Abstract
Background
Coevolution between modern aphids and their primary obligate, bacterial endosymbiont, Buchnera aphidicola, has been previously reported at different classification levels based on molecular phylogenetic analyses. However, the Buchnera genome remains poorly understood within the Rhus gall aphids.
Results
We assembled the complete genome of the endosymbiont Buchnera in 16 aphid samples, representing 13 species in all six genera of Rhus gall aphids by shotgun genome skimming method. We compared the newly assembled genomes with those from GenBank to comprehensively investigate patterns of coevolution between the bacteria Buchnera and their aphid hosts. Buchnera genomes were mostly collinear, and the pan-genome contained 684 genes, in which the core genome contained 256 genes with some lineages having large numbers of tandem gene duplications. There has been substantial gene-loss in each Buchnera lineage. We also reconstructed the phylogeny for Buchnera and their host aphids, respectively, using 72 complete genomes of Buchnera, along with the complete mitochondrial genomes and three nuclear genes of 31 corresponding host aphid accessions. The cophylogenetic test demonstrated significant coevolution between these two partner groups at individual, species, generic, and tribal levels.
Conclusions
Buchnera exhibits very high levels of genomic sequence divergence but relative stability in gene order. The relationship between the symbionts Buchnera and its aphid hosts shows a significant coevolutionary pattern and supports complexity of the obligate symbiotic relationship.
Similar content being viewed by others
Background
Insects are the most diverse and abundant class of animals on earth and are associated with a remarkable range of symbiotic microorganisms. Many insects are well known to have co-diversified with intracellular bacterial symbionts, or endosymbionts, including nearly all groups of phloem sap-sucking insects [1,2,3]. Among plant-sap-sucking insects, aphids comprise around 4000 species mainly distributed throughout the temperate regions of the globe [4] and constitute a monophyletic group, superfamily Aphidoidea, within the order Hemiptera [5]. Most aphids have an obligate, mutualistic relationship with the symbiotic bacteria, Buchnera aphidicola (Proteobacteria: Gammaproteobacteria: Enterobacteriaceae) inhabiting specialized cells called bacteriocytes, which occur in the aphid’s abdominal haemocoel [6,7,8]. Buchnera aphidicola is required for host development, growth, and reproduction [9, 10]. Buchnera provides the host aphid with nutrition such as amino acids, vitamins, and sterols, which are all necessary for normal development and reproduction but cannot be synthesized by the aphids and are deficient in their phloem sap diet [11,12,13]. In turn, the aphid provides Buchnera with nutrients, including nonessential amino acids and carbohydrates that are abundant in the phloem diet or produced by the aphid host [10, 14]. The bacteria are also completely dependent on the aphid for vertical transmission through maternal lineages [3, 11]. Recently, comparative whole-genome sequence analysis of the pea aphid, Acyrthosiphon pisum, and its primary endosymbiont revealed that these two obligate mutualists were fully interdependent for the biosynthesis of amino acids, and the two genomes formed a highly integrated metabolic collaboration [13, 15,16,17]. The relationship between Buchnera and their host aphids is at least 150 million years old [18, 19] and has been considered one of the best-studied cases of symbiosis and coevolution [20,21,22,23,24].
The term “coevolution” was first introduced in a study on butterflies and their plant hosts in 1964 [25]. Co-phylogenetic analysis of the host and parasite trees have been the main method for the study of coevolution with rapid development of molecular phylogenetic techniques [26]. The degree of congruence reflects whether parasites and their hosts have undergone co-diversification, in which one organismal partner triggers diversification in the other, or if they have random associations in their evolutionary history [27, 28]. Co-diversification may be investigated by comparing the topologies of phylogenetic trees of the host and parasite lineages. The topologies might be congruent if co-diversification has occurred, and the timing of diversification in both lineages should also be correlated in a co-diversification scenario [26, 29].
Different strains of this symbiotic bacteria Buchnera within different aphid hosts have been named as one species, i.e., Buchnera aphidicola [20]. The co-diversification between Buchnera and its host aphid has been investigated in prior studies at different taxonomic scales. These studies mainly used the 16S ribosomal RNA gene for phylogenetic reconstruction and focused on closely related species of aphids or intra-species lineages [21, 22, 30,31,32,33]. Recently, Arab and Lo (2021) suggested a highly significant correlation of molecular rates between the genomes of Buchnera and the mitochondrial genomes of their hosts [34]. These analyses usually supported parallel evolution and co-diversification in the aphid-Buchnera associations and suggested that the Buchnera gene sequences could be used as molecular markers to define the species and evolutionary relationships of the aphids [21, 30].
Since the complete genome of Buchnera aphidicola sp. strain APS from the pea aphid (Acyrthosiphon pisum) was first sequenced and analyzed [15], so far, there have been 73 complete genomes of Buchnera reported in GenBank from 56 aphid species (Additional file 1: Table S1), belonging to the family Aphididae and representing eight subfamilies, i.e., Aphidinae, Eriosomatinae, Lachninae, Calaphidinae, Phyllaphidinae, Hormaphidinae, Thelaxinae, and Anoeciinae [15, 19, 35,36,37,38,39,40]. In total, the Buchnera aphidicola genomes range from 412 to 646 kb in length, thus comprising one of the smallest known cellular genomes of symbiotic bacteria [34, 36, 38, 41,42,43]. Buchnera has experienced drastic shrinkage in genome size, retaining only essential genes for its specialized lifestyle, and genome shrinkage may be ongoing [15, 38, 44,45,46].
The Buchnera genome remains poorly understood within the Rhus gall aphids of the subtribe Melaphidina (Aphididae: Eriosomatinae: Fordini) [5, 47, 48]. The Melaphidina aphids live obligately on species of sumac plants (Rhus subgenus Rhus, Anacardiaceae) as the primary host for part of their life cycle to induce a large gall on a developing leaf or shoot [49]. The gall is rich in tannin and, therefore, effective in traditional medicine for stopping dysentery, bleeding, coughing, sweating, and rectal prolapse [49]. Also, tannins are extracted from the galls and further chemically synthesized as a series of ester compounds, which are widely applied as additive component in various fields, such as medicines, electronics, chemical products, oils, inks, dyeing, and foods [50]. The Melaphidina aphids, like other aphids, are believed to have an obligate, mutualistic relationship with their endosymbiotic Buchnera aphidicola [4, 18, 51, 52]. The genomes of Buchnera in Melaphidina have not been studied comprehensively, and only two genome sequences of Buchnera strains from Melaphidina were published [38]. Yet, little is known about the co-evolutionary patterns between Rhus gall aphids and their primary symbiont Buchnera.
In this study, we sequenced the complete genomes of Buchnera aphidicola in Rhus gall aphids and investigated the co-evolution of Buchnera and its host aphids within a phylogenomic framework. We obtained 15 complete genomes of Buchnera aphidicola in 17 samples of the 16 Rhus gall aphid species (including three subspecies and two individuals for the species Nurudea yanoniella) and Chaetogeoica yunlongensis, a species in the Fordina subtribe sistering to Rhus gall aphids. Each complete genomes of Buchnera aphidicola is derived from only one species or subspecies of Rhus gall aphids, including all six genera and the 13 species recognized within Melaphidina. We combined these newly generated genomes with the existing molecular sequences for B. aphidicola in other aphids from GenBank to construct the Buchnera phylogeny, along with the aphid phylogeny based on complete mitochondrial genome and three nuclear genes to investigate coevolution and/or co-diversification of these endosymbionts and their host aphids, while we especially focused on Rhus gall aphids and their symbiont Buchnera. Our work aims to yield new insights into biodiversity of Buchnera and into the coevolutionary patterns of the aphid-bacterial system.
