Abstract
Background
Vitamin D status has been implicated in COVID-19 disease. The objective of the COVID-VIT-D trial was to investigate if an oral bolus of cholecalciferol (100,000 IU) administered at hospital admission influences the outcomes of moderate-severe COVID-19 disease. In the same cohort, the association between baseline serum calcidiol levels with the same outcomes was also analysed.
Methods
The COVID-VIT-D is a multicentre, international, randomised, open label, clinical trial conducted throughout 1 year. Patients older than 18 years with moderate-severe COVID-19 disease requiring hospitalisation were included. At admission, patients were randomised 1:1 to receive a single oral bolus of cholecalciferol (n=274) or nothing (n=269). Patients were followed from admission to discharge or death. Length of hospitalisation, admission to intensive care unit (ICU) and mortality were assessed.
Results
In the randomised trial, comorbidities, biomarkers, symptoms and drugs used did not differ between groups. Median serum calcidiol in the cholecalciferol and control groups were 17.0 vs. 16.1 ng/mL at admission and 29.0 vs. 16.4 ng/mL at discharge, respectively. The median length of hospitalisation (10.0 [95%CI 9.0–10.5] vs. 9.5 [95%CI 9.0–10.5] days), admission to ICU (17.2% [95%CI 13.0–22.3] vs. 16.4% [95%CI 12.3–21.4]) and death rate (8.0% [95%CI 5.2–12.1] vs. 5.6% [95%CI 3.3–9.2]) did not differ between the cholecalciferol and control group. In the cohort analyses, the highest serum calcidiol category at admission (>25ng/mL) was associated with lower percentage of pulmonary involvement and better outcomes.
Conclusions
The randomised clinical trial showed the administration of an oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve the outcomes of the COVID-19 disease. A cohort analysis showed that serum calcidiol at hospital admission was associated with outcomes.
Trial registration
COVID-VIT-D trial was authorised by the Spanish Agency for Medicines and Health products (AEMPS) and registered in European Union Drug Regulating Authorities Clinical Trials (EudraCT 2020-002274-28) and in ClinicalTrials.gov (NCT04552951).
Similar content being viewed by others
Background
The “classical effects” of vitamin D on the bone and mineral metabolism are well established [1, 2]. However, in the last two decades, many “non-classical” actions of vitamin D on the immune system [3] that may contribute to a better defensive response against several bacterial and viral infections have been described [4,5,6,7].
Deficiency of vitamin D, assessed by serum calcidiol levels, is common, particularly in the elderly and frail, and it has been associated with higher morbidity and mortality [8,9,10,11,12]. The information on a possible beneficial role of vitamin D comes from randomised trials, experimental and clinic-epidemiological association studies, and reviews [13,14,15,16,17,18,19,20]. The meta-analyses of randomised clinical trials on vitamin D and respiratory infections and chronic diseases show no consensus on the effects of vitamin D supplementation [21,22,23].
Therefore, the COVID-VIT-D trial was designed to investigate if a single oral bolus of 100,000 IU of cholecalciferol administered at hospital admission could influence the outcomes of patients with COVID-19 disease. In addition, the study also aimed to find out if vitamin D status at hospital admission (serum calcidiol concentration) influenced the pulmonary involvement at admission and the outcomes of the disease.
Methods
Study design and dosing
The COVID-VIT-D was a randomised, open label, multicentre, international clinical independent trial designed and coordinated by the Bone and Mineral Research Unit of Hospital Universitario Central de Asturias (HUCA), Oviedo, Spain, carried out in 12 centres from four countries (Spain, Argentina, Guatemala and Chile), not supported by any pharmaceutical company. In clinical practice, the current dose of cholecalciferol used in different countries to maintain optimal serum calcidiol levels with no risk of hypercalcemia, either as a dietary supplement or as a prescribed supplement, ranged between 15,000 and 50,000 IU, administered daily or monthly. Thus, in order to achieve the optimal serum calcidiol levels in a few days [23], minimising the risks of hypercalcaemia [24,25,26,27,28], in agreement with the Spanish Agency for Medicines and Health products (AEMPS), which is part of the European Agency of Medicines (AEM), responsible for the authorization of clinical trials, it was decided to administer a single oral bolus of 100,000 IU of cholecalciferol.
Participants
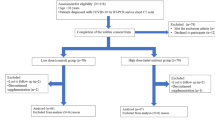
Eligible participants were aged 18 years or above requiring hospitalisation for moderate-severe COVID-19 disease who consented the participation in the study, 570 patients were invited to participate (Fig. 1), finally 543 patients (cholecalciferol n=274, control n=269) from four countries that were admitted and discharged from hospital since April 4, 2020, to April 22, 2021, were analysed (Argentina; six centres N=295, Spain; four centres N=173, Guatemala; one centre N=47, Chile; one centre N=28). Patients with dementia or not able to communicate, tested negative for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) despite clinical findings compatible with COVID-19 disease, pregnant and lactating women, patients who received any form of vitamin D in the previous 3 months and allergic to vitamin D were excluded.
