Chinese children. Sleep Biol Rhythms. 2014;12(3):187–93.
Lyu J, Ye X, Chen Y, Xia Y, Zhu J, Tong S, Yin Y, Qu J, Li S. Children’s sleep may depend on maternal sleep duration during pregnancy: a retrospective study. Nat Sci Sleep. 2020;12:197–207.
Zambrana IM, Vollrath ME, Sengpiel V, Jacobsson B, Ystrom E. Preterm delivery and risk for early language delays: a sibling-control cohort study. Int J Epidemiol. 2016;45(1):151–9.
Ma J, Li S, Jiang F, Jin X, Shen X, Li F. Co-sleeping and childhood enuresis in China. J Dev Behav Pediatr. 2014;35(1):44–9.
Barta A. New and revised ICD-10-CM obstetric guidelines. J AHIMA. 2013;84(11):70–2.
Versteeg M, Kappe RF, Knuiman C. Predicting student engagement: the role of academic belonging, social integration, and resilience during COVID-19 emergency remote teaching. Front Public Health. 2022;10:849594.
Pringle KG, Lee YQ, Weatherall L, Keogh L, Diehm C, Roberts CT, Eades S, Brown A, Smith R, Lumbers ER, et al. Influence of maternal adiposity, preterm birth and birth weight centiles on early childhood obesity in an Indigenous Australian pregnancy-through-to-early-childhood cohort study. J Dev Orig Health Dis. 2019;10(1):39–47.
Aronsen S, Conway R, Lally P, Roberts A, Croker H, Beeken RJ, Fisher A. Determinants of sleep quality in 5835 individuals living with and beyond breast, prostate, and colorectal cancer: a cross-sectional survey. J Cancer Surviv. 2021:1–3.
Garfield V. The association between body mass index (BMI) and sleep duration: where are we after nearly two decades of epidemiological research? Int J Environ Res Public Health. 2019;16(22):4327.
Williamson AL, Derado J, Barney BJ, Saunders G, Olsen IE, Clark RH, Lawson ML. Longitudinal BMI growth curves for surviving preterm NICU infants based on a large US sample. Pediatrics. 2018;142(3).
Tennant PWG, Murray EJ, Arnold KF, Berrie L, Fox MP, Gadd SC, Harrison WJ, Keeble C, Ranker LR, Textor J, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. Int J Epidemiol. 2021;50(2):620–32.
Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300.
Takahashi M, Wang G, Adachi M, Jiang F, Jiang Y, Saito M, Nakamura K. Differences in sleep problems between Japanese and Chinese preschoolers: a cross-cultural comparison within the Asian region. Sleep Med. 2018;48:42–8.
Liu Z, Wang G, Geng L, Luo J, Li N, Owens J. Sleep patterns, sleep disturbances, and associated factors among Chinese urban kindergarten children. Behav Sleep Med. 2016;14(1):100–17.
Wang G, Xu G, Liu Z, Lu N, Ma R, Zhang E. Sleep patterns and sleep disturbances among Chinese school-aged children: prevalence and associated factors. Sleep Med. 2013;14(1):45–52.
Li W, Wang G, Yu Z, Ip P, Leng Y, Zhang Y, Zhao J, Zhang J, Jiang Y, Deng Y, et al. Grandparental care and sleep disturbances in preschool children: a population-based prospective cohort study. Sleep Med. 2021;82:165–71.
Lewien C, Genuneit J, Meigen C, Kiess W, Poulain T. Sleep-related difficulties in healthy children and adolescents. BMC Pediatr. 2021;21(1):82.
Romeo DM, Leo G, Lapenta L, Leone D, Turrini I, Brogna C, Gallini F, Cota F, Vento G, Mercuri E. Sleep disorders in low-risk preschool very preterm children. Sleep Med. 2019;63:137–41.
Durankus F, Aladag Ciftdemir N, Vatansever Ozbek U, Duran R, Acunas B. Comparison of sleep problems between term and preterm born preschool children. Sleep Med. 2020;75:484–90.
Manacero S, Nunes ML. Longitudinal study of sleep behavior and motor development in low-birth-weight preterm children from infancy to preschool years. J Pediatr (Rio J). 2021;97(1):44–51.
Li SF, Wang Y, Li GF, Zhao YY, Chen L, Zhang S. Diurnal rhythm of fetal heart rate in third trimester of pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2018;53(12):849–54.
Seron-Ferre M, Valenzuela GJ, Torres-Farfan C. Circadian clocks during embryonic and fetal development. Birth Defects Res C Embryo Today. 2007;81(3):204–14.
Granat M, Lavie P, Adar D, Sharf M. Short-term cycles in human fetal activity. I. Normal pregnancies. Am J Obstet Gynecol. 1979;134(6):696–701.
Johnson DA, Billings ME, Hale L. Environmental determinants of insufficient sleep and sleep disorders: implications for population health. Curr Epidemiol Rep. 2018;5(2):61–9.
Mileva-Seitz VR, Bakermans-Kranenburg MJ, Battaini C, Luijk MP. Parent-child bed-sharing: the good, the bad, and the burden of evidence. Sleep Med Rev. 2017;32:4–27.
