Abstract
Background
Sex hormones have been suggested to play a role in colorectal cancer (CRC), but their influence on early initiation of CRC remains unknown.
Methods
We retrospectively examined the associations with risk of CRC precursors, including conventional adenomas and serrated polyps, for plasma estrone, estradiol, free estradiol, testosterone, free testosterone, sex hormone-binding globulin (SHBG), and the ratio of estradiol to testosterone among 5404 postmenopausal women from the Nurses’ Health Study I and II. Multivariable logistic regression was used to calculate the odds ratio (OR) and 95% confidence intervals (CI). Given multiple testing, P < 0.005 was considered statistically significant.
Results
During 20 years of follow-up, we documented 535 conventional adenoma cases and 402 serrated polyp cases. Higher concentrations of SHBG were associated with lower risk of conventional adenomas, particularly advanced adenomas (multivariable OR comparing the highest to the lowest quartile, 0.40, 95% CI 0.24–0.67, P for trend < 0.0001). A nominally significant association was found for SHBG with lower risk of large serrated polyps (≥ 10 mm) (OR, 0.47, 95% CI 0.17–1.35, P for trend = 0.02) as well as free estradiol and free testosterone with higher risk of conventional adenomas (OR, 1.54, 95% CI 1.02–2.31, P for trend = 0.03 and OR, 1.33, 95% CI 0.99–1.78, P for trend = 0.03, respectively).
Conclusions
The findings suggest a potential role of sex hormones, particularly SHBG, in early colorectal carcinogenesis.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is highly heterogeneous and can arise from distinct precursor lesions, including conventional adenomas and serrated polyps [1]. In contrast to the conventional pathway characterized by chromosomal instability, the serrated pathway features BRAF mutation, CpG island methylator phenotype, and microsatellite instability [2]. According to the 5th edition of the WHO criteria, serrated polyps include hyperplastic polyp (HP), sessile serrated lesion (SSL), SSL with dysplasia, traditional serrated adenoma (TSA), and unclassified serrated adenoma [3]. The serrated pathway is proposed to mainly originate from HPs and transit to SSLs or TSAs before progression to dysplasia and cancer [4, 5], although there is the potential for in situ development of SSLs [6]. Of particular concern, serrated polyps are flat and difficult to remove by endoscopy and have potential for rapid growth, disproportionately contributing to interval CRCs diagnosed after a negative screening test [7].
CRC incidence is lower in women than men across all ages. Also, it appears that women are more likely to develop serrated polyps than men [8, 9]. While the reasons for these sex differences remain unclear, sex hormones have been postulated as a potential explanation [10]. Exposure to exogenous sex hormones, including oral contraceptives [11, 12] and postmenopausal hormone therapy [13, 14], has been linked to lower risk of CRC in some but not all studies [15, 16]. A recent meta-analysis of cohort studies found that hormone replacement therapy, but not oral contraceptives, was associated with lower risk of conventional adenoma [17]. Of note, due to first-pass metabolism by the liver, exogenous hormones may exert certain biological effects different from those of endogenous estrogens [18].
Current data on endogenous sex hormones and CRC risk are sparse and yield inconsistent results. Two studies reported no association for estrone or estradiol [19, 20], while others found positive [21, 22] or inverse associations for these hormones [23]. Similar inconsistency has been observed for CRC with other components of the sex hormone axis, including testosterone and sex hormone-binding globulin (SHBG).
In women, testosterone is mainly produced by the ovaries (even after menopause), adrenal glands, and peripheral conversion of androgen precursors produced by the adrenals and ovaries [24]. Experimental evidence suggests that testosterone may promote the development of colonic adenoma [25]. SHBG is a glycoprotein synthesized in the liver and regulates sex hormone bioactivity through binding with high affinity to circulating testosterone and estradiol [26]. To our knowledge, no epidemiologic studies have yet examined circulating levels of sex hormones in relation to conventional adenomas or serrated polyps.
Therefore, we performed this retrospective study to evaluate the associations of prediagnostic sex hormones with risk of conventional adenoma and serrated polyp among 5404 postmenopausal women from the Nurses’ Health Study (NHS) I and II. We assessed estrone, estradiol, free estradiol, testosterone, free testosterone, and SHBG, as well as the ratio of estradiol to testosterone, which reflects the activity of aromatase conversion from testosterone to estradiol.