Criteria for hospitalisation/intensive unit care admission
Criteria for hospitalisation were radiological evidence of pulmonary involvement compatible with the COVID-19 disease (bilateral multifocal ground-glass opacities > 50%), and/or moderate-severe flu-like syndrome having oxygen saturation lower than 94% breathing room air and/or additional risk factors (hypertension, diabetes, chronic pulmonary and cardiac diseases, or other serious risk factors). Criteria for intensive care unit (ICU) admission were oxygen saturation lower than 93% on high flow oxygen therapy with FiO2 of 70% and/or severe haemodynamic instability.
Ethics considerations
The ethics committees of all participating centre approved the study. Due to the COVID-19 pandemic and in order to avoid unnecessary exposure to the SARS-CoV-2 virus, all ethics committees authorised verbal consent. The trial was conducted according to the ethical principles of the Declaration of Helsinki.
Data collection and randomisation
At the time of hospital admission, serum calcidiol and other biomarkers were measured (Table 1). Patients were randomised to receive a single oral bolus of 100,000 IU of cholecalciferol, (cholecalciferol group) or nothing (control group). The case sheet of each patient included in the study had a note informing the patient was included in the COVID-VIT-D trial, but there was no information about the arm in which the patient was included (active or control). This information was withheld in the list of randomisation of each centre. Furthermore, the serum calcidiol levels at admission was blinded for the medical staff who managed the patients. All patients received other therapies according to local protocols. Randomisation was performed individually in each centre using a computer-generated list with a 1:1 ratio, and data included in the study were collected in a database. The text of the verbal consent, the database in which patients were identified using different numbers per each centre and patient, and the randomisation lists of the 12 centres were produced and distributed by the HUCA coordinating centre which monthly received the updated database from all participating centres.
Follow-up
Patients were followed from hospital admission to discharge or death during their hospitalisation period; there was no follow-up after the hospital discharge. Demographics, comorbidities, symptoms, biochemical parameters, chest X-ray and/or computed axial tomography, clinical evolutionary data, types of therapy received during the hospitalisation, admission to ICU and death were collected in the database. The data used in this report were those necessary for the present analyses (29 variables and 14 items, Table 1), selected from the complete database distributed to all centres, which included 53 variables and 38 items (Additional file 1: Table S1).
Outcomes
The end points of the COVID-VIT-D trial were three outcomes of the COVID-19 disease: length of hospitalisation, admission to the ICU and mortality. In the cohort analyses, the relationship between serum calcidiol at admission with (a) pulmonary involvement and (b) with the same three outcomes of the trial was assessed.
Clinical trial registration
The COVIT-VIT-D was authorised as a low-intervention clinical trial by the AEMPS and registered in the European Union Drug Regulating Authorities Clinical Trials (EudraCT 2020-002274-28) and in ClinicalTrials.gov (NCT04552951). Protocol details can be found in the Additional file 2 [1, 3, 5, 6, 8, 10,11,12,13, 17, 18, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53].
Laboratory analyses and imaging techniques
Serum calcidiol was measured locally in each centre by electrochemiluminiscence (Cobas e601/e801, Roche Diagnostics) or chemiluminiscence immunoassay (Architect 2000, Abbott and Atellica Solution, Siemens). C-reactive protein (CRP), albumin, lactate dehydrogenase, interleukin-6 (IL-6), haemoglobin, leucocytes, ferritin, calcium and phosphate were measured by autoanalyser (Roche diagnostics, Mindray, Beckman Coulter, Wiener lab, BioMérieux, Abbott, Werfen, Radiometer and Siemens). SARS-CoV-2 status was investigated in nasopharyngeal swabs using either polymerase chain reaction test (PCR) or antigen tests.
Pulmonary involvement was evaluated by pulmonary X-ray and/or pulmonary computed axial tomography (CAT). In the database three categories were considered: positive (pneumonia), negative (no pneumonia) and doubtful (not clearly positive but not normal) (Table 1). In this analysis, doubtful patients were considered positive.
Statistical analyses
Continuous variables were described by using median and interquartile range (IQR), and categorical variables were summarised using absolute and relative frequencies. Differences between groups were tested using the Kruskal-Wallis or Mann-Whitney test for continuous variables, and chi-squared test or Fisher’s exact test (frequencies less than five), for categorical variables.
Patients were described according to initial calcidiol levels (≤10, 10–15, 15–20, 20–25 and >25 ng/mL). The association between the serum calcidiol levels at hospital admission and length of hospitalisation was assessed using linear regression analysis. Binary logistic regression was used to study the association between calcidiol levels and pulmonary involvement and Cox regression was used for admission to ICU, and mortality. Multivariate adjustments with ten variables: demographics (N=2), comorbidities (N=5) and serum biochemical parameters (N=3) were performed in patients in whom at least 70% of these variables were collected. A complete set of gender, age-matched and control group analyses were performed. All statistical analyses were done using R statistical software version 4.0.4.