Results
Buchnera genomics and phylogenomics
All the newly sequenced bacterial genomes are available from the SRA database of NCBI as raw data, from that we assembled and annotated genomes for 15 newly sequenced Buchnera samples, which were deposited and available in GenBank (Table 1). For seven of the 15 genomes, we were able to assemble a single circular chromosome with one contig and other nine with more than one contig (Table 1). There were two Buchnera samples in Kaburagia rhusicola ovatirhusicola and K. r. ovogallis strain for which we did not recover sequences of Buchnera even though these species are assumed to contain the symbionts (Codes R9 and R11 in Table 1). These aphids and bacterial symbionts will be subject in future investigation to determine whether these results are due to poor bacterial preservation or unexpected biological loss of the association.
We downloaded 57 genomes of Buchnera in other aphids from GenBank (Additional file 1: Table S1) as well as our current Buchnera genomes to compare and analyze their general characters. As a result, we found that the 72 Buchnera genomes ranged from 412,404 to 645,850 bp in length with a strong bias towards A + T (71.9 to 79.9%). All the symbiont genomes in Rhus gall aphids ranged from 604,433 to 626,129 bp and contained 532 to 550 protein-coding genes, 31 to 33 tRNAs, one ncRNA, one tmRNA, and three rRNAs. These genomes of Buchnera in different aphid species are relatively stable in terms of gene order, in contrast to the high levels of nucleotide divergence.
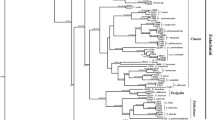
Based the concatenated protein-coding gene dataset, we constructed a maximum likelihood (ML) phylogenetic tree for all the 72 Buchnera samples with Escherichia coli, Shigella sp., and Candidatus Ishikawaella capsulata as outgroups (Fig. 1a). The tree well supported the monophyly of each subfamily sampled in this study. We observed a seven-gene (thyA, lgt, lysA, lysS, lysU, prfB, and ygfZ) inversion across all Buchnera genomes within the subfamily “Eriosomatinae” clade (Fig. 2 and Table 2), and we also found a four-gene (leuA, leuB, leuC, and leuD) rearrangement with three different orders in different subfamilies, i.e., ABCD in Anoecia oenotherae from the subfamily Anoeciinae, and DCBA in Therioaphis trifolii + Sarucallis kahawaluokalani + Stegophylla sp. from the subfamily Calaphidinae and Phyllaphidinae, respectively, while ADCB was observed in the tribe Fordini from the subfamily Eriosomatinae. Moreover, the genes trpG and trpE were inserted in a different location of the Buchnera genomes within Eriosomatinae and Calaphidinae + Phyllaphidinae aphids, and the genes ibpA and ibpB also presented in different positions within Buchnera of some aphids in subfamily Aphidinae and Calaphidinae + Phyllaphidinae. Despite stability of gene order, there has been substantial gene loss across each subfamily. Buchnera corresponding to each aphid subfamily had different numbers of gene losses shared that are still retained in any sampled Buchnera taxon within that group to date, i.e., Eriosomatinae, Calaphidinae, and Aphidinae (Table 2). There are 15 gene losses shared among all members of the “Lachninae” clade, 18 gene losses shared among the “Phyllaphidinae” clade, two gene losses shared among the “Hormaphidinae” clade, and one gene loss shared among each of the “Thelaxinae” and “Anoeciinae” clades.
Tanglegram depicting the associations of Buchnera (a) and host aphids (b) by TreeMap analysis. Stars at the branches represent bootstrap support of ML 100% and BI 1.00. The red dot on the internal node represents the significance of their congruence according to the TreeMap analysis with darker red being of greater congruence. The colors correspond to the subfamilies of host aphids represented and their respective parasite species, Blue: Aphidinae, Purple: Eriosomatinae, Green: Lachninae, Brown: Calaphidinae, Yellow: Phyllaphidinae, Pink: Anoeciinae, Cyan: Thelaxinae, Light green: Hormaphidinae. Code of Buchnera aphidicola are the same as Table 1 and Additional file 1: Table S1
Genomes in circular genome diagram (outer to inner, organized in phylogenetic order). Aphid hosts: S. graminum, M. persicae, U. ambrosiae, A. pisum, T. salignus, C. cofinis, C. tujafilina, C. cedri, C. pseudotaxifoliae, C. strobi, C. fornacula, B. pistaciae, R20 (N. shiraii), R22 (M. rhois), R1 (S. chinensis), S. chinensis, R4 (F. choui), R8 (M. elongallis), and R7 (K. r. ensigallis)
Genomes of the outgroup taxa, Escherichia coli (4.6 Mbp) and Shigella sp. PAMC 28760 (4.5 Mbp), are almost ten times as large as any of the genomes of Buchnera. Escherichia coli and Shigella sp. contain all the genes that are part of the Buchnera pan-genome, which has 684 genes. Ishikawaella also has a reduced genome (700 Kbp) but does not have the conserved gene order that the Buchnera genomes have nor does the Ishikawaella genome contain all genes of the Buchnera pan genome (Fig. 3), indicating that it has its own history of gene loss. We identified a core genome of 256 shared genes in the sampled species of Buchnera. However, the high level of nucleotide sequence divergence shows that some putatively homologous genes do not align at the nucleotide level. The atp gene family, which contains up to eight members (atpA-atpH), is notably absent from all members of the “Lachninae” clade, as is the mur gene family (murA-murD, murF, murG, and murI). GC content (Table 1 and Additional file 1: Table S1) for Buchnera samples ranged from 20.1 to 28.1%. For E. coli and Shigella, GC content are both 50.8%, but 30.2% for Ishikawaella.
Venn diagrams showing shared gene content of Buchnera samples. a Five representatives from the ingroup. b Five representatives from Rhus gall aphid species (purple clade in Fig. 1). c Three outgroup species and one ingroup taxon
The RAxML tree showed three major clades of Buchnera with high support corresponding to the subfamilies of aphid hosts (Fig. 1a). The relationship among the Buchnera strains in different aphid subfamilies is as follows: Buchnera in—(Aphidinae, (Eriosomatinae, (Lachninae + Anoeciinae + Thelaxinae + Hormaphidinae + Phyllaphidinae + Calaphidinae))), to which we referred as the Buchnera-Aphidinae, Eriosomatinae, and LATHPC clades, respectively. In the case of the LATHPC clade, our sampling comprised only one or two genomes for each of the five subfamilies except for Lachninae, and there were some bacterial taxa for which there were no corresponding aphid sequences available. The branching patterns of the Buchnera tree completely correspond to the classification of the aphids (Fig. 1a).
Aphid phylogenetics
Our samples consisted of three subfamilies and 31 species from the family Aphididae with two species from the two families Phylloxeridae and Adelgidae as outgroups (Table 3). The combined, aligned mitochondrial genomes (13 protein-coding genes and two rRNAs) represented 13,159 bp and the three nuclear genes comprised a matrix of 4,531 bp in length. We concatenated the 15 mtDNA genes with the three nuclear datasets based on the results of an incongruence length difference (ILD) test (p = 0.33 > 0.01) and the concatenated sequences had 17,690 bp.