Methods
Study population
The NHSI and NHSII are two US prospective cohort studies that included 121,700 registered female nurses aged 30–55 years since 1976 and 116,686 female nurses aged 25–42 years since 1989, respectively. The enrolled women completed a questionnaire at study baseline and were mailed follow-up questionnaires biennially to update their lifestyle and medical information, with cumulative follow-up rates greater than 90% in both cohorts [27]. In NHSI, 32,826 women donated blood samples via overnight courier on ice packs in 1989–1990, and in NHSII, 29,611 provided blood samples using a similar method in 1996–1999. Upon arrival, samples were centrifuged, aliquoted, and stored in liquid nitrogen freezers immediately [28]. Demographic, dietary, and lifestyle profiles of women who provided blood samples were generally similar to those who did not [29].
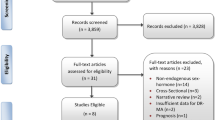
The current study initially included 8055 NHSI and 4574 NHSII women with available sex hormone data (i.e., estrone, estradiol, testosterone, and SHGB) predominately from previous nested case-control studies of various outcomes conducted between 1997 and 2014 [30]. We excluded premenopausal women and those whose biomarker data were considered as outliers by the generalized extreme studentized deviate many-outlier approach [31] and who had a history of cancer (except non-melanoma skin cancer), colorectal polyp, or inflammatory bowel disease at the time of blood draw, or had no lower gastrointestinal endoscopy after blood donation (Additional file 1: Figure S1 and Table S1). A total of 5404 postmenopausal women were included in the final analysis. Participants showed similar demographic and lifestyle characteristics compared to all postmenopausal women who provided a blood sample in the two cohorts (Additional file 1: Table S2).
The study was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and those of participating registries as required.
Assessment of circulating biomarkers
Plasma concentrations of estrone, estradiol, and testosterone were measured in the Molecular lab at the Mayo Clinic (Rochester, MN) using the turbulent flow liquid chromatography tandem mass spectrometry. SHBG was assayed in Dr. Rifai’s Lab at the Children’s Hospital (Boston, MA) using a competitive electrochemiluminescence immunoassay. Levels of C-peptide, a marker of insulin secretion, were determined by using enzyme-linked immunosorbent assay in Dr. Pollak’s laboratory at McGill University (Montreal, QC, Canada). Quality control samples were randomly interspersed among the case-control samples. Laboratory personnel were blinded to quality control, case-control status, or any other participant information. The median intra-assay coefficient of variation from quality control samples ranged from 6.8 to 12.0% for the studied biomarkers [30]. To control for variations across batches, we recalibrated hormone concentrations within each cohort to the value of an “average batch” using the method proposed by Rosner and colleagues [32]. Free estradiol and testosterone concentrations were calculated by a validated algorithm based on total estradiol or testosterone, SHBG, an assumed constant for normal albumin concentrations, and affinity constants of SHBG and albumin for estradiol or testosterone [33]. The ratio of total estradiol to total testosterone was also calculated.
Assessment of covariates
Covariate data were collected through self-administered questionnaires at baseline and during follow-up biennially. In the current study, we used covariate data from the questionnaires closest to blood draw, including body mass index (BMI), smoking status, alcohol consumption, Alternate Healthy Eating Index (AHEI), physical activity, family history of CRC, regular use of aspirin, and postmenopausal hormone use. These covariates are plausible risk factors for colorectal polyps and CRC [34, 35] and might be associated with sex hormone levels [36, 37]. BMI was calculated as body weight in kilograms divided by the square of height in meters. Physical activity was assessed by the product sum of the metabolic equivalent values of each specific recreational activity and hours spent on that activity per week [38]. Positive family history of CRC was defined as report of a CRC diagnosis in a parent or sibling. Consistent with our previous studies [39, 40], regular aspirin use was defined as use of at least two standard-equivalent tablets per week. Alcohol consumption and AHEI were derived from validated food frequency questionnaires. AHEI is a dietary score measuring the adherence to a dietary pattern characterized by foods and nutrients strongly associated with risk of chronic disease [41].
