Abstract
Background
Sex differences in microRNA (miRNA) expression profiles have been found across multiple tissues. Skeletal muscle is one of the most sex-biased tissues of the body. MiRNAs are necessary for development and have regulatory roles in determining skeletal muscle phenotype and have important roles in the response to exercise in muscle. Yet there is limited research into the role and regulation of miRNAs in the skeletal muscle at baseline and in response to exercise, a well-known modulator of miRNA expression. The aim of this study was to investigate the effect of sex on miRNA expression in the skeletal muscle at baseline and after an acute bout of high-intensity interval exercise. A total of 758 miRNAs were measured using Taqman®miRNA arrays in the skeletal muscle of 42 healthy participants from the Gene SMART study (23 males and 19 females of comparable fitness levels and aged 18–45 years), of which 308 were detected. MiRNAs that differed by sex at baseline and whose change in expression following high-intensity interval exercise differed between the sexes were identified using mixed linear models adjusted for BMI and Wpeak. We performed in silico analyses to identify the putative gene targets of the exercise-induced, sex-specific miRNAs and overrepresentation analyses to identify enriched biological pathways. We performed functional assays by overexpressing two sex-biased miRNAs in human primary muscle cells derived from male and female donors to understand their downstream effects on the transcriptome.
Results
At baseline, 148 miRNAs were differentially expressed in the skeletal muscle between the sexes. Interaction analysis identified 111 miRNAs whose response to an acute bout of high-intensity interval exercise differed between the sexes. Sex-biased miRNA gene targets were enriched for muscle-related processes including proliferation and differentiation of muscle cells and numerous metabolic pathways, suggesting that miRNAs participate in programming sex differences in skeletal muscle function. Overexpression of sex-biased miRNA-30a and miRNA-30c resulted in profound changes in gene expression profiles that were specific to the sex of the cell donor in human primary skeletal muscle cells.
Conclusions
We uncovered sex differences in the expression levels of muscle miRNAs at baseline and in response to acute high-intensity interval exercise. These miRNAs target regulatory pathways essential to skeletal muscle development and metabolism. Our findings highlight that miRNAs play an important role in programming sex differences in the skeletal muscle phenotype.
Similar content being viewed by others
Background
Biological sex is a fundamental characteristic that has profound effects on physiological and molecular factors influencing nearly all complex traits [1, 2]. Sex differences arise from a combination of interacting factors such as differences in exposure to sex hormones, sex chromosome complement, epigenetic mechanisms and non-coding RNA programming. Whilst sex hormones and the sex chromosomes are the major drivers of sex differences, the potential of non-coding RNAs, specifically microRNAs (miRNAs), in programming sex differences in muscle and other tissues is starting to come into focus [3, 4].
MiRNAs are short (~ 20 nucleotides) single-stranded RNA molecules that primarily act by repressing a target mRNA molecule and reducing the translation and expression of the corresponding protein [5]. As regulators of gene and protein expression, they play a crucial role in a variety of biological processes, such as cell development, cell proliferation, differentiation, apoptosis and cellular signalling. Sex differences in miRNA expression profiles have been found across multiple tissue including pancreatic islets [6], peripheral blood, brain and mucosa tissues [3]. There is, however, limited research into whether there are sex differences in miRNA expression in the skeletal muscle and what upstream mechanisms may be mediating these differences. Yet, the skeletal muscle is one of the top sexually differentiated tissues as it has the second highest number of genes (up to 3000) that are differentially expressed between the sexes [7, 8]. This indicates a potential role of fine-tuning mechanisms, such as miRNAs, which could be crucial in programming sex differences in gene expression profiles and the resulting phenotype in muscle.