Independent analyses of the concatenation of the three nuclear genes and mitochondrial genes yielded topologies with high support for clades, especially comprising species within the same genus, but the relationships among deep nodes had low bootstrap support (BS < 60). The four phylogenetic analyses, i.e., ML and Bayesian inference (BI) with and without partitioning, of the concatenated, or total-evidence, dataset showed high support for the monophyly of all three subfamilies (Aphidinae, Eriosomatinae, and Lachninae) by the Bayesian posterior probabilities (PP) and BS (BI-PP = 1.00 and ML-BS = 100), and the tribes within each subfamily, except for Tuberolachnini and Enlachnini in Lachninae with only one species used in our sampling, i.e., Tuberolachnus salignus and Cinara tujafilina. In all trees, the six genera of Melaphidina aphids composed five generally well-supported clades: Nurudea, Melaphis, Schlechtendalia, Floraphis, and Meitanaphis + Kaburagia. There was some incongruence among the concatenated trees generated using the four different approaches. Notably, Lachninae was sister to a clade of Aphidinae and Eriosomatinae in all trees with high support (BI-PP = 1.00 and ML-BS = 100) except the ML tree with no partitioning, in which Lachninae and Aphidinae were sisters but with low support (ML-BS = 59). Our results also yielded differences in relationships within the highly supported clade of Acyrthosiphon + Sitobion, Diuraphis, and Myzus (BI-PP = 1.0 and ML-BS > 88) (Additional file 2: Figs. S1, S2 and S3).
Therefore, we showed the BI tree resulting from the analysis with partitioning among genes (Fig. 1b). Notably, the Bayesian trees reconstructed under the GTR + G model may be more robust estimates of relationships and evolutionary distances due to base compositional biases inferred by IQTree [57]. The BI topology showed that the three subfamilies formed three clades with well-support (BI-PP = 1.00 and ML-BS = 100), and Aphidinae is closer to Eriosomatinae than to Lachninae with high BI-PP = 0.97, but low ML-BS = 60 (Fig. 1b). The four species Acyrthosiphon pisum, Sitobion avenae, Myzus persicae, and Diuraphis noxia of the tribe Macrosiphini grouped as a clade with high support as well as the six species of the tribe Aphidini, whose monophyly was well supported (BI-PP = 1.00 and ML-BS = 97). The two tribes Tuberolachnini and Enlachnini only include one species Tuberolachnus salignus and Cinara tujafilina, respectively, which grouped as a high-supported clade.
Cophylogenetic analyses of Aphid-Buchnera
Based on topology-based cophylogenetic analyses by TreeMap and Jane, evolution of Buchnera was generally in accordance with their aphid hosts at individual, species, generic, and tribal levels (Figs. 1 and 4 and Table 4). A disagreement between trees results from the position of the subfamily Lachninae (Fig. 1), which is sister to the group of Aphidinae + Eriosomatinae in the aphid tree (BI-PP = 1.00 and ML-BS = 100), but Buchnera in Lachninae is closer to the Buchnera species in Eriosomatinae in the bacterial tree. We detected three duplication and host switch events between Myzus and Acyrthosiphon + Sitobion, Melaphis and Schlechtendalia, two genera of Lachninae, which group was well supported (100%) in both the Buchnera and aphid tree. Also, one duplication and host switch event was detected between the two genera Melaphis and Schlechtendalia species at the genus level in the tribe Fordini.
The cophylogeny of aphid and Buchnera from Jane at the different level with the reconciled trees based on the aphid tree (BI) and Buchnera tree (ML): a species; b genus; c tribe. Black and blue maps indicate the phylogenies of the aphid and Buchnera, respectively. Hollow red circles indicate co-diversification events; solid red and yellow circles indicate duplications; arrows indicate host switch events; dotted lines indicate loss events
The distance-based methods for cophylogenetic inference showed significant global fit for all four reconstructions of the aphid tree compared with the bacterial tree (p = 0.001 for ParaFit and p ≤ 0.001 for PACo), and the signal of global congruence was significant (Fig. 5). However, the analyses based on the aphid trees without partitioning showed considerably better global fit with the tree of bacteria than the analyses based on trees reconstructed with partitioning. For both ParaFit and PACo, the ML analyses without partitions yielded the best fit with the bacterial tree, while BI with partitions showed the least good fit. In the PACo, trees reconstructed without partitions led to associations showing slightly positive effects on the global fit, while analyses with partitions showed much larger negative effects on global fit. Each individual associations were analyzed by the individual host–parasite (H-P) link test in ParaFit. Both ML and BI analyses with and without partitions showed 24 of the 30 aphid-Buchnera links and had a significant coevolution relationship with ParaFit1 or ParaFit2 (p value ≤ 0.05). The largest Aphids-Buchnera residuals in all the four PACo analyses were Cinara tujafilina and Tuberolachnus salignus, indicating that these two species had the weakest signal of cophylogeny with their symbiont Buchnera.
Discussion
Buchnera comparative genomics
Buchnera is endosymbiotic within host species throughout the aphid lineage. Even though Buchnera is recognized to include a single species, B. aphidicola, it exhibits extremely high genetic diversity as a lineage of Gammaproteobacteria and is at least 50 million years old based on the Buchnera accessions studied so far [19, 38, 58, 59]. While there has been substantial evolutionary change in Buchnera (see Additional file 3: Dataset S1 for pairwise identity across homologous regions) with gene losses and gains (Table 2 and Additional file 3: Dataset S1), the arrangement of genes appears extremely stable (cite our data) [59]. In prior studies, two rearrangements and one inversion were detected in the sequenced genomes of Buchnera in Eriosomatinae, Calaphidinae, Phyllaphidinae, and Anoeciinae [15, 35, 38, 60,61,62,63]. In this study, we found rearrangement of ibpA and ibpB in Calaphidinae + Phyllaphidinae, despite sampling one or two species from each of the subfamilies Calaphidinae, Phyllaphidinae, and Anoeciinae.
Our analysis on the Buchnera pan-genome for 72 taxa contained 684 genes, and the core genome contained 256 genes. We calculated gene gains and losses of each clade by comparing with core genome of other clades, rather than with the inferred ancestral genome. We investigated gene gains by comparing the Buchnera core genomes comprising only those genes shared by all sampled species to pan-genomes of other clades. To reconstruct the ancestral genome of Buchnera, van Ham et al. (2003) identified 601 ancestral protein-coding genes based on three genomes of Buchnera [35], while Chong et al. (2019) inferred 616 ancestral protein-coding genes based on 39 complete genomes [38]. Although we have expanded the sampling of Buchnera compared to these prior studies, there are many unsampled or as yet unknown strains of Buchnera. van Ham et al. (2003) suggested that gene loss in Buchnera continued among extant lineages at a slower rate, but there is no evidence that the gene gains are occurring [35]. Here, we found up to five gene gains based on each subfamily clade, and they all exist in Escherichia coli genome, four of which have the same relative positions as in Buchnera genome, while only the position of the ydiN gene showed obvious rearrangement comparing with E. coli. Therefore, we speculated that the gained four genes in different subfamily were relative to their loss in other subfamilies. The rearrangement of ydiN gene might be from regaining through loss, horizontal gene transfer from the host or other bacteria, etc. However, the evidence suggests that genes related to leucine (leuABCD) and tryptophan (trpEG) biosynthesis have undergone multiple movements between plasmid and chromosome locations, finding on the variable locations of these genes in previous and this study [15, 38, 60,61,62].
We performed a comparative genomic analysis of Escherichia coli and Shigella sp. PAMC 28760 representing related free-living bacteria, which were also belong to the family Enterobacteriaceae. The genome length of Buchnera in Aphididae hosts is much smaller than that of the two genomes of E. coli (4.6 Mbp) and Shigella (4.5 Mbp), which points to the very distinct evolutionary trajectory of these bacteria towards genome reduction [15, 35, 38]. It has been reported that Buchnera has some of the smallest genomes in bacteria sequenced to date and is lacking many of the genes that would enable them to live freely and, thus, be cultured. For example, Charles et al. (2011) investigated the reduction of membrane transporter genes in Buchnera, which are essential for shared metabolic networks [56]. These authors showed that, overall, Buchnera had lost membrane transporter genes, but the decreased diversity and limited substrate specificity of those retained probably facilitated essential movement of metabolic products. Moreover, genes involved in heat tolerance may have been purged from the genomes of Buchnera, yielding a greater heat sensitivity, which may, in turn, constrain the ecological and geographic ranges of the aphid hosts [64].