Role of the funding source
This study was not supported by any pharmaceutical company.
Results
Comparison between the cholecalciferol and control group
The demographics and comorbidities are shown in Table 2. Overall, the median age was 58.0 years (Argentina 57.0, Spain 62.0, Guatemala56.0, Chile61.5), and the 65.0% were males. Hypertension (43.8%), diabetes (24.7%) and cardiovascular disease (21.2%) were the most frequent comorbidities. Pulmonary involvement was diagnosed in 83.1% of the admitted patients. Fever (71.5%), cough (66.5%), weakness (62.2%), dyspnoea (54.0%) and headache (34.6%) were the most frequent symptoms.
The biochemical parameters at admission are depicted in Table 3. Median serum calcidiol did not differ by sex, but differences by countries were observed (Argentina16.0, Spain 13.4, Guatemala24.1, Chile 19.5 ng/mL). Table 4 shows the percentages of different types of drugs received during hospitalisation.
Effect of cholecalciferol on the outcomes
There were no differences in the three outcomes studied between the cholecalciferol and the control group; median length of hospitalisation 10.0 [95%CI 9.0–10.5] vs. 9.5 [95%CI 9.0–10.5] days, admission to ICU 17.2% [95%CI 13.0–22.3] vs. 16.4% [95%CI 12.3–21.4], and death 8.0% [95%CI 5.2–12.1] vs. 5.6% [95%CI 3.3–9.2], respectively (Figs. 2, 3 and 4). Thirty-seven patients died (22 in the cholecalciferol and 15 in the control groups). In the cholecalciferol group, the effect-modification by vitamin D levels was tested and there were no differences in outcomes related to the variation in serum calcidiol levels.
At hospital discharge, the most frequent symptoms were cough (28.9%), weakness (15.3%) and dyspnoea (13.6%) (Additional file 1: Table S2). In the cholecalciferol group, serum calcidiol was higher compared with the control group 29.0 vs. 16.4ng/mL, p=0.000), respectively. No other differences were observed in the biochemical parameters (Additional file 1: Table S3 and Fig. 5).
Cohort analysis by calcidiol levels at hospital admission
Patients in the lowest calcidiol category (≤ 10 ng/mL) were older than patients in the higher category (> 25 ng/mL, Additional file 1: Table S4). In the five comorbidities analysed, no significant differences were observed among the calcidiol categories (Additional file 1: Table S4). Significant differences in C-reactive protein, serum albumin, haemoglobin, calcium and phosphate were found among the five calcidiol categories, but no differences were observed in the remaining parameters (Additional file 1: Table S5). Similar differences were found in the age-matched analyses (Additional file 1: Table S6).
A greater percentage of pulmonary involvement at admission was observed in the lowest compared with the highest calcidiol category (92.7% [95% CI 85.1–96.8] vs.70.1% [95%CI 59.2–79.2], Additional file 1: Table S7). A higher rate in the ICU admission was observed in patients with the lowest calcidiol levels, which was highly significant after age-matched analyses (Additional file 1: Table S7). There were no significant differences in the time of hospitalisation and death rate by calcidiol levels.
Serum calcidiol at admission >25 ng/mL was associated with a lower risk of pulmonary involvement at admission (OR 0.21[95%CI 0.08–0.60]), less days of hospitalisation (−3.69[95%CI −6.47–0.90] days) and lower risk of ICU admission (HR 0.35[95%CI 0.13–0.95]) compared with serum calcidiol ≤10 ng/mL after adjustment by demographics, comorbidities and laboratory parameters (Table 5). The associations remained significant after the age-matched analyses. There was no association between serum calcidiol and mortality (Table 5).
Additional analyses can be found in the Additional file 1: Tables S8-S13.
Discussion
The results of the trial showed that there were no differences in the outcomes of the COVID-19 disease between patients who received a single oral bolus of 100,000 IU of cholecalciferol at hospital admission compared with those who did not receive it. A cohort analysis showed that serum calcidiol at hospital admission was associated with outcomes.
As expected, demographics, comorbidities, pulmonary involvement, symptoms, biochemical parameters, serum calcidiol levels and types of drugs receive during the hospitalisation were well balanced in the cholecalciferol and control groups (Tables 2, 3 and 4). Even though a single dose of cholecalciferol achieved a significant increment of serum calcidiol level at discharge (+12.0 ng/ml), no differences in outcomes were observed.