Males and females display distinct muscle phenotypes. On a functional level, females have lower muscle mass and strength compared to males [9, 10]. On the molecular level, there are intrinsic sex differences in fibre type proportions, satellite cell numbers and substrate metabolism [11]. These functional and molecular differences result in females being more susceptible to the metabolic and functional decline associated with both age-related muscle loss and prolonged periods of disuse due to sedentary lifestyle or muscle pathologies [9, 11]. Exercise is a potent skeletal muscle stimulus and is therefore one of the most effective lifestyle management therapies for preventing age-related muscle loss and metabolic conditions, such as type 2 diabetes mellitus (T2DM) and cardiovascular disease [12]. Males and females display differences in musculoskeletal, cardiovascular, and metabolic responses to the same exercise regime [13]. These differences have been partially attributed to differences in sex hormones [14, 15], transcription factors [16] and more recently DNA methylation [17, 18]. Exercise is well known to modulate microRNA expression [19], but there is limited literature investigating the potential differences between males and females in the skeletal muscle miRNA expression following an acute exercise bout. Therefore, the aim of this study was to investigate the influence of sex on miRNA expression in the skeletal muscle at baseline and after an acute bout of high-intensity interval exercise.
Results
There were no significant differences in age or body mass index (BMI). Males had a higher aerobic capacity (VO2peak), lactate threshold (LT) and Wpeak than females. As expected, males also had greater levels of both free and total testosterone than females, whilst females had greater SHBG and estradiol levels than males (Table 1).
The expression levels of miRNAs at baseline are different between males and females
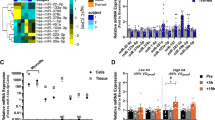
At resting baseline (pre-exercise), the levels of 148 miRNAs were different between males and females in the skeletal muscle; 147 of these miRNAs displayed a lower expression in males compared with females and one miRNA displayed a higher expression in males compared to females (Fig. 1A).
The skeletal muscle microRNA profile displays differences at baseline and a differential response to exercise between males and females. A Volcano plot: differences in microRNA expression levels between males compared to female at baseline. B Venn diagram: Overlap between miRNAs that were altered 3 h after an acute bout of exercise in females (female up-regulated miRNAs) and males (males up-regulated or down-regulated miRNAs). C Volcano plot: Interaction analysis revealed sex differences in miRNA response to an acute bout of exercise. Each point represents a microRNA. Red points indicate an increase in miRNA expression in males compared to females. Blue points indicate a decrease in miRNA expression in males compared to females. Black dots were not significant. Significance was set at FDR adjusted p value < 0.05. N = 44. Details of the specific miRNAs can be found in Additional file 1: Tables S1-5
The response of miRNAs expression following exercise is different between males and females
The male and female cohort were first investigated separately to identify the miRNAs that were altered in response to high-intensity interval exercise in each sex. In the female cohort, four miRNAs were up-regulated, and no miRNAs were down-regulated in response to an acute bout of exercise (Fig. 1B, blue). In the male cohort, 148 miRNAs were up-regulated (Fig. 1B, yellow) and two miRNAs were down-regulated in response to an acute bout of exercise (Fig. 1B, green). One miRNA, miR-223-3p, was up-regulated in both males and females in response to exercise, whilst the remaining miRNAs were differentially altered specific to each sex in response to exercise. Secondly, we conducted interaction analysis to identify the miRNAs that were differentially induced in males vs females in response to exercise. We found a total of 111 miRNAs whose response to an acute bout of exercise significantly differed between the sexes (Fig. 1C).
Sex-biased miRNAs target genes are enriched for pathways relating to muscle-related processes
Overrepresentation analysis (ORA) was performed to identify the gene pathways targeted by the sex-biased miRNAs at baseline and those that differed in response to exercise between the sexes. This analysis was conducted on genes that were considered expressed in the skeletal muscle based on RNA sequencing data from the GTEx portal [20]. Overall, 123 Gene Ontology (GO) terms were overrepresented in the baseline analysis and 118 GO terms were overrepresented in the interaction analysis (Additional file 1: Tables S6, S7, S8). Similar pathways were targeted at both baseline and in response to exercise. Altogether, the GO pathways broadly belonged to the ‘biological processes’ categories including ‘muscle cell development’, ‘muscle contraction’, ‘muscle system processes’ and ‘muscle cell differentiation’ (Fig. 2A and Additional file 1: Tables S7, S8). In agreement with this, we found enrichment in the WikiPathway term ‘striated muscle contraction pathway’ and Reactome pathway terms ‘striated muscle contraction’, ‘ion homeostasis’ and ‘glycogen breakdown’ (q < 0.05) (Fig. 2B).