In this study, we included the genomes of the same four strains studied by Charles et al. (2011; A. pisum, S. graminum, B. pistaciae, and C. cedri) [56]. What is striking is that the genes lost in Buchnera of the aphid subfamily Lachninae (or the “Lachninae” clade) are present in all the other Buchnera lineages sampled in this study. Within the “Lachninae” Buchnera, the most of genes lost have protein products that are membrane transporters. These include (1) the F-ATP synthase complex, revealing that these Buchnera do not use this pathway to drive ATP synthesis; (2) members of the ATP-dependent mur ligases (murA-murD, murF, murG, murI), which are key in the synthesis of peptidoglycan, or the bacterial cell walls [65]; (3) the glucose/mannitol phosphotransfer-driven group translocators superfamily (PTS) transport system, which could necessitate that Buchnera import carbon sources to build up essential amino-acid backbones for its host from other transporters. In contrast, Buchnera strains from other aphid subfamilies possess all complete PTS systems for the importation of glucose and mannitol, which are the two main carbon sources detected in the cytoplasm of bacteriocytes [66].
We especially found the peptidoglycan-associated lipoprotein (Pal) encoded only in the genome of Buchnera from the aphid subfamily Eriosomatinae in our current study. The protein Pal was anchored in the outer membrane (OM) of Gram-negative bacteria and interacts with Tol proteins [56]. But no inner membrane Tol proteins have been found in any strains of Buchnera. The role of Pal protein in Eriosomatinae remains unknown, but its features suggested the possibility of neo-functionalization.
More work is needed to include other lineages related to Buchnera and Candidatus Ishikawaella capsulata, a special intestinal bacterium and a sister group of the obligate intracellular symbiote Buchnera in aphids [38], that might be able to tell us more about the evolutionary processes leading to their acquisition as a symbiont and genome reduction. Gene repertoire and elevated evolutionary rate of Ishikawaella were strikingly similar to Buchnera [67], providing a possible case of similar evolutionary patterns and convergence of some gene. This work represents one of several recent and ongoing efforts to expand the number of sequenced genomes of Buchnera (i.e., 72 included in this study).
Call for Buchnera taxonomic delimitations
The large number of available genomes represents considerable genetic resources for taxonomic revision of Buchnera. In cases where bacteria cannot be cultured, a description may serve as the type according to Rule 18a of the International Code of Nomenclature of Bacteria [68], and, notably, this rule was cited in the original description of Buchnera aphidicola [20]. The genomic data for Buchnera could be utilized as the basis for delimiting species, even though the breadth of genomic variation allowable within a single bacterial species may be much larger than that in eukaryotes [69], depending on the species concept [70].
Now that there are a sizable number of sequenced genomes of Buchnera (i.e., 72 included in this study), we call for a reassessment and further studies on the taxonomy of the group. Such taxonomic reassessment and debate may result in an expansion of the number of species within Buchnera. The reassessment needs to further expand the taxon sampling and it is possible that other taxonomically divergent relatives of Buchnera may be discovered (with genomes reduced intermediately between E. coli and Buchnera).
Aphid and Buchnera co-evolution
As an obligate endosymbiont, Buchnera aphidicola provides aphid hosts with several essential nutrients, and numerous studies have indicated the congruent phylogenetic relationship between the endosymbiont and the aphids [19, 22, 71, 72]. Most of previous studies focused on lower taxonomic levels and/or several genes (e.g., closely related species or intraspecific lineages). Here, we constructed the phylogenetic relationship with the broadest taxonomic sampling of whole-genome sequences across Buchnera on diverse host lineages and of complete mitochondrial genome and three nuclear genes across the host aphids (Hemiptera: Aphididae).
The cophylogenetic analyses demonstrated significant patterns of coevolution between Buchnera and its aphid hosts. However, the patterns are more complex than simply shared branching order. In fact, the most robustly reconstructed evolutionary trees for aphids using data partitioning [73] compared to the bacterial tree showed significant coevolutionary relationships (Table 4 and Figs. 1, 4, and 5), but analyses for individual associations showed negative (and often significant) impacts on global fit, which have a higher value than most individual associations of no-partitioned data. Prior studies have regarded this complex specific relationship inferred using distance-based cophylogenetic methods as representing one or more host switches [74, 75].
However, this seems unlikely in our case as visual inspection of the aphid and bacterial trees with TreeMap (Fig. 1b) suggests that branching orders among the hosts and endosymbionts are highly congruent. Thus, the significantly negative relationships may reflect differing, non-random tempos of evolution that are detectable using the distance-based methods. In particular, a large number of gene losses in Buchnera in comparison with phylogeny of interspecific aphids has also suggested that bacterial evolution may be fast while that in aphid may be slow and vice versa [45]. While testing this hypothesis will necessitate dated phylogenies, it is logical in that shared periods of rapid evolution could make the shared metabolic network unstable and, thus, be maladaptive [76]. Somewhat similarly, temporal patterns may emerge as rapid evolution in one symbiotic partner triggers rapid evolution in the other, constantly perpetuating and stabilizing the fragile obligate relationship on a dynamic evolutionary landscape [77].
The topology of Buchnera was somewhat inconsistent with that of aphid hosts, which implied that duplication and host switch events might happen. Actually, we examined and found that Melaphis and Schlechtendalia with their Buchnera had duplication and host switch events in the group of Rhus gall aphids. Additionally, the strong support in the subfamily Aphidinae detected one duplication and host switch events between Myzus and Acyrthosiphon + Sitobion. These aphids exhibited both sexual and parthenogenetic reproduction [49]. It is speculated that the “duplication and host switch” of Buchnera may occur through hybridization during sexual reproduction in aphids. Overall, our results highlight the complexity of the obligate symbiotic relationship between aphids and bacteria and have generated coevolutionary hypothesis for this intriguing system that merit additional studies.
Methods
Buchnera aphidicola as the endosymbiotic bacteria has never been successfully cultured. Thus, we employed high-throughput genome skimming sequencing technology to simultaneously generate sequence data for the host aphids and endosymbiotic Buchnera.
Isolation and sequencing of genome DNA
We collected galls on Rhus from China except for the galls of the North American aphid species, Melaphis rhois, which we collected from the USA (Table 1). Aphids from the same gall represent parthenogenetic clones, and these comprise a sample. We stored samples from each gall in 75% ethanol and 100% ethanol for identification and DNA extraction purposes, respectively. Our 17 samples of the Rhus gall aphids included all six genera and the 13 species recognized within Melaphidina [5, 47, 48]. We also collected and sampled galls of Chaetogeoica yunlongensis representing the sister subtribe Fordina collected from the host plant Pistacia chinensis. We deposited voucher specimens at the School of Life Science of Shanxi University in China.
We extracted genomic DNA from five aphid individuals within each sampled Rhus gall by immersing them in distilled water for at least 36 h and then using a DNeasy extraction kit (QIAGEN, Valencia, CA). Our extractions for the aphids also included the DNA of the endosymbiotic bacteria. We quantified the total DNA yields and assessed quality using NanoDrop-2000 (Thermo Fisher Scientific) before sending the samples partially to the Genomic Sequencing and Analysis Facility (GSAF), University of Texas, Austin, for library construction and genomic sequencing. DNAs were sheared into ~ 500 bp fragments using the Covaris M220 Focused-ultrasonicator to prepare the library with a TruSeq Nano DNA library preparation kit (Illumina, FC-121–4003). Genomic sequencing comprised paired-end reads of 2 × 150 bp generated on an Illumina NextSeq sequencer with an insert size of 400 bp. We filtered the raw data using Trimmomatic v. 0.35 with default settings (ILLUMINACLIP:Tru Seq3-PE:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36) [78].