Similar results to our study were obtained in a recently published Brazilian study in hospitalised patients with moderate-severe COVID-19 disease, in which the administration of 200,000 IU of cholecalciferol did not lead to reduction in hospital stay, mechanical ventilation, patients admitted to ICU and mortality [54]. However, this study had some limitations such as a higher prevalence of diabetes, hypertension and obesity in the group of patients that received vitamin D [55].
Both studies have similarities and differences, the more relevant were the duration of hospitalisation, 2.5 days shorter and the serum calcidiol at admission and discharge 4.3 ng/mL and 15.4 higher, respectively, compared with our study, likely due to the higher dose of cholecalciferol administered in the former (a single oral dose of 200,000 IU). In both trials, patients with COVID-19 disease who require hospitalisation, showed a significant increment in serum calcidiol during the hospital stay which was not able to render outcome benefits.
Apart from the two large trials discussed above, other open-label trial with lower number of participants (n = 76) has been published [15], but the authors did not provide information related with vitamin D status at baseline, in addition, the drug administration schedule and the formulation of vitamin D used was different to the Brazilian and our study. They used an activated form of vitamin D, (calcifediol −25(OH)D3−, 0.532 mg administered orally on day one, followed by 0.266 mg on days three and seven, and then 0.266 weekly until discharge). The differences between both studies and the lower total number of participants, considering both studies together (n = 316) and deaths (n =17), prevented to combine them in further analyses and drew the attention to the importance of our study to investigate the role of vitamin D administered at hospital admission, in the management of COVID-19 disease.
The present COVID-VIT-D trial is so far the largest multicentre international trial designed to investigate the impact of the use of a single oral bolus of non-active vitamin D in clinical outcomes of moderate-severe COVID-19 disease in hospitalised patients, like the Brazilian trial [54], the result of the COVID-VIT-D trial was negative and similar results with the use of vitamin D have been observed in previous trials performed in other infectious diseases [18, 27, 56,57,58,59,60]. However, the lack of response of bolus versus daily dosing of vitamin D in several diseases, such as respiratory infections including the COVID-19 disease, is a matter of controversy [19, 61].
The results of the cohort analysis showed that higher calcidiol at admission was associated with less pulmonary involvement and better clinical outcomes. However, in the cohort analysis, there are multiple overlapping risk factors that can play an important role as confounders, such as age, diabetes, hypertension, cardiovascular disease, obesity and chronic obstructive pulmonary disease. Many of them were included in the multivariate adjustments, but still other non-measured confounders could have contributed to residual confounding. Furthermore, this cohort analysis may be subject to bias because the population recruited for the study was heterogeneous, i.e., different countries with uneven socioeconomic issues and health system coverage, and different latitudes that can influence calcidiol levels through different sun exposures [62].
According to the results of the cohort analyses, we could think that other factors such as the time that cholecalciferol may need to achieve its full modulatory function to reinforce the immune system could have played a positive role. In fact, a bolus dosing of 100,000 IU of cholecalciferol significantly increases serum calcidiol levels in a few days [23], but it may not be able to obtain the long-term systemic effects of calcitriol on the antimicrobial proteins such as cathelicidin, defensins or regulatory T cells [19, 23]. If this is the case, cholecalciferol should be given in advance, before the full COVID-19 disease is established, to promote a more effective immunological background for protection against the SARS-Cov-2 virus infection. However, this possible explanation remains in the speculative area.
The COVID-VIT-D study has some limitations; the time between the onset of symptoms and the administration of vitamin D was not analysed, and because it was an open label trial not controlled by placebo, it cannot be considered level-one evidence. However, the study has several important strengths, including its international nature (performed in 12 centres from four countries in two continents north and south of the equator), and the large number of patients recruited for the trial. The expertise of the HUCA Spanish coordinating centre in leading European and Latin American studies [63,64,65] was useful to design a study as simple and complete as possible, taking into account the difficulties of the pandemic and the strategic limitations of the participating centres.
Conclusions
In summary, the results of the COVID-VIT-D trial demonstrated that in the moderate-severe COVID-19 disease that needs hospitalisation, a single oral bolus of cholecalciferol (100,000 IU), administered at admission did not improve the outcomes of the disease compared with patients who did not receive it. A cohort analysis showed that high serum calcidiol level at hospital admission was associated with better outcomes.
Availability of data and materials
The data underlying this article will be shared upon reasonable request to the corresponding author.
Abbreviations
- AEMPs:
-
Spanish Agency for Medicines and Health Products
- BMI:
-
Body mass index
- CAT:
-
Computed axial tomography
- CRP:
-
C-reactive protein
- HUCA:
-
Hospital Universitario Central de Asturias
- ICU:
-
Intensive care unit
- IL-6:
-
Interleukin 6
- PCR:
-
Polymerase chain reaction
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome CoronaVirus 2
References
Holick MF, Vitamin D. Physiology, molecular biology, and clinical applications: Totowa. NJ: Humana Press; 2010.
Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11):1656.