Gene set enrichment analysis of the skeletal muscle enriched gene targets of sex-biased miRNAs that differed in response to an exercise bout. A Top enriched gene ontology (GO) terms belonging to the ‘biological processes’ category. B Enriched Reactome pathways. Significance was set at FDR adjusted p value < 0.05. N = 44. Details of enriched pathways can be found in Additional file 1: Tables S7, S8
Neither chromosomal location nor circulating sex hormones explained the basis of the sex-biased miRNAs expression in the skeletal muscle
We next investigated the possible basis of sex-biased miRNAs expression in the skeletal muscle. A Fisher test was performed to investigate whether sex-biased miRNA genes (baseline and interaction analysis) were more likely to be encoded on the sex chromosomes compared to the autosomes. There was no difference in likelihood of the sex-specific miRNA gene being located on the sex chromosomes or autosomes at baseline or after an acute bout of exercise (Additional file 2: Fig. S2). Sex hormones can regulate gene expression via signalling directly through sex hormone response elements located across the genome, and there are known hormone response elements in the promoters of some miRNAs [21, 22]. We therefore sought to understand whether circulating sex hormone concentration was associated with miRNAs expression levels detected in the muscle. There were no significant correlations between circulating estradiol or total testosterone levels and the expression of sex-specific miRNAs within each sex. Only one miRNA, hsa-miR-613, was negatively associated with free testosterone (fT) after FDR adjustment in males (Additional file 1: Tables S9).
There was enrichment of specific transcription factors (TF) with binding sites that are located near the transcription start site (TSS) of sex-biased miRNAs
Transcription factors (TFs) have been shown to have sex-divergent targeting patterns across multiple tissues including the skeletal muscle [16]. We therefore sought to understand whether certain TF were more likely to regulate the sex-biased miRNAs in the muscle. For differentially expressed miRNAs at baseline, 23 TF binding sites were significantly enriched including TP53 (located near TSS of 35 sex-biased miRNAs), NFKB1 (located near TSS of 27 sex-biased miRNAs), NFE2L2 (located near TSS of 18 sex-biased miRNAs), SMAD3 (located near TSS of 17 sex-biased miRNAs) and TGFB1 (located near TSS of 14 sex-biased miRNAs) (Additional file 1: Tables S10). For sex-biased miRNAs in response to exercise, there was enrichment for 11 TF binding sites in the putative transcriptional regulatory region of the miRNAs. These included TP53 (located near TSS of 26 sex-biased miRNAs), NFKB1 (located near TSS of 23 sex-biased miRNAs), SMAD3 (located near TSS of 15 sex-biased miRNAs) and TGFB1 (located near TSS of 13 sex-biased miRNAs) (Additional file 1: Tables S11).
Overexpression of selected sex-biased miRNAs in skeletal muscle primary cell lines
All five miR-30 family members (miR-30a-5p, miR-30b-5p, miR-30c-5p and miR-30d-5p/-3p) displayed a sex-specific response to exercise (Additional file 1: Table S5). Two candidates in this family were selected, miR-30a-5p and miR-30c-5p, as they have yet to be studied in the skeletal muscle in the context of sex. Hsa-miRNA-30a-5p (miR-30a) and hsa-miRNA-30c-5p (miR-30c) were overexpressed in three female and three male primary skeletal muscle lines. Overexpression of miR-30a and miR-30c was confirmed by quantifying transcript levels using qPCR (Fig. 3A, B) and led to a 70-fold increase in miR-30a and 80-fold increase in miR-30c.We then conducted RNA sequencing to understand the transcriptome profile changes driven by these miRNAs and to explore the putative biological pathways.