Buchnera genomics and phylogenomics
We assembled shotgun sequence data using SPAdes v. 3.7.1 [79] with kmers 21, 33, 55, 77, 99, and 127. Since we used the whole body of aphid individuals to extract the genomic DNA and sequenced, also with the abundance of symbionts Buchnera in aphid cells, these assemblies included the complete genomes of the bacterial endosymbionts. For those genomes whose contiguous circular genomes were not able to be completely generated but with several contigs, we used the mapping function in Geneious to filter out the aphid contigs by referring to the closest complete Buchnera genomes [80]. For gene counting comparisons, contigs were arranged in the most possible conservative way, i.e., orientation was assumed to be collinear with reference to the complete single circular contig genomes. We annotated the bacterial genomes with Prokka v. 1.12 [81].
For comparative genomic and co-phylogenetic analysis, we downloaded complete genomes of Buchnera from GenBank with corresponding aphid sequences. In total, there are 73 genomes of Buchnera aphidicola in 56 species of aphids reported in GenBank. Usually, there were the similar genomic organization for the same species, so we compared and selected one genome of Buchnera aphidicola in each aphid species except for the aphid Acyrthosiphon pisum, which published two genomes but with relative high variability (GenBank accession no. CP002303 and NC_011833). All the 57 publicly available genome sequences and our dataset of 15 newly sequenced genomes were shown in Additional file 1: Table S1. The gene losses and gains were counted with respect to the Buchnera core genome and pan-genome. We chose this method because there are almost certainly unsampled or yet unknown taxa, which would be important to consider if we were to calculate the gene history of Buchnera with reference to its sister taxon. We also performed a comparative genomic analysis of Buchnera with three outgroups: Escherichia coli, Shigella sp. PAMC 28760 (representing related free-living bacteria), and Candidatus Ishikawaella capsulata (a special intestinal bacterium and a sister group of the obligate intracellular symbiote Buchnera of aphids [38]).
We performed phylogenetic analyses using 144 protein-coding genes that were present in the genomes of all the 75 Buchnera species and three outgroup taxa [40]. Each protein-coding gene dataset was aligned using MAFFT version 7 [82] with Translation Align implemented in Geneious 10.2.4 with default settings. We generated partitioning schemes for these shared genes in PartitionFinder2 [83, 84] and performed a partitioned analysis in RAxML v. 8.2.7 [85] under the GTRGAMMA model with 1000 full bootstrap replicates. We also used the bacterial sequence data to generate Venn diagrams of genome content with a web-tool from the University of Gent (http://bioinformatics.psb.ugent.be/webtools/Venn/), and we visualized circular genome plots using CGView [86].
Aphid genes and phylogenetic analysis
We constructed the aphid phylogeny by using the 17 mitogenomes of Fordini species sampled in this study (16 Rhus gall aphids and one species Chaetogeoica yunlongensis living on Pistacia chinensis) and combining the mitogenomes from GenBank representing 12 additional species of the family Aphididae, belonging to two subfamilies, Aphidinae and Lachninae, and four tribes: Aphidini, Macrosiphini, Tuberolachnini and Eulachnini. We used two species, Daktulosphaira vitifoliae and Adelges laricis, from the other aphid superfamilies, Phylloxeroidea and Adelgoidea, as outgroups. We also obtained the three nuclear markers, 18S, EF-1α, and LWO from the clean reads of the shotgun skimming genome by remapping the reads to alignments of available sequences in GenBank and also performing de novo re-assemblies of reads in SPAdes for checking and correcting the degenerate bases in our previous report [53]. Thus, the reconstructed phylogenetic relationships of host aphids included 31 samples from 30 species, including six genera and 13 species, and employed 15 mitochondrial and three nuclear genes (Table 3).
We extracted 13 protein-coding genes (COI-COIII, ATP6, ATP8, ND1-ND6, ND4L, Cytb) and two rRNA loci (12S and 16S) from all the aphid mitogenomes and performed the alignments by the program MAFFT v7.2 as well as the nuclear sequences [82].
The mitochondrial genes are maternally inherited and represent a non-recombining locus, so we concatenated them as a dataset. We used the partition homogeneity test [87] in PAUP* [88] to determine the appropriateness of combining the mitochondrial and nuclear genes as one dataset. Based on the above test (ILD, p = 0.33 > 0.01), we concatenated the 15 mitochondrial genes and the three nuclear genes into a total-evidence matrix. We analyzed the total-evidence matrix in four ways by using the ML and BI with and without partitioning by gene. We performed the ML analyses in IQTree [89] with internal model selection based on ModelFinder [90] according to the Bayesian Information Criterion. We accomplished BIs for all individual genes, the combined nuclear genes, and the combined mitochondrial genes using MrBayes v. 3.2.3 [91, 92] on the high-performance computing cluster available via the Cipres Science Gateway (https://www.phylo.org). The BIs with and without partitioning by gene each consisted of two simultaneous, independent runs of 10 million generations of one cold chain and five hot chains with sampling every 1000 generations. For BI, we applied the GTR + G model [93], which may be more robust to base compositional biases inferred in IQTree [57]. Following BI, we examined the output in Tracer v. 1.6 [94] to verify that simultaneous runs had each reached stationarity according to effective sample sizes (ESS) > 200 and to assure the suitability of a 10% burnin. We performed the 10% burnins and combined trees from simultaneous BI runs in Log Combiner, and we summarized the trees on the maximum clade credibility tree with median branch lengths using Tree Annotator, both of the BEAST v. 1.8 package [95].
Cophylogenetic analyses of the endosymbiont Buchnera and its host aphids
To determine the degree to what aphids and their endosymbiotic bacteria have coevolved, we performed cophylogenetic analyses based on our reconstructed phylogenetic trees. Cophylogenetic analyses are either event-based and utilize tree topology or distance-based and utilize tree shape [96]. In general, distance-based methods compare distance matrices derived from tree branches transformed using principal coordinates. We performed event-based cophylogenetic analysis using TreeMap v3.0 [97] and Jane v4 [98] to determine whether there is a significant match between Buchnera and aphid trees at different taxonomic levels, e.g., individuals, species, genus and tribe, and what is the best explanation for any differences between the two trees.
In TreeMap, the algorithm maximizes the number of both co-diversification events and evolutionary events regarded as more complex, such as diversification of the parasite without diversification in the host (duplication), diversification of the host without diversification in the parasite (“failure to diverge”), loss of the host-parasite association (loss), and host switching. Within TreeMap, we tested the least costly reconciliation against a number of randomly generated trees to determine if the result obtained is statistically significant.
We ran Jane v4 to determine the coevolutionary events among co-diversification and duplication, loss, failure to diverge, and duplication followed by host switching likely accounted for the patterns of Buchnera-aphid associations. This analysis assigns a range of costs to each coevolutionary event and attempts to identify the most parsimonious solution that minimizes the costs. The event-costs that we used in running Jane were co-speciation = 0, duplication = 1, duplication and host switching = 2, loss = 1, failure to diverge = 1.
We performed distance-based cophylogenetic analyses in ParaFit [26] implemented in the ape v.5.3 package [99] for R and PACo v.0.3.2 [100] implemented as a R package [101] using the BI tree resulting from the analysis with partitioning among genes. ParaFit and PACo differed in how they made comparisons between the distance matrices of the two organismal partners [100]. For this analysis, our input data included only bacterial species in aphid hosts represented in the aphid phylogeny, and all other aphids were removed from the distance matrices. In ParaFit, we applied a “lingoes” correction [102] to negative eigenvalues within distance matrices transformed as principal coordinates, and we used the transformed and corrected matrices to determine global fit between the aphid and bacterial trees. We tested the significance of the fit using 999 permutations comprising randomization of the associations between aphid and bacterial species. ParaFit assesses the significance of each H-P association, using the ParaFitLink1 and ParaFitLink2 statistic. For PACo, we performed a square root transformation of the bacterial distance matrix prior to obtaining principal coordinates to circumvent negative eigenvalues. We then determined global fit and measured significance using 1000 permutations of aphid-bacterial associations. PACo generates residuals of the Procrustean fit, which describes the contribution of each individual H-P association to the global fit (smaller residuals means a more congruence). We performed the ParaFit and PACo analyses on all four trees resulting from the BI and ML analyses of the combined sequence data to account for weakly supported topological incongruence outside of Melaphidina and differences in branch lengths.