Illescas-Montes R, Melguizo-Rodríguez L, Ruiz C, Costela-Ruiz VJ. Vitamin D and autoimmune diseases. Life Sci. 2019;233:116744.
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408.
Coussens AK, Martineau AR, Wilkinson RJ. Anti-inflammatory and antimicrobial actions of vitamin d in combating TB/HIV. Scientifica. 2014;2014:903680.
Alvarez N, Aguilar-Jimenez W, Rugeles MT. The potential protective role of vitamin D supplementation on HIV-1 infection. Front Immunol. 2019;10:2291.
Sudfeld CR, Mugusi F, Muhihi A, Aboud S, Nagu TJ, Ulenga N, et al. Efficacy of vitamin D3 supplementation for the prevention of pulmonary tuberculosis and mortality in HIV: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2020;7(7):e463–e71.
Cannata-Andia JB, Gomez AC. Vitamin D deficiency: a neglected aspect of disturbed calcium metabolism in renal failure. Nephrol Dial Transplant. 2002;17(11):1875–8.
National Heart L, Blood Institute PCTN, Ginde AA, Brower RG, Caterino JM, Finck L, et al. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. New Engl J Med. 2019;381(26):2529–40.
Merker M, Amsler A, Pereira R, Bolliger R, Tribolet P, Braun N, et al. Vitamin D deficiency is highly prevalent in malnourished inpatients and associated with higher mortality: a prospective cohort study. Medicine (Baltimore). 2019;98(48):e18113.
Amrein K, Parekh D, Westphal S, Preiser JC, Berghold A, Riedl R, et al. Effect of high-dose vitamin D3 on 28-day mortality in adult critically ill patients with severe vitamin D deficiency: a study protocol of a multicentre, placebo-controlled double-blind phase III RCT (the VITDALIZE study). BMJ open. 2019;9(11):e031083.
Johansson H, Odén A, Kanis J, McCloskey E, Lorentzon M, Ljunggren Ö, et al. Low serum vitamin D is associated with increased mortality in elderly men: MrOS Sweden. Osteoporos Int. 2012;23(3):991–9.
Yang J, Ou-Yang J, Huang J. Low serum vitamin D levels increase the mortality of cardiovascular disease in older adults: a dose-response meta-analysis of prospective studies. Medicine (Baltimore). 2019;98(34):e16733.
Jimenez-Sousa MA, Martinez I, Medrano LM, Fernandez-Rodriguez A, Resino S. Vitamin D in human immunodeficiency virus infection: influence on immunity and disease. Front Immunol. 2018;9:458.
Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcala Diaz JF, Lopez Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751.
Hernandez JL, Nan D, Fernandez-Ayala M, Garcia-Unzueta M, Hernandez-Hernandez MA, Lopez-Hoyos M, et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2021;106(3):e1343–e53.
Naves-Díaz M, Cabezas-Rodríguez I, Barrio-Vázquez S, Fernández E, Díaz-López JB, Cannata-Andía JB. Low calcidiol levels and risk of progression of aortic calcification. Osteoporos Int. 2012;23(3):1177–82.
Zittermann A, Ernst JB, Prokop S, Fuchs U, Dreier J, Kuhn J, et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J. 2017;38(29):2279–86.
Mazess RB, Bischoff-Ferrari HA, Dawson-Hughes B. Vitamin D: bolus is bogus-a narrative review. JBMR Plus. 2021;5(12):e10567.
Nogues X, Ovejero D, Pineda-Moncusí M, Bouillon R, Arenas D, Pascual J, et al. Calcifediol treatment and COVID-19-related outcomes. J Clin Endocrinol Metab. 2021;106(10):e4017–e27.
Ganmaa D, Enkhmaa D, Nasantogtokh E, Sukhbaatar S, Tumur-Ochir KE, Manson JE. Vitamin D, respiratory infections, and chronic disease: Review of meta-analyses and randomized clinical trials. J Intern Med. 2022;291(2):141–64.
Jolliffe DA, Camargo CA Jr, Sluyter JD, Aglipay M, Aloia JF, Ganmaa D, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276–92.
Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract. 2014;20(4):341–51.
Malihi Z, Wu Z, Lawes CMM, Scragg R. Adverse events from large dose vitamin D supplementation taken for one year or longer. J Steroid Biochem Mol Biol. 2019;188:29–37.
Ginde AA, Blatchford P, Breese K, Zarrabi L, Linnebur SA, Wallace JI, et al. High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc. 2017;65(3):496–503.
Jetty V, Glueck CJ, Wang P, Shah P, Prince M, Lee K, et al. Safety of 50,000-100,000 units of vitamin D3/week in vitamin D-deficient, hypercholesterolemic patients with reversible statin intolerance. North Am J Med Sci. 2016;8(3):156–62.
Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179(9):843–50.
Vieth R. Chapter 57 - The pharmacology of vitamin D. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. 3rd ed. San Diego: Academic; 2011. p. 1041–66.