Ascertainment of colorectal polyps
Ascertainment of colorectal polyps in the NHSI and NHSII has been described in detail previously [35]. Briefly, on each biennial questionnaire, participants were asked whether they had undergone a colonoscopy or sigmoidoscopy and whether any colorectal polyp had been diagnosed in the past 2 years. When a participant reported a polyp diagnosis, written consent was to obtain to review her endoscopic and pathologic records. Study physicians, who were unaware of exposure information, confirmed the diagnosis and extracted data on histology, size, number, and anatomic location of polyps. Conventional adenomas included tubular, tubulovillous and villous adenomas, and adenomas with high-grade dysplasia. Advanced adenomas were defined as at least one adenoma of ≥ 10 mm in diameter or with advanced histology (tubulovillous/villous histological features or high-grade or severe dysplasia) [42]. Serrated polyps comprised HPs and mixed/serrated adenomas. The latter consisted of both mixed polyps (those with both adenomatous and hyperplastic changes in histology) and polyps with any serrated diagnosis (e.g., serrated adenomas, serrated polyps, and sessile serrated adenoma/polyps). If a participant had more than one adenoma or serrated polyp in a sublocation (proximal colon, distal colon, or rectum) in an endoscopy, the histology of the most advanced lesion and the size of the largest polyp were used for that sublocation. Although we did not conduct systematic record review for individuals who reported to have undergone an endoscopy but no polyps, our previous validation study in a random sample of 114 women indicated an accuracy of 97% for self-reported negative endoscopies [43].
Statistical analysis
The current study only included postmenopausal women who had undergone at least one lower endoscopy since blood draw. If a woman reported more than one endoscopy during the study period, multiple records were used in the analysis. Participants were censored at the time of the first diagnosis of colorectal polyps, death, or the end of follow-up (June 1, 2012, for the NHSI and June 1, 2011, for the NHSII), whichever occurred first. To account for multiple records per participant, we applied an Andersen-Gill data structure with a new record for each 2-year follow-up during which a participant underwent an endoscopy [44].
We log transformed the concentrations of sex hormones to improve normality. Multivariable logistic regression for clustered data (PROC GENMOD) was used to account for repeated observations (i.e., multiple endoscopies) and to compute odds ratios (ORs) of conventional adenoma and serrated polyp, categorically according to quartiles and continuously per one standard deviation (SD) increment in hormone concentrations, with adjustment for potential confounders (plausible risk factors for colorectal polyps and CRC and might be associated with sex hormone levels). P for trend was calculated by a Wald test. To maximize the ability for confounding control, we calculated the averages of body mass index, physical activity, alcohol consumption, and AHEI using the two adjacent questionnaires administered most proximately to blood draw. For missing data of covariates on a questionnaire (all less than 2%), we carried forward available information from prior questionnaires. To test for potential non-linearity, we performed restricted cubic spline analyses and used a likelihood ratio test to compare the model with the linear term only to the model with both the linear and spline terms. No strong statistical evidence for non-linearity was found. We also compared the biomarker associations between conventional adenoma and serrated polyp through a case-only analysis and calculated P for heterogeneity [45].
We further performed separate analyses according to histopathological features of polyps. Sensitivity analyses were performed among postmenopausal women who were not using hormone therapy and among those who were selected as controls in the source case-control studies. In a subset of participants who also had C-peptide measurements (n = 2330), we additionally controlled for C-peptide levels. Moreover, we analyzed the association of sex hormones with polyp risk according to the median time interval since blood draw (< 11 years, ≥ 11 years).
To account for multiple hypothesis testing, we used a stringent α level of 0.005 as recommended by Benjamin et al. to reduce the chance findings or so-called false positives [46]. All statistical tests were two-sided and conducted in SAS version 9.4 software (SAS Institute Inc., Cary, NC).
Results
During 20 years of follow-up of 5404 postmenopausal women in the NHSI and NHSII, we documented 535 cases of conventional adenoma and 402 cases of serrated polyp, including 109 cases having synchronous conventional adenomas and serrated polyps. The median time interval between blood draw and polyp diagnosis was 11.0 years (interquartile range, 6.7–14.1). As shown in Table 1, compared to women without any polyps, those who developed conventional adenomas or serrated polyps were more likely to have a higher BMI, report a family history of CRC, smoke more cigarettes, drink more alcohol, have a lower AHEI, and were less likely to be physically active at blood draw. A modest/high correlation was found among sex hormones (Additional file 1: Table S3).