Availability of data and materials
The raw DNA sequencing data, individual annotated genes, and complete genomes have been submitted to National Center for Biotechnology Information (NCBI) under BioProject accession PRJNA1025172 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1025172) [103]. Full details are available in Table 1. Other Buchnera genomes analyzed were acquired from previous studies [14, 35,36,37,38,39,40, 52, 64, 67, 104,105,106,107,108,109,110] or downloaded from NCBI (https://www.ncbi.nlm.nih.gov; Additional file 1: Table S1).
References
Allen JM, Reed DL, Perotti MA, Braig HR. Evolutionary relationships of “Candidatus Riesia spp.,” endosymbiotic Enterobacteriaceae living within hematophagous primate lice. Appl Environ Microbiol. 2007;73:1659–64.
Conord C, Despres L, Vallier A, Balmand S, Miquel C, Zundel S, Lemperiere G, Heddi A. Long-term evolutionary stability of bacterial endosymbiosis in curculionoidea: additional evidence of symbiont replacement in the dryophthoridae family. Mol Biol Evol. 2008;25:859–68.
Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–90.
Dixon AFG, Kindlmann P. Role of plant abundance in determining the abundance of herbivorous insects. Oecologia. 1990;83:281–3.
Heie OE. The Aphidea of Fennoscandia and Denmark I. General Part. The families Mindaridae, Hormaphididae, Thelaxidae, Anoeciidae, and Pemphigidae. Fauna Entomologica Scandinavica. Klampenborg: Scandinavian Science Press; 1980.
Moran NA, von Dohlen CD, Baumann P. Faster evolutionary rates in endosymbiotic bacteria than in cospeciating insect hosts. Appl Environ Microbiol. 1995;73:1659–64.
Wilkinson TL, Fukatsu T, Ishikawa H. Transmission of symbiotic bacteria Buchnera to parthenogenetic embryos in the aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). Arthropod Struct Dev. 2003;32:241–5.
Moya A, Peretó J, Gil R, Latorre A. Learning how to live together: genomic insights into prokaryote-animal symbioses. Nat Rev Genet. 2008;9:218–9.
Buchner P. Endosymbiosis of animals with plant microorganisms. New York: Interscience Publishers; 1965.
Baumann P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol. 2005;59:155–89.
Baumann P, Baumann L, Lai CY, Rouhbaksh D, Moran NA, Clark MA. Genetics, physiology and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids. Annu Rev Microbiol. 1995;49:55–94.
Shigenobu S, Wilson AC. Genomic revelations of a mutualism: the pea aphid and its obligate bacterial symbiont. Cell Mol Life Sci. 2011;68:1297–309.
Hansen AK, Moran NA. Aphid genome expression reveals host-symbiont cooperation in the production of amino acids. Proc Natl Acad Sci U S A. 2011;108:2849–54.
Cassone BJ, Wenger JA, Michel PA. Whole genome sequence of the soybean aphid endosymbiont Buchnera aphidicola and genetic differentiation among biotype-specific strains. J Genomics. 2015;3:85–94.
Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407:81–6.
International Aphid Genomics Consortium. Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010;8:e1000313.
Colella S, Parisot N, Simonet P, Gaget K, Duport G, Baa-Puyoulet P, Rahbé Y, Charles H, Febvay G, Callaerts P, et al. Bacteriocyte reprogramming to cope with nutritional stress in a phloem sap feeding Hemiptera, the pea aphid Acyrthosiphon pisum. Front Physiol. 2018;9:1498.
Moran NA, Munson MA, Baumann P, Ishikawa H. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc R Soc Lond B. 1993;253:167–71.
Tamas I, Klasson L, Canbäck B, Näslund AK, Eriksson AS, Wernegreen JJ, Sandström JP, Moran NA, Andersson SGE. 50 million years of genomic stasis in endosymbiotic bacteria. Science. 2002;296:2376–9.
Munson MA, Baumann P, Kinsey MG. Buchnera gen. nov. and Buchnera aphidicola sp. nov. a taxon consisting of the mycetocyte-associated, primary endosymbionts of aphids. Int J Syst Bacteriol. 1991;41:566–8.
Jousselin E, Desdevised Y, Coeur d’acier A. Fine-scale cospeciation between Brachycaudus and Buchnera aphidicola: bacterial genome helps define species and evolutionary relationships in aphids. Proc Biol Sci. 2009;276:187–96.
Chen R, Wang Z, Chen J, Jiang LY, Qiao GX. Insect-bacteria parallel evolution in multiple-co-obligate-aphid association: a case in Lachninae (Hemiptera: Aphididae). Sci Rep. 2017;7:10204.
Smith TE, Moran NA. Coordination of host and symbiont gene expression reveals a metabolic tug-of-war between aphids and Buchnera. Proc Natl Acad Sci U S A. 2020;117:2113–21.
Xu TT, Chen J, Jiang LY, Qiao GX. Diversity of bacteria associated with Hormaphidinae aphids (Hemiptera: Aphididae). Insect Sci. 2021;28:165–79.
Ehrlich PR, Raven PH. Butteries and plants: a study in coevolution. Evolution. 1964;18:586–608.
Legendre P, Desdevises Y, Bazin E. A statistical test for host-parasite coevolution. Syst Biol. 2002;51:217–34.
Hafner MS, Sudman PD, Villablanca FX, Spradling TA, Demastes JW, Nadler SA. Disparate rates of molecular evolution in cospeciating hosts and parasites. Science. 1994;265:1087–90.
Page RDM. Parallel phylogenies: reconstructing the history of host-parasite assemblages. Cladistics. 1994;10:155–73.
Page RDM. Clocks, clades, and cospeciation: comparing rates of evolution and timing of cospeciation events in host-parasite assemblages. Syst Zool. 1991;40:188–98.
Clark MA, Moran NA, Baumann P, Wernegreen JJ. Cospeciation between bacterial endosymbionts (Buchnera) and a recent radiation of aphids (Uroleucon) and pitfalls of testing for phylogenetic congruence. Evolution. 2000;54:517–25.
Funk DJ, Helbling L, Wernegreen JJ, Moran NA. Intraspecific phylogenetic congruence among multiple symbiont genomes. Proc Biol Sci. 2000;267:2517–21.
Peccoud J, Simon JC, McLaughlin HJ, Moran NA. Post-Pleistocene radiation of the pea aphid complex revealed by rapidly evolving endosymbionts. Proc Natl Acad Sci U S A. 2009;106:16315–20.
Liu L, Huang XL, Zhang RL, Jiang LY, Qiao GX. Phylogenetic congruence between Mollitrichosiphum (Aphididae: Greenideinae) and Buchnera indicates insect-bacteria parallel evolution. Syst Entomol. 2013;38:81–92.
Arab DJ, Lo N. Evolutionary rates are correlated between Buchnera endosymbionts and the mitochondrial genomes of their aphid hosts. J Mol Evol. 2021;89:238–48.
van Ham R, Kamerbeek J, Palacios C, Rausell C, Abascal F, Bastolla U, Fernández JM, Jiménez L, Postigo M, Silva FJ, et al. Reductive genome evolution in Buchnera aphidicola. Proc Natl Acad Sci U S A. 2003;100:581–6.