Díaz López JB, Cannata-Andía JB. El amplio espectro de la activación del receptor de vitamina D. In: Cannata-Andía JB, editor. Alteraciones del metabolismo óseo y mineral en la enfermedad renal crónica: avances en patogenia, diagnóstico y tratamiento. Barcelona Wolters Kluwer: Lippincott Williams & Wilkins; 2010. p. 275–9.
Alvarez-Hernández D, Gómez-Alonso C, Cannata-Andía JB. Vitamin D supplementation: what is right? Clin Cases Miner Bone Metab. 2006;3:71–5.
Grant WB. Review of recent advances in understanding the role of vitamin D in reducing cancer risk: breast, colorectal, prostate, and overall cancer. Anticancer Res. 2020;40(1):491–9.
Haykal T, Samji V, Zayed Y, Gakhal I, Dhillon H, Kheiri B, et al. The role of vitamin D supplementation for primary prevention of cancer: meta-analysis of randomized controlled trials. J Community Hosp Intern Med Perspect. 2019;9(6):480–8.
Perge P, Boros AM, Gellér L, Osztheimer I, Szilágyi S, Tahin T, et al. Vitamin D deficiency predicts poor clinical outcomes in heart failure patients undergoing cardiac resynchronization therapy. Dis Markers. 2019;2019:4145821.
Grandi NC, Breitling LP, Vossen CY, Hahmann H, Wüsten B, März W, et al. Serum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart disease. Am Heart J. 2010;159(6):1044–51.
Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. New Engl J Med. 2019;380(1):33–44.
Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study : a randomized clinical trial. JAMA Cardiol. 2017;2(6):608–16.
Roffe-Vazquez DN, Huerta-Delgado AS, Castillo EC, Villarreal-Calderón JR, Gonzalez-Gil AM, Enriquez C, Garcia-Rivas G, Elizondo-Montemayor L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int J Mol Sci. 2019;20(22):5811.
Pinzone MR, Di Rosa M, Malaguarnera M, Madeddu G, Focà E, Ceccarelli G, et al. Vitamin D deficiency in HIV infection: an underestimated and undertreated epidemic. Eur Rev Med Pharmacol Sci. 2013;17(9):1218–32.
Mansueto P, Seidita A, Vitale G, Gangemi S, Iaria C, Cascio A. Vitamin D deficiency in HIV infection: not only a bone disorder. BioMed Res Int. 2015;2015:735615.
Ayelign B, Workneh M, Molla MD, Dessie G. Role of vitamin-D supplementation in TB/HIV co-infected patients. Infect Drug Resist. 2020;13:111–8.
Dusso AS. Molecular biology of Vitamin D: genomic and nongenomic actions of vitamin D in chronic kidney disease. Switzerland: Springer International Publishing Switzerland; 2016.
Liu D, Fang YX, Wu X, Tan W, Zhou W, Zhang Y, et al. 1,25-(OH)(2)D(3)/Vitamin D receptor alleviates systemic lupus erythematosus by downregulating Skp2 and upregulating p27. Cell Commun Signal. 2019;17(1):163.
Gassen NC, Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A, et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat Commun. 2019;10(1):5770.
Ginde AA, Brower RG, Caterino JM, Finck L, Banner-Goodspeed VM, Grissom CK, et al. Early high-dose vitamin D(3) for critically ill, vitamin D-deficient patients. N Engl J Med. 2019;381(26):2529–40.
Metzger M, Stengel B. Epidemiology of vitamin D deficiency in chronic kidney disease. Switzerland: Springer International Publishing Switzerland; 2016.
Sudfeld CR, Mugusi F, Aboud S, Nagu TJ, Wang M, Fawzi WW. Efficacy of vitamin D(3) supplementation in reducing incidence of pulmonary tuberculosis and mortality among HIV-infected Tanzanian adults initiating antiretroviral therapy: study protocol for a randomized controlled trial. Trials. 2017;18(1):66.
Martínez-Alonso M, Dusso A, Ariza G, Nabal M. Vitamin D deficiency and its association with fatigue and quality of life in advanced cancer patients under palliative care: a cross-sectional study. Palliat Med. 2016;30(1):89–96.
Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, Guinsburg A, Marelli C, Rodriguez-Puyol D, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74(8):1070–8.
Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–37.
Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95(1):91–100.
Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ (Clinical research ed). 2014;348:g1903.
Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. Bmj. 2019;366:l4673.
Gaksch M, Jorde R, Grimnes G, Joakimsen R, Schirmer H, Wilsgaard T, et al. Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 2017;12(2):e0170791.
Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. Jama. 2021;325(11):1053–60.
Pal R, Banerjee M, Bhadada SK, Shetty AJ, Singh B, Vyas A. Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. J Endocrinol Invest. 2022;45(1):53–68.