miRNA overexpression in skeletal muscle primary cells. QRT-PCR analysis of miR-30a/30c expression levels containing either A miR-30a or B miR-30c. Fold change normalised back to negative control. *P < 0.05. Data is expressed as mean ± SD. N = 6. C Heatmap of top 26 differentially expressed genes clustered by sex at baseline. Details of the specific genes can be found in Additional file 1: Table S1
Overexpression of miRNA-30a resulted in differences in sex-specific gene responses
At baseline, and without miRNA overexpression, 26 genes were differentially expressed between primary muscle cells cultured from male and female donors (Fig. 3C).
Overexpression of miR-30a resulted in differences in gene expression that were partly specific to the sex of the donor cells. In male-derived primary muscle cell lines, 18 genes were up-regulated and 23 genes were down-regulated with overexpression of miR-30a (Fig. 4A). In female-derived primary muscle cell lines, 16 genes were up-regulated and 23 were down-regulated with overexpression of miR-30a (Fig. 4B). Of these, 28 genes were similarly altered between male and female cell lines in response to overexpression of miR-30a and 24 genes were altered uniquely between male and female cell lines in response to overexpression of miR-30a (Fig. 4C, Additional file 2: Tables S2-5).
Differentially expressed genes with overexpression of miRNA-30a in human primary skeletal muscle. MA-plot of gene differentially expressed with overexpression of miR-30a in A males (N = 3) or B females (N = 3). C Venn diagram comparing the comparing DE genes with overexpression of miR-30a between the sexes. Significance at FDR adjusted p value set at p < 0.05. The complete list of genes can be found in Additional file 2: Tables S2-5
Overexpression of miRNA-30c resulted in differences in sex-specific gene responses
Similar to miR-30a, overexpression of miR-30c resulted in differences in gene expression that were partly specific to the sex of the donor cells. In male-derived primary muscle cell lines, 13 genes were up-regulated and 12 genes were down-regulated with overexpression of miR-30c (Fig. 5A). In female-derived primary muscle cell lines, 10 were up-regulated and 11 were down-regulated with overexpression of miR-30c (Fig. 5B). Of these, 10 genes were similarly altered between male and female cell lines in response to overexpression of miR-30c (Fig. 5C). A total of 26 genes were altered uniquely between male and female cell lines in response to overexpression of miR-30c (Fig. 5C, Additional file 2: Table S6-7).
Differentially expressed genes with overexpression of miRNA-30c in human primary skeletal muscle. MA-plot of gene differentially expressed with overexpression of miR-30c in A males (N = 3) or B females (N = 3). C Venn Diagram comparing the comparing DE genes with overexpression of miR-30c between the sexes. Significance at FDR adjusted p value set at p < 0.05. The complete list of genes can be found in Additional file 2: Tables S6-9
Discussion
MiRNAs are necessary for skeletal muscle development [23] and have regulatory roles in determining skeletal muscle phenotype [24], which is one of the most sex-divergent tissues. Few studies have examined the effect of sex on the skeletal muscle miRNAome. Thus, in a screening approach, we conducted miRNA microarrays to profile the skeletal muscle miRNA signature across males and females at baseline and after an acute bout of high-intensity interval exercise. We found profound sex differences in the muscle miRNA profile, with an overwhelming number of miRNAs exhibiting a lower expression in males when compared to females. Three hours after an acute bout of high-intensity interval exercise, there were a greater number of differentially expressed miRNAs in males compared to females. Sex-biased miRNA gene targets were enriched for muscle-related processes such as myocyte proliferation and differentiation and metabolic pathways, indicating that miRNAs may play a role in programming the physiological sex differences in skeletal muscle pre- and post-exercise. We then investigated the potential upstream mechanisms, including chromosome location, sex hormones and transcription factors that may explain the basis of sex differences in the miRNAome. Neither chromosomal location of the miRNA gene nor circulating sex hormones explained sex differences in miRNAs expression in the skeletal muscle. The sex-biased miRNAs were enriched for 23 transcription factor (TFs) binding sites at baseline and 11 TFs after an acute bout of high-intensity interval exercise, indicating the potential role of specific TFs in regulating sex-biased miRNA expression. We further profiled two sex-biased miRNAs to elucidate the genes targeted by these miRNAs. Overexpression of miRNA-30a and miRNA-30c in human primary skeletal muscle cells resulted in changes to gene expression profiles that were partly specific to the sex of the cell donor. Altogether, this indicates that sex not only plays a role in regulating the expression of miRNAs but also in regulating their gene targeting patterns.