Pérez-Brocal V, Gil R, Ramos S, Lamelas A, Postigo M, Michelena JM, Silva FJ, Moya A, Latorre A. A small microbial genome: the end of a long symbiotic relationship? Science. 2006;314:312–3.
Lamelas A, Gosalbes MJ, Moya A, Latorre A. New clues about the evolutionary history of metabolic losses in bacterial endosymbionts, provided by the genome of Buchnera aphidicola from the aphid Cinara tujafilina. Appl Environ Microbiol. 2011;77:4446–54.
Chong RA, Park H, Moran NA. Genome evolution of the obligate endosymbiont Buchnera aphidicola. Mol Biol Evol. 2019;36:1481–9.
Kutsukake M, Moriyama M, Shigenobu S, Meng XY, Nikoh N, Noda C, Kobayashi S, Fukatsu K. Exaggeration and cooption of innate immunity forsocial defense. Proc Natl Acad Sci U S A. 2019;116:8950–9.
Monnin D, Jackson R, Kiers ET, Bunker M, Ellers J, Henry LM. Parallel evolution in the integration of a co-obligate aphid symbiosis. Curr Biol. 2020;30:1949–57.
Nakabachi A, Yamashita A, Toh H, Ishikawa H, Dunbar HE, Moran NA, Hattori M. The 160-kilobase genome of the bacterial endosymbiont Carsonella. Science. 2006;314:267.
McCutcheon JP, Moran NA. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc Natl Acad Sci U S A. 2007;104:19392–7.
McCutcheon JP, McDonald BR, Moran NA. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Natl Acad Sci U S A. 2009;106:15394–9.
Brinza L, Viñuelas J, Cottret L, Calevro F, Rahbé Y, Febvay G, Duport G, Colella S, Rabatel A, Gautier C, et al. Systemic analysis of the symbiotic function of Buchnera aphidicola, the primary endosymbiont of the pea aphid Acyrthosiphon pisum. C R Biol. 2009;332:1034–49.
Chong RA, Moran NA. Evolutionary loss and replacement of Buchnera, the obligate endosymbiont of aphids. ISME J. 2018;12:898–908.
Martínez-Cano DJ, Bor G, Moya A, Delaye L. Testing the domino theory of gene loss in Buchnera aphidicola: the relevance of epistatic interactions. Life (Basel). 2018;8:17.
Blackman RL, Eastop VF. Aphids on the world’s crops: an identification guide. Chichester: John Wiley and Sons; 1984.
Remaudière G, Remaudière M. Catalogue of the world’s Aphididae (Homoptera Aphidoidea). Paris: Institut National de la Recherche Agronomique; 1997.
Zhang GX, Qiao GX, Zhong TS, Zhang WY. Fauna Sinica Insecta (Vol.14), Homoptera: Mindaridae and Pemphigidae. Beijing: Science Press; 1999.
Li ZG, Yang WY, Xia DJ. Study on the Chinese gallnuts. For Res. 2003;16:760–7.
Moran NA, Plague GR, Sandstrom JP, Wilcox JL. A genomic perspective on nutrient provisioning by bacterial symbionts of insects. Proc Natl Acad Sci U S A. 2003;100:14543–8.
Zhang Y, Su X, Harris AJ, Caraballo-Ortiz MA, Ren ZM, Zhong Y. Genetic structure of the bacterial endosymbiont Buchnera aphidicola from its host aphid Schlechtendalia chinensis and evolutionary implications. Curr Microbiol. 2018;75:309–15.
Ren ZM, von Dohlen CD, Harris AJ, Dikow RB, Su X, Wen J. Congruent phylogenetic relationships of Melaphidina aphids (Aphididae: Eriosomatinae: Fordini) according to nuclear and mitochondrial DNA data with taxonomic implications on generic limits. PLoS One. 2019;14:e0213181.
Liang YK, Wen J, Ren ZM. Complete mitochondrial genome of Rhus gall aphid Meitanaphis microgallis (Hemiptera: Aphididae: Eriosomatinae). Mitochondrial DNA B Resour. 2019;4:2363–4.
Ren ZM, Harris AJ, Dikow RB, Ma EB, Zhong Y, Wen J. Another look at the phylogenetic relationships and intercontinental biogeography of eastern Asian – North American Rhus gall aphids (Hemiptera: Aphididae: Eriosomatinae): evidence from mitogenome sequences via genome skimming. Mol Phylogenet Evol. 2017;117:102–10.
Charles H, Balmand S, Lamelas A, Cottret L, Pérez-Brocal V, Burdin B, Latorre A, Febvay G, Colella S, Calevro F, et al. A genomic reappraisal of symbiotic function in the Aphid/Buchnera symbiosis: reduced transporter sets and variable membrane organisations. PLoS ONE. 2011;6:e29096.
Song H, Sheffield NC, Cameron SL, Miller KB, Whiting MF. When phylogenetic assumptions are violated: base compositional heterogeneity and among-site rate variation in beetle mitochondrial phylogenomics. Syst Entomol. 2010;35:429–48.
Gil R, Sabater-Munoz B, Latorre A, Silva FJ, Moya A. Extreme genome reduction in Buchnera spp.: toward the minimal genome needed for symbiotic life. Proc Natl Acad Sci USA. 2002;99:4454–8.
Clark MA, Moran NA, Baumann P. Sequence evolution in bacterial endosymbionts having extreme base compositions. Mol Biol Evol. 1999;16:1586–98.
Bracho AM, Martínez-Torres D, Moya A, Latorre A. Discovery and molecular characterization of a plasmid localized in Buchnera sp. bacterial endosymbiont of the aphid Rhopalosiphum padi. J Mol Evol. 1995;41:67–73.
Rouhbakhsh D, Lai CY, von Dohlen CD, Clark MA, Baumann L, Baumann P, Moran NA, Voegtlin DJ. The tryptophan biosynthetic pathway of aphid endosymbionts (Buchnera): genetics and evolution of plasmid-associated anthranilate synthase (trpEG) within the Aphididae. J Mol Evol. 1996;42:414–21.
Baumann L, Baumann P, Moran N, Sandstrom JP, Thao ML. Genetic characterization of plasmids containing genes encoding enzymes of leucine biosynthesis in endosymbionts (Buchnera) of aphids. J Mol Evol. 1999;48:77–85.
Gil R, Sabater-Muñoz B, Perez-Brocal V, Silva FJ, Latorre A. Plasmids in the aphid endosymbiont Buchnera aphidicola with the smallest genomes. A puzzling evolutionary story. Gene. 2006;370:17–25.
Zhang B, Leonard SP, Li YY, Moran NA. Obligate bacterial endosymbionts limit thermal tolerance of insect host species. Proc Natl Acad Sci U S A. 2019;116:24712–8.
Smith CA. Structure, function and dynamics in the mur family of bacterial cell wall ligases. J Mol Evol. 2006;362:640–55.
Thomas GH, Zucker J, Macdonald SJ, Sorokin A, Goryanin I, Douglas AE. A fragile metabolic network adapted for cooperation in the symbiotic bacterium Buchnera aphidicola. BMC Syst Biol. 2009;3:24.
Nikoh N, Hosokawa T, Oshima K, Hattori M, Fukatsu T. Reductive evolution of bacterial genome in insect gut environment. Genome Biol Evol. 2011;3:702–14.
Lapage SP, Sneath PHA, Lessel EF, Skerman VBD, Seeliger HPR, Clark WA. International code of nomenclature of bacteria (1975 Revision). Washington DC: Bacteriological Code American Society for Microbiology; 1990.