Paul IM, Savage JS, Anzman-Frasca S, Marini ME, Mindell JA, Birch LL: INSIGHT responsive parenting intervention and infant sleep. Pediatrics 2016;138(1).
Wilson KE, Miller AL, Lumeng JC, Chervin RD. Sleep environments and sleep durations in a sample of low-income preschool children. J Clin Sleep Med. 2014;10(3):299–305.
Talge NM, Holzman C, Wang J, Lucia V, Gardiner J, Breslau N. Late-preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics. 2010;126(6):1124–31.
Arpi E, Ferrari F. Preterm birth and behaviour problems in infants and preschool-age children: a review of the recent literature. Dev Med Child Neurol. 2013;55(9):788–96.
Dong Y, Chen SJ, Yu JL. A systematic review and meta-analysis of long-term development of early term infants. Neonatology. 2012;102(3):212–21.
Arpino C, Compagnone E, Montanaro ML, Cacciatore D, De Luca A, Cerulli A, Di Girolamo S, Curatolo P. Preterm birth and neurodevelopmental outcome: a review. Childs Nerv Syst. 2010;26(9):1139–49.
Walfisch A, Wainstock T, Beharier O, Landau D, Sheiner E. Early term deliveries and the risk of pediatric obstructive sleep apnoea in the offspring. Paediatr Perinat Epidemiol. 2017;31(2):149–56.
Smithers LG, Searle AK, Chittleborough CR, Scheil W, Brinkman SA, Lynch JW. A whole-of-population study of term and post-term gestational age at birth and children’s development. BJOG. 2015;122(10):1303–11.
Galal M, Symonds I, Murray H, Petraglia F, Smith R. Postterm pregnancy. Facts Views Vis Obgyn. 2012;4(3):175–87.
Chand S, Salman A, Abbassi RM, Siyal AR, Ahmed F, Leghari AL, Kabani AS, Ali S. Factors leading to meconium aspiration syndrome in term- and post-term neonates. Cureus. 2019;11(9): e5574.
Yurtcu N, Caliskan C, Celik S. Serum melatonin as a biomarker for assessment of late-term and postterm pregnancies in women without spontaneous onset of labor. Z Geburtshilfe Neonatol. 2021;225(06):499–505.
Peltz JS, Rogge RD, Connolly H. Parents still matter: the influence of parental enforcement of bedtime on adolescents' depressive symptoms. Sleep. 2020;43(5).
Bruni O, Lo Reto F, Miano S, Ottaviano S. Daytime behavioral correlates of awakenings and bedtime resistance in preschool children. Suppl Clin Neurophysiol. 2000;53:358–61.
Larsen KL, Erp LA, Jordan M, Jordan SS. Bedtime routines of young children, parenting stress, and bedtime resistance: mediation models. Child Psychiatry Hum Dev. 2021:1–9.
Moore BA, Friman PC, Fruzzetti AE, MacAleese K. Brief report: evaluating the Bedtime Pass Program for child resistance to bedtime–a randomized, controlled trial. J Pediatr Psychol. 2007;32(3):283–7.
Schierding W, Antony J, Karhunen V, Vaarasmaki M, Franks S, Elliott P, Kajantie E, Sebert S, Blakemore A, Horsfield JA, et al. GWAS on prolonged gestation (post-term birth): analysis of successive Finnish birth cohorts. J Med Genet. 2018;55(1):55–63.
Acknowledgements
We thank Guixia Chen, Yao Lin, Hongyan Guan, Bolin Cui, Minhui Cao, Lan Zhang, Tingting Wang, Wenjing Wang, and Xujie Mao for their help with the data acquisition. We are grateful to the class teachers in all participating kindergartens for distributing the notification to parents to complete the online questionnaire.
Funding
This study was supported by the National Natural Science Foundation of China (81673179), the Science and Technology Commission of Shanghai Municipality (18140903100, 19140903100), Shanghai Municipal Health Commission (2020YJZX0213), Pudong Municipal Health Commission (PW2020D-11).
Author information
Authors and Affiliations
Contributions
The corresponding authors (WD & JH) had full access to all the data in the study and had final responsibility for the decision to submit for publication. Study concept and design: JL, WD, JH. Acquisition of data: HL, LW, JZ. Administrative, technical, and material support: HL, LW, JZ. Analysis and interpretation of data: JL, JAG, ALB, WD, JH. Drafting of the manuscript: JL, WD. Critical revision of the manuscript for important intellectual content: JL, JAG, ALB, WD, JH. Statistical analysis: JL, JH. All author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shanghai First Maternity and Infant Hospital (KS18156). All information acquired was kept confidential and was only accessible by the researchers.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. A directed acyclic graph (DAG) describing the relationship between gestational age with daily sleep hours and CSHQ score. Green lines represent paths associated with variables on the causal pathway and were not included in adjusted models.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lyu, J., Groeger, J.A., Barnett, A.L. et al. Associations between gestational age and childhood sleep: a national retrospective cohort study. BMC Med 20, 253 (2022). https://doi.org/10.1186/s12916-022-02443-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02443-9