Hueniken K, Aglipay M, Birken CS, Parkin PC, Loeb MB, Thorpe KE, et al. Effect of high-dose vitamin D supplementation on upper respiratory tract infection symptom severity in healthy children. Pediatr Infect Dis J. 2019;38(6):564–8.
Tukvadze N, Sanikidze E, Kipiani M, Hebbar G, Easley KA, Shenvi N, et al. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;102(5):1059–69.
Bjorkhem-Bergman L, Missailidis C, Karlsson-Valik J, Tammelin A, Ekstrom L, Bottai M, et al. Vitamin D supplementation to persistent carriers of MRSA-a randomized and placebo-controlled clinical trial. Eur J Clin Microbiol Infect Dis. 2018;37(9):1735–44.
Ganmaa D, Uyanga B, Zhou X, Gantsetseg G, Delgerekh B, Enkhmaa D, et al. Vitamin D supplements for prevention of tuberculosis infection and disease. New Engl J Med. 2020;383(4):359–68.
Zhang J, Chen C, Yang J. Effectiveness of vitamin D supplementation on the outcome of pulmonary tuberculosis treatment in adults: a meta-analysis of randomized controlled trials. Chin Med J. 2019;132(24):2950–9.
Camargo CA, Sluyter J, Stewart AW, Khaw KT, Lawes CMM, Toop L, et al. Effect of monthly high-dose vitamin D supplementation on acute respiratory infections in older adults: a randomized controlled trial. Clin Infect Dis. 2020;71(2):311–7.
Kasahara AK, Singh RJ, Noymer A. Vitamin D (25OHD) serum seasonality in the United States. PLoS One. 2013;8(6):e65785.
Naves-Diaz M, Passlick-Deetjen J, Guinsburg A, Marelli C, Fernandez-Martin JL, Rodriguez-Puyol D, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant. 2011;26(6):1938–47.
Díaz Lopez JB, Jorgetti V, Caorsi H, Ferreira A, Palma A, Menéndez P, et al. Epidemiology of renal osteodystrophy in Iberoamerica. Nephrol Dial Transplant. 1998;13(Suppl 3):41–5.
Fernández-Martín JL, Canteros A, Alles A, Massari P, Cannata-Andía J. Aluminum exposure in chronic renal failure in iberoamerica at the end of the 1990s: overview and perspectives. Am J Med Sci. 2000;320(2):96–9.
Acknowledgements
We acknowledge the participation of Álvarez Menéndez, Francisco (Hospital Universitario Central de Asturias –HUCA- and Instituto de Investigación Sanitaria del Principado de Asturias -ISPA-, Oviedo, Spain); de la Iglesia Fanjul, Ignacio (HUCA); Dusso, Adriana (Whasington University in St. Louis, MO, USA); Feito Díaz, Estefanía (HUCA); Galiano García, María Reyes (HUCA); González Álvarez, María Fernanda (HUCA); Llaneza Faedo, Mónica (HUCA); Lozano Vázquez, Ana (HUCA); Melón García, Santiago (HUCA); Naves López, María Teresa (HUCA); Telenti Asensio, Mauricio (HUCA); Oddo, Sebastián (Hospital Independencia, Santiago del Estero, Argentina); Fernández, Paula Antonella (Hospital Militar Central Cirujano Mayor Dr. Cosme Argerich -HMC-, Buenos Aires, Argentina); González Paganti, Analia Luciana (HMC); Tarracina, Luciana Laura (HMC); Pelayo Terán, José María (Hospital El Bierzo, Ponferrada, Spain); Tierra Rodríguez, Ana María (Hospital El Bierzo); Rodríguez Manzano, Isabel (Hospital El Bierzo); Chea Vine, Rosa (Hospital Barros Luco Trudeau –HBLT- and Universidad de Chile –U Chile-, Santiago, Chile); Palma Onetto, Carolina (HBLT and U Chile); Zamora Ferrari, Daniela (HBLT and U Chile); Pérez Ortega, Juan (Fundación para la Investigación Biosanitaria de Andalucía Oriental –FIBAO-, Granada, Spain). The cholecalciferol was friendly donated to HUCA by Gebro Pharma from April to July 2020. The full list of COVID-VIT-D trial collaborators is depicted in the Additional file 3.
Funding
This study was not supported by any pharmaceutical company. The groups involved in the study received research support from Fondo Europeo de Desarrollo Regional (FEDER), Plan de Ciencia, Tecnología e Innovación 2013–2017 y 2018–2022 del Principado de Asturias (GRUPIN 14-028, IDI-2018-000-152, IDI/2021/000080). Red Cooperativa en Salud REDinREN y RICORS2040 del Instituto de Salud Carlos III (RD12/0021/1023; RD16/0009/0017;RD21/0005/0019). Instituto de Salud Carlos III (ISCIII)-Fondo de Investigación Sanitaria: PI17/00715, PI17/00384, PI17/02181, PI19/00532, PI20/00633, PI20/00753. Ayuda para la formación de Profesorado Universitario (FPU). Programa de Ayudas “Severo Ochoa” para la Formación en investigación y Docencia del Principado de Asturias, convocatoria 2019. Universidad de Oviedo, España. Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Fundación para la Investigación y la Innovación Biosanitaria de Asturias (FINBA). Fundación Renal Íñigo Álvarez de Toledo (FRIAT).