Tables 2 and 3 show the associations of sex hormones with conventional adenomas and serrated polyps, respectively. Higher concentrations of SHBG were associated with lower risk of conventional adenomas (P for trend < 0.0001), with the multivariable OR of 0.47 (95% CI 0.35–0.64) comparing the highest to the lowest quartile. A suggestive inverse association was also found for SHBG with serrated polyps (OR, 0.72, 95% CI 0.51–1.02, P for trend = 0.01). By contrast, higher levels of free estradiol and free testosterone were nominally associated with increased risk of conventional adenomas (OR, 1.54, 95% CI 1.02–2.31, P for trend = 0.03 and OR, 1.33, 95% CI 0.99–1.78, P for trend = 0.03, respectively). No association was found for estrone, total estradiol, total testosterone, or the ratio of total estradiol to testosterone.
Given the established malignant potential of advanced conventional adenoma and large serrated polyp, we then focused on these high-risk lesions and examined their associations with sex hormones (Table 4). SHBG was inversely associated with risk of advanced conventional adenomas (P for trend < 0.0001), with the OR comparing extreme quartiles of 0.40 (95% CI 0.24–0.67). A nominally significant association was also observed for SHBG and lower risk of large serrated polyps (OR, 0.47, 95% CI 0.17–1.35, P for trend = 0.02). In addition, higher concentrations of free testosterone were suggestively associated with an increased risk of advanced conventional adenomas (OR, 1.72, 95% CI 1.08–2.73, P for trend = 0.02).
The associations remained essentially unchanged when we restricted the analysis to postmenopausal women who were not taking hormone therapy at the time of blood draw (Additional file 1: Table S4) or control participants of the original case-control studies (Additional file 1: Table S5). When additionally adjusting for C-peptide levels in model 2, the associations of free estradiol and testosterone with conventional adenoma were attenuated to null (P for trend = 0.42 and 0.27, respectively), while the associations of SHBG with conventional adenoma and serrated polyp remained essentially unchanged (P for trend = 0.0003 and 0.01, respectively) (Additional file 1: Table S6). In the stratified analysis by the time interval since blood draw, the inverse association between SHBG and adenoma was statistically significant in both subgroups (< and ≥ 11 years) (Additional file 1: Table S7).
Discussion
In this retrospective analysis of 5404 postmenopausal women in the NHS cohorts, we found that higher concentrations of SHBG were associated with lower risk of conventional adenomas, particularly advanced adenomas. In addition, a suggestive association was found for SHBG with lower risk of large serrated polyps and free estradiol and free testosterone with higher risk of conventional adenomas. These data indicate the potential roles of sex hormones in the early stages of colorectal carcinogenesis.
Several epidemiologic studies have evaluated the association between circulating levels of SHBG and CRC risk. A case-control study nested in 4 US prospective cohorts showed an inverse association between plasma SHBG and CRC risk in men even after adjusting for BMI and C-peptide, but no association in postmenopausal women [19]. Additional two studies of postmenopausal women reported null results [20, 22]; however, the numbers of CRC patients in these studies were relatively small (n < 200) and the statistical power might be limited. In contrast, an investigation of 401 postmenopausal women with CRC enrolled in the Women’s Health Initiative Clinical Trial found a positive association between SHBG levels and CRC risk [23]. The reasons for the conflicting results remain unknown and may relate to the differences in the study population, assaying methods, and covariate adjustments [23]. In line with the free hormone hypothesis, the current study showed an inverse correlation between SHBG and free estradiol and testosterone. Furthermore, we found that higher SHBG levels were associated with lower risk of conventional adenomas, particularly advanced lesions. In addition to modulating sex hormone balance, in vitro evidence suggests that SHBG may suppresses inflammation and lipid accumulation in macrophages and adipocytes independent of testosterone and estradiol [47]. Besides, observational and Mendelian randomization studies have consistently linked lower SHBG levels to an increased risk of insulin resistance and type 2 diabetes, which are important risk factors for CRC, supporting the beneficial role of SHBG in mitigating metabolic perturbations [48, 49]. The observed associations for SHBG in our study remained robust to adjustment for both BMI and C-peptide, suggesting an independent role of SHBG in early colorectal carcinogenesis. Further mechanistic investigations are warranted.