Levin BR, Bergstrom CT. Bacteria are different: observations, interpretations, speculations, and opinions about the mechanisms of adaptive evolution in prokaryotes. Proc Natl Acad Sci U S A. 2000;97:6981–5.
Riley MA, Lizotte-Waniewski M. Population genomics and the bacterial species concept. In: Gogarten MB, Gogarten JP, Olendzenski LC, editors. Methods in Molecular Biology (Vol. 532), Horizontal Gene Transfer. Paterson: Humana Press; 2009. p. 367–77.
Martinez-Torres D, Buades C, Latorre A, Moya A. Molecular systematics of aphids and their primary endosymbionts. Mol Phylogenet Evol. 2001;20:437–49.
Nováková E, Hypša V, Klein J, Foottit RG, von Dohlen CD, Moran NA. Reconstructing the phylogeny of aphids (Hemiptera: Aphididae) using DNA of the obligate symbiont Buchnera aphidicola. Mol Phylogenet Evol. 2013;68:42–54.
Nylander JAA, Ronquist F, Huelsenbeck JP, Nieves-Aldrey JL. Bayesian phylogenetic analysis of combined data. Syst Biol. 2004;53:447–67.
Navaud O, Barbacci A, Taylor A, Clarkson JP, Raffaele S. Shifts in diversification rates and host jump frequencies shaped the diversity of host range among Sclerotiniaceae fungal plant pathogens. Mol Ecol. 2018;27:1309–23.
Santiago-Alarcon D, Rodríguez-Ferraro A, Parker PG, Ricklefs RE. Different meal, same flavor: cospeciation and host switching of haemosporidian parasites in some non-passerine birds. Parasit Vectors. 2014;7:286.
Saikkonen K, Wäli P, Helander M, Faeth SH. Evolution of endophyte–plant symbioses. Trends Plant Sci. 2004;9:275–80.
Bennett GM, Moran NA. Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc Natl Acad Sci U S A. 2015;112:10169–76.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20.
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–77.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9.
Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–9.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80.
Lanfear R, Calcott B, Kainer D, Mayer C, Stamatakis A. Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evol Biol. 2014;14:82.
Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 2016;34:772–3.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3.
Stothard P, Wishart DS. Circular genome visualization and exploration using CGView. Bioinformatics. 2004;21:537–9.
Farris JS, Källersjö M, Kluge AG, Bult C. Testing significance of incongruence. Cladistics. 1995;10:315–9.
Swofford DL. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Massachusetts: Sinauer Associates; 2002.
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol Biol Evol. 2015;32:268–74.
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–9.
Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–4.
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42.
Huelsenbeck J, Rannala B. Frequentist properties of Bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Syst Biol. 2004;53:904–13.
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst Biol. 2018;67(5):901–4.
Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214.
Avino M, Ng GT, He YY, Renaud MS, Jones BR, Poon AFY. Tree shape-based approaches for the comparative study of cophylogeny. Ecol Evol. 2018;9:6756–71.
Charleston MA. TreeMap 3b. A Java program for cophylogeny mapping. 2012. http://www.sydney.edu.au/engineering/it/~mcharles/software/treemap/. Accessed 22 Feb 2023.
Conow C, Fielder D, Ovadia Y, Libeskind-Hadas R. Jane: a new tool for the cophylogeny reconstruction problem. Algorithms Mol Biol. 2010;5:16.
Paradis E, Schliep K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35:526–8.
Balbuena JA, Míguez-Lozano R, Blasco-Costa I. PACo: a novel procrustes application to cophylogenetic analysis. PLoS ONE. 2013;8:e61048.
Balbuena JA, Poisot T, Hutchinson M, Cagua F. Package ‘PACo’: procrustes application to cophylogenetic analysis (version 0.3.2). 2017. https://www.uv.es/cophylpaco/. Accessed 22 Feb 2023.
Lingoes JC. Some boundary conditions for a monotone analysis of symmetric matrices. Psychometrika. 1971;36:195–203.
Liang YK, Dikow RB, Su X, Wen J, Ren ZM. Comparative genomics of the primary endosymbiont Buchnera aphidicola in aphid hosts and their coevolutionary relationships. NCBI BioProject accession PRJNA1025172; 2023. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1025172.
Chen WB, Shakir S, Bigham M, Richter A, Fei ZJ, Jander G. Genome sequence of the corn leaf aphid (Rhopalosiphum maidis Fitch). Gigascience. 2019;8:giz033.
Mathers TC, Mugford ST, Hogenhout SA, Tripathi L. Genome sequence of the banana aphid, Pentalonia nigronervosa Coquerel (Hemiptera: Aphididae) and its symbionts. G3 (Bethesda). 2020;10:4315–21.
Jiang Z, Jones DH, Khuri S, Tsinoremas NF, Wyss T, Jander G, Wilson AC. Comparative analysis of genome sequences from four strains of the Buchnera aphidicola Mp endosymbion of the green peach aphid, Myzus persicae. BMC Genomics. 2013;14:917.
Moran NA, McLaughlin HJ, Sorek R. The dynamics and time scale of ongoing genomic erosion in symbiotic bacteria. Science. 2009;323:379–82.
MacDonald SJ, Thomas GH, Douglas AE. Genetic and metabolic determinants of nutritional phenotype in an insect-bacterial symbiosis. Mol Ecol. 2011;20:2073–84.
Degnan PH, Ochman H, Moran NA. Sequence conservation and functional constraint on intergenic spacers in reduced genomes of the obligate symbiont Buchnera. PLoS Genet. 2011;7: e1002252.
Riley M, Abe T, Arnaud MB, Berlyn MK, Blattner FR, Chaudhuri RR, Glasner JD, Horiuchi T, Keseler IM, Kosuge T, et al. Escherichia coli K-12: a cooperatively developed annotation snapshot–2005. Nucleic Acids Res. 2006;34:1–9.
Acknowledgements
We thank Smithsonian Institution for providing computation on the Smithsonian Institution High Performance Cluster (SI/HPC), Hydra (https://doi.org/10.25572/SIHPC).
Funding
This work was financially supported by the National Natural Science Foundation of China (31870366, 31170359), Shanxi International Science and Technology Cooperation Project (201803D421051), Research Project Supported by Shanxi Scholarship Council of China (2020–018), and the National High Technology Research and Development “863” Program (2014AA021802).
Author information
Authors and Affiliations
Contributions
The project was conceived and supervised by Z.R., J.W., and R.D. Z.R. and J.W. collected samples, finished the experiments, and organized the main text. Y.L. and R.D., contributing equally to this work as co-first authors, conducted bioinformatic analyses, drafted the manuscript, and prepared the tables and figures. Z.R., R.D., J.W., and X.S. guided the manuscript development and revision. All the authors participated in the drafting or revising of the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12915_2024_1934_MOESM2_ESM.docx
Additional file 2: Figures S1-S3. Fig. S1. The BI tree of aphids without partitioning. Stars at the branches represent bootstrap support of BI 1.00. The colors correspond to the subfamilies of host aphids represented, Blue: Aphidinae, Purple: Eriosomatinae, Green: Lachninae. Fig. S2. The ML tree of aphids with partitioning. Stars at the branches represent bootstrap support of ML 100%. The colors correspond to the subfamilies of host aphids represented, Blue: Aphidinae, Purple: Eriosomatinae, Green: Lachninae. Fig. S3. The ML tree of aphids without partitioning. Stars at the branches represent bootstrap support of ML 100%. The colors correspond to the subfamilies of host aphids represented, Blue: Aphidinae, Purple: Eriosomatinae, Green: Lachninae.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liang, Y., Dikow, R.B., Su, X. et al. Comparative genomics of the primary endosymbiont Buchnera aphidicola in aphid hosts and their coevolutionary relationships. BMC Biol 22, 137 (2024). https://doi.org/10.1186/s12915-024-01934-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12915-024-01934-w