Author information
Authors and Affiliations
Consortia
Contributions
These are the contributions of the authors according to CRediT (Contributor Roles Taxonomy): 1. Conceptualization: JBCA, NCL, SP, JC, WD; 2. Data curation: ADS, PF, CPA, MND, WD, JLFM; 3. Formal analysis: ADS, MND, JLFM; 4. Funding acquisition: JBCA; 5. Investigation: ADS, PF, CPA, PHP, RM, GHI, CAC, CB, VSP, JPP, IMR, JCV, MDG, CGA, WD; 6. Project administration: JBCA, JLFM; 7. Methodology: JBCA, PF, MND, WD; 8. Resources: ADS, PF, CPA, PHP, RM, NCL, SP, GHI, CAC, CB, VSP, JPP, IMR, JCV, MDG, CGA MND, WD, JLFM; 9. Software: ADS, JLFM; 10 Supervision: JBCA, PF, NCL, SP, MND, WD; 11. Validation: JBCA, JC, JLFM; 12. Visualisation: JBCA, NCL, SP; Writing-original draft: JBCA, JLFM; 14. Writing-review and editing: JBCA, NCL, JC. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The ethics committees of all participating centre approved the study. Due to the COVID-19 pandemic and in order to avoid unnecessary exposure to the SARS-CoV-2 virus, all ethics committees authorized verbal consent. The trial was conducted according to the ethical principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The following authors received research grants fees, grants for congress attending, courses and collaborations by the following entities: Jorge B. Cannata-Andía from Amgen, Kyowa-Kirim and Vifor Pharma; Ricardo Mouzo from Takeda, Otsuka, Nipro, Sanofi-Aventis, Amgen and the Senefro Foundation; Natalia Carrillo-López from Ministerio de Ciencia e Innovación (MICINN)/Instituto de Salud Carlos III (ISCIII); Sara Panizo from MICINN/ISCIII and Luis Hernando gran from Fundación Renal Íñigo Álvarez de Toledo; Carolina Ballarino from Pfizer, Takeda and Sanofi-Aventis; Jacqueline Pefaur-Penna from Novartis and Sanofi-Aventis; Jesús Calviño-Varela from Baxter, Otsuka, Palex, Astra, Vifor and Chiesi; Carlos Gómez-Alonso from Amgen, UCB, Stada, Grünenthal, Gebro Pharma, FAES, Kiowa-Kirin and Laboratorios Rubió; John Cunningham from Amgen, Merck and Vifor Pharma; Manuel Naves-Díaz from MICINN/ISCIII, Amgen, UCB, Kyowa-Kirim, Stada, Italfármaco, Gebro Pharma, Rubió, Gedeon Richter, Grünenthal and FEIOMM and José L. Fernández-Martín from MICINN/ISCIII. The rest of authors are not aware of any additional relationship, funding or financial holdings.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Variables collected in the COVID-VIT-D trial. T. Table S2. Symptoms at discharge. Table S3. Biochemical parameters at discharge. Table S4. Demographic, comorbidities, and serum calcidiol categories at hospital admission. Table S5. Relevant biochemical parameters and serum calcidiol categories at hospital admission. Table S6. Relevant biochemical parameters and serum calcidiol categories at hospital admission in age-matched patients. Table S7. Pulmonary involvement at admission and outcomes according to serum calcidiol categories. Table S8. Types and number of drugs received during the hospitalization and serum calcidiol categories at hospital admission. Table S9. Demographic, comorbidities, and serum calcidiol categories at admission in age-matched patients. Table S10. Types and number of drugs received during the hospitalization and serum calcidiol categories at hospital admission in age-matched patients. Table S11. Relevant biochemical parameters and serum calcidiol categories at hospital admission in the control group (No cholecalciferol). Table S12. Types and number of drugs received during the hospitalization and serum calcidiol categories at hospital admission in the control group (No cholecalciferol). Table S13. Pulmonary involvement at admission and outcomes according to initial serum calcidiol categories in the control group (No cholecalciferol).
Additional file 2.
Original trial protocol.
Additional file 3.
COVID-VIT-D collaborators and affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cannata-Andía, J.B., Díaz-Sottolano, A., Fernández, P. et al. A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D—a randomised multicentre international clinical trial. BMC Med 20, 83 (2022). https://doi.org/10.1186/s12916-022-02290-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02290-8