In support of our findings for free estradiol and conventional adenoma, two independent studies have reported positive associations between endogenous levels of estrogens and CRC risk in postmenopausal women [21, 22]. However, these findings are inconsistent with three other studies, including two with null results [19, 20] and one reporting inverse associations for total/free estradiol and estrone [23]. The reasons for the divergent results may be partly attributed to the different assay methods. It has been noted that radioimmunoassay in prior studies is less specific than liquid chromatography tandem mass spectrometry and therefore the levels of estrogens measured by radioimmunoassay are likely to be overestimated [22, 23], while electrochemiluminescence may not be able to detect the very low levels in postmenopausal women [20]. Mechanistically, evidence from in vitro and in vivo studies, although somewhat inconsistent, has suggested the mitogenic and tumorigenic effects of estradiol on colorectal cells [50, 51]. In our study, further adjustment for C-peptide attenuated the association of free estradiol with conventional adenoma, suggesting that the effects may be related to insulin resistance. There is a perception that high levels of circulating estradiol is an indicator of estrogen resistance concomitant with insulin resistance [52].
Regarding endogenous testosterone, a nested case-control study of Japanese postmenopausal women showed a positive association between plasma total testosterone and CRC [20]. Moreover, a joint analysis of the NHS and Women’s Health Study found an OR of 1.43 for CRC (95% CI 0.82–2.50) comparing postmenopausal women in the highest quartile of total testosterone to those in the lowest quartile [19]. Since most testosterone is bound to SHBG or albumin, the free portion (less than 2%) is hypothesized to be most biologically active [53].We observed a suggestive positive association between free testosterone and risk of total and advanced conventional adenoma, supporting a pro-CRC effect of testosterone in postmenopausal women. Although the biological mechanisms remain unclear, testosterone has been shown to stimulate growth of colon cancer cells in vitro and the effect can be inhibited by anti-androgens [54]. Evidence from animal models also indicates that testosterone may promote formation of colorectal adenomas [25]. Additionally, testosterone can exert adverse metabolic effects in women by influencing fat mass expansion and distribution, insulin signaling, and lipid metabolism [55]. Given the established role of adiposity in CRC [56, 57], testosterone may also promote CRC through its metabolic effects.
The major strengths of our study include long-term follow-up and detailed data on covariates that allow for robust confounding control. Moreover, we ascertained polyp diagnosis through medical record review and collected detailed histopathologic information of both conventional adenomas and serrated polyps. Several limitations also should be acknowledged. First, because of the evolving nature and lack of consensus on the diagnostic criteria for specific subtypes of serrated polyps, HPs were difficult to separate from SSLs and TSAs in pathologic records. However, prior data showed that serrated polyps ≥ 10 mm in size, which were likely SSLs, were strongly associated with CRC [58]. Second, although existing evidence indicates that sex hormones are generally stable over at least a 4-year period [59, 60], the current study had only a one-time blood measure for each participant, which may not well reflect a longer period of exposure. Third, our study participants were all female health professionals and almost entirely whites. Further investigations in men and racially diverse populations are needed.
Conclusions
We observed an inverse association of SHBG with risk of overall conventional adenomas and advanced adenomas, as well as a suggestive positive association for free estradiol and free testosterone. Our findings indicate a potential role of sex hormones in early stages of colorectal carcinogenesis. Further studies are needed to confirm our findings and assess the clinical utility of sex hormones for CRC risk stratification.
Availability of data and materials
The NHSI and NHSII datasets can be made available upon reasonable request for scientific collaborations. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu).
Abbreviations
- AHEI:
-
Alternate Healthy Eating Index
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- HP:
-
Hyperplastic polyp
- MET:
-
Metabolic equivalent of task
- NHS:
-
The Nurses’ Health Study
- OR:
-
Odds ratio
- SD:
-
Standard deviation
- SHBG:
-
Sex hormone-binding globulin
- SSL:
-
Sessile serrated lesion
References
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93.
Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42(1):1–10.
Pai RK, Mäkinen MJ, Rosty C. Colorectal serrated lesions and polyps. In: Nagtegaal ID, Arends MJ, Odze RD, Lam AK, editors. WHO classification of tumours of the digestive system. Lyon: IARC Press; J Natl Cancer Inst. 2015;107(10): djv210.
Rashtak S, Rego R, Sweetser SR, Sinicrope FA. Sessile serrated polyps and colon cancer prevention. Cancer Prev Res (Phila). 2017;10(5):270–8.
Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138(6):2088–100.
Holme O, Bretthauer M, Eide TJ, Loberg EM, Grzyb K, Loberg M, Kalager M, Adami HO, Kjellevold O, Hoff G. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut. 2015;64(6):929–36.
Sweetser S, Smyrk TC, Sinicrope FA. Serrated colon polyps as precursors to colorectal cancer. Clin Gastroenterol Hepatol. 2013;11(7):760–7 quiz e754–765.
Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, Simms LA, Young J, James M, Montgomery GW, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131(5):1400–7.
Carr NJ, Mahajan H, Tan KL, Hawkins NJ, Ward RL. Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol. 2009;62(6):516–8.
Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: estrogen pathway in colorectal cancer. Clin Cancer Res. 2013;19(21):5842–8.
Fernandez E, La Vecchia C, Balducci A, Chatenoud L, Franceschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84(5):722–7.
Lin KJ, Cheung WY, Lai JY, Giovannucci EL. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer. 2012;130(2):419–30.
Green J, Czanner G, Reeves G, Watson J, Wise L, Roddam A, Beral V. Menopausal hormone therapy and risk of gastrointestinal cancer: nested case-control study within a prospective cohort, and meta-analysis. Int J Cancer. 2012;130(10):2387–96.
Johnson JR, Lacey JV Jr, Lazovich D, Geller MA, Schairer C, Schatzkin A, Flood A. Menopausal hormone therapy and risk of colorectal cancer. Cancer Epidemiol Biomark Prev. 2009;18(1):196–203.
Simon MS, Chlebowski RT, Wactawski-Wende J, Johnson KC, Muskovitz A, Kato I, Young A, Hubbell FA, Prentice RL. Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol. 2012;30(32):3983–90.
Charlton BM, Wu K, Zhang X, Giovannucci EL, Fuchs CS, Missmer SA, Rosner B, Hankinson SE, Willett WC, Michels KB. Oral contraceptive use and colorectal cancer in the Nurses’ Health Study I and II. Cancer Epidemiol Biomark Prev. 2015;24(8):1214–21.
Song J, Jin Z, Han H, Li M, Guo Y, Guo H, Guo W, He J. Hormone replacement therapies, oral contraceptives, reproductive factors and colorectal adenoma risk: a systematic review and dose-response meta-analysis of observational studies. Colorectal Dis. 2019;21(7):748–59.
Goodman MP. Are all estrogens created equal? A review of oral vs. transdermal therapy. J Womens Health (Larchmt). 2012;21(2):161–9.
Lin JH, Zhang SM, Rexrode KM, Manson JE, Chan AT, Wu K, Tworoger SS, Hankinson SE, Fuchs C, Gaziano JM, et al. Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol. 2013;11(4):419–24 e411.
Mori N, Sawada N, Iwasaki M, Yamaji T, Goto A, Shimazu T, Inoue M, Murphy N, Gunter MJ, Tsugane S. Circulating sex hormone levels and colorectal cancer risk in Japanese postmenopausal women: the JPHC nested case-control study. Int J Cancer. 2019;145(5):1238–44.
Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Howard BV, Wylie-Rosett J, Anderson GL, Ho GY, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68(1):329–37.
Clendenen TV, Koenig KL, Shore RE, Levitz M, Arslan AA, Zeleniuch-Jacquotte A. Postmenopausal levels of endogenous sex hormones and risk of colorectal cancer. Cancer Epidemiol Biomark Prev. 2009;18(1):275–81.
Murphy N, Strickler HD, Stanczyk FZ, Xue X, Wassertheil-Smoller S, Rohan TE, Ho GY, Anderson GL, Potter JD, Gunter MJ. A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. J Natl Cancer Inst. 2015;107(10).
Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab. 1986;15(2):213–28.
Amos-Landgraf JM, Heijmans J, Wielenga MC, Dunkin E, Krentz KJ, Clipson L, Ederveen AG, Groothuis PG, Mosselman S, Muncan V, et al. Sex disparity in colonic adenomagenesis involves promotion by male hormones, not protection by female hormones. Proc Natl Acad Sci U S A. 2014;111(46):16514–9.
Thaler MA, Seifert-Klauss V, Luppa PB. The biomarker sex hormone-binding globulin - from established applications to emerging trends in clinical medicine. Best Pract Res Clin Endocrinol Metab. 2015;29(5):749–60.
Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, Chavarro JE. Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health. 2016;106(9):1573–81.
Bertrand KA, Giovannucci E, Liu Y, Malspeis S, Eliassen AH, Wu K, Holmes MD, Laden F, Feskanich D. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br J Nutr. 2012;108(10):1889–96.
Hunter DJ, Hankinson SE, Hough H, Gertig DM, Garcia-Closas M, Spiegelman D, Manson JE, Colditz GA, Willett WC, Speizer FE, et al. A prospective study of NAT2 acetylation genotype, cigarette smoking, and risk of breast cancer. Carcinogenesis. 1997;18(11):2127–32.
Hang D, Kvaerner AS, Ma W, Hu Y, Tabung FK, Nan H, Hu Z, Shen H, Mucci LA, Chan AT, et al. Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals. Am J Clin Nutr. 2019;109(3):635–47.
Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25(2):165–72.
Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653–66.
Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–10.
Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AV, Andrews KS, Bandera EV, Spees CK, Robien K, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245–71.
He X, Wu K, Ogino S, Giovannucci EL, Chan AT, Song M. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology. 2018;155(2):355–73 e318.
Brand JS, Chan MF, Dowsett M, Folkerd E, Wareham NJ, Luben RN, van der Schouw YT, Khaw KT. Cigarette smoking and endogenous sex hormones in postmenopausal women. J Clin Endocrinol Metab. 2011;96(10):3184–92.
Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015;16(7):581–606.
Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF, Paffenbarger RS Jr. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80.
Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–42.
Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294(8):914–23.
Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18.
Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–57.
Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095–105.
Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat. 1982;10:1100–20.
Wang M, Spiegelman D, Kuchiba A, Lochhead P, Kim S, Chan AT, Poole EM, Tamimi R, Tworoger SS, Giovannucci E, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35(5):782–800.
Benjamin DJ, Berger JO, Johannesson M, Nosek BA, Wagenmakers EJ, Berk R, Bollen KA, Brembs B, Brown L, Camerer C, et al. Redefine statistical significance. Nat Hum Behav. 2018;2(1):6–10.
Yamazaki H, Kushiyama A, Sakoda H, Fujishiro M, Yamamotoya T, Nakatsu Y, Kikuchi T, Kaneko S, Tanaka H, Asano T. Protective effect of sex hormone-binding globulin against metabolic syndrome: in vitro evidence showing anti-inflammatory and lipolytic effects on adipocytes and macrophages. Mediat Inflamm. 2018;2018:3062319.
Le TN, Nestler JE, Strauss JF 3rd, Wickham EP 3rd. Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol Metab. 2012;23(1):32–40.
Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361(12):1152–63.
Gilligan LC, Rahman HP, Hewitt AM, Sitch AJ, Gondal A, Arvaniti A, Taylor AE, Read ML, Morton DG, Foster PA. Estrogen activation by steroid sulfatase increases colorectal cancer proliferation via GPER. J Clin Endocrinol Metab. 2017;102(12):4435–47.
Foster PA. Oestrogen and colorectal cancer: mechanisms and controversies. Int J Color Dis. 2013;28(6):737–49.
Mauvais-Jarvis F. Is estradiol a biomarker of type 2 diabetes risk in postmenopausal women? Diabetes. 2017;66(3):568–70.
Khosla S. Editorial: Sex hormone binding globulin: inhibitor or facilitator (or both) of sex steroid action? J Clin Endocrinol Metab. 2006;91(12):4764–6.
Tutton PJ, Barkla DH. The influence of androgens, anti-androgens, and castration on cell proliferation in the jejunal and colonic crypt epithelia, and in dimethylhydrazine-induced adenocarcinoma of rat colon. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;38(3):351–5.
Schiffer L, Arlt W, O’Reilly MW. Understanding the role of androgen action in female adipose tissue. Front Horm Res. 2019;53:33–49.
Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86(3):s836–42.
Jinjuvadia R, Lohia P, Jinjuvadia C, Montoya S, Liangpunsakul S. The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J Clin Gastroenterol. 2013;47(1):33–44.
Hiraoka S, Kato J, Fujiki S, Kaji E, Morikawa T, Murakami T, Nawa T, Kuriyama M, Uraoka T, Ohara N, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology. 2010;139(5):1503–10 1510 e1501–1503.
Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2-3-year period. Cancer Epidemiol Biomark Prev. 1995;4(6):649–54.
Dashti SG, Viallon V, Simpson JA, Karahalios A, Moreno-Betancur M, English DR, Gunter MJ, Murphy N. Explaining the link between adiposity and colorectal cancer risk in men and postmenopausal women in the UK Biobank: a sequential causal mediation analysis. Int J Cancer. 2020;147(7):1881–94.
Acknowledgements
We would like to thank the participants and staff of the Nurses’ Health Study and the Nurses’ Health Study II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding
This work was supported by the American Cancer Society Mentored Research Scholar Grant (MRSG-17-220-01 - NEC to MS); by the US National Institutes of Health grants (P01 CA87969, UM1 CA186107 to MJ Stampfer; U01 CA176726, P01 CA55075 to WC Willett; R01 CA49449, R01 CA67262 to SE Hankinson; R01 HL088521 to KM Rexrode; U01 CA167552 to LA Mucci and WC Willett; P50 CA127003 to CS Fuchs; K24 DK098311, R01 CA137178, R01 CA202704, R01 CA176726 to ATC; R01 CA151993, R35 CA197735 to SO; R21 CA230873 to KW; K99 CA215314, R00 CA215314 to MS); and by grants from National Key R&D Program of China (2017YFC0908300 to DH), National Natural Science Foundation of China (81973127 to DH), Natural Science Foundation of Jiangsu Province (BK20190083 to DH), the American Institute for Cancer Research (KW), The Project P Fund for Colorectal Cancer Research, The Dana-Farber Harvard Cancer Center (through the Nodal Award 2016-02 to SO), Bennett Family Fund, and The Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. ATC is a Stuart and Suzanne Steele MGH Research Scholar. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
DH performed the statistical analysis and drafted the manuscript. XH, ASK, ATC, KW, SO, ZH, HS, and ELG were involved in the acquisition, analysis, and interpretations of data. MS was responsible for study design. All authors critically assessed, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from all participants in this study. The institutional review boards of Brigham and Women’s Hospital and Harvard T.H. Chan School Public Health approved the study protocol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Flowchart of study participant selection. Table S1. Cases and controls included in this study by their original study status in the NHSI and NHSII. Table S2. Baseline characteristics of all postmenopausal women at blood draw and those included in the current study. Table S3. Age-adjusted Spearman correlation coefficients between plasma sex hormones and body mass index (BMI) in postmenopausal women from the NHSI and NHSII. Table S4. Associations of plasma sex hormones with conventional adenoma, serrated polyp, and advanced lesions in postmenopausal women not taking hormone therapy at blood draw from the NHSI and NHSII. Table S5. Associations of plasma sex hormones with conventional adenoma and serrated polyp in postmenopausal women who were selected as controls in the source case-control studies from the NHSI and NHSII. Table S6. Associations of plasma sex hormones with conventional adenoma and serrated polyp after further adjustment for plasma C-peptide levels in postmenopausal women from the NHSI and NHSII. Table S7. Associations of plasma sex hormones with conventional adenoma and serrated polyp in postmenopausal women according to the median time interval since blood draw.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hang, D., He, X., Kværner, A.S. et al. Plasma sex hormones and risk of conventional and serrated precursors of colorectal cancer in postmenopausal women. BMC Med 19, 18 (2021). https://doi.org/10.1186/s12916-020-01895-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-020-01895-1