To our knowledge, this is the most extensive miRNA profiling analysis that has been completed in the skeletal muscle comparing males and females at rest or following an exercise intervention. Research into sex-specific differences in response to acute or chronic training adaptations is limited by the paucity of robust studies [13], especially in clinical settings. It is however established that some functional adaptations (VO2max, substrate metabolism) are sex-specific [13, 25]. In line with our findings, our group and others have also reported sex differences in proteome [26] and transcriptome [27], but not DNA methylome [26], in response to training. The response to acute and chronic exercise training is however complex, and males and females differ in some, but not all, aspects of physiological and molecular training adaptations. We reported 148 and 111 miRNAs displaying a sex bias at baseline and in response to an acute bout of high-intensity interval exercise, respectively. At baseline, an overwhelming majority of miRNAs were more highly expressed in females when compared to males including two myo-miRNAs (muscle-enriched miRNAs), miR-133b and miR-1-3p. This is in line with previous literature showing that expression levels of miR-133b were markedly higher in females compared to males at baseline [28]. Our findings also suggest that, after an acute bout of high-intensity interval exercise, males exhibited a greater change in the miRNA profile compared to females. In males, the myo-miRs-133a/b and 1-3p were up-regulated in response to acute exercise, which is in line with most [19, 29, 30], but not all studies [31]. The discrepancies between our data and Silver et al. [31] may be due to the differences in the intensity of the exercise bout between the studies (high-intensity interval exercise vs moderate intensity exercise), which can alter miRNA levels differentially [32]. In females, we observed no changes in the expression of miR-133a/b or miR-1-3p in response to acute exercise, in line with previous findings [31]. MiR-133a/b is under androgenic control in males but not females, which may explain these sex-specific responses [28]. While miR-133a/b did not associate with testosterone (free or total T) in the male cohort, it is also strongly modulated by aerobic fitness, which may override the regulatory effects of androgens in males [28]. According to Rapp et al. [33], our cohort was situated approximately in the 90th percentile for relative VO2peak norms, indicating a high aerobic fitness, which may provide an explanation for this lack of association. Altogether, these data demonstrate the influence of sex on the miRNAome in muscle at both baseline and in response to acute exercise.
The sex chromosome complement (i.e. XX in biological females and XY in biological males) may contribute to sex differences in miRNA expression, as the X chromosome contains a higher density of miRNA genes compared to the Y chromosome [34, 35]. While many miRNAs genes are inactivated on the X chromosome, others are known to “escape” X inactivation and therefore would be expected to be more highly expressed in females compared to males [35,36,37]. Therefore, we investigated whether the sex-biased miRNA expression could be related to the genetic differences between the sexes. The current data did not indicate an overrepresentation of miRNAs genes on the X chromosome either at baseline or after high-intensity interval exercise which is consistent with findings in brain, colorectal tissue, blood and cord blood tissues [3]. These findings support the idea that sex chromosomes may not be the major contributor to the origin of sex-biased miRNA expression in the skeletal muscle. It should be noted that our findings were limited to miRNAs represented in the microarray array and therefore may not apply to the entire miRNAome of the skeletal muscle.
Sex steroid hormones can regulate miRNA expression directly via binding to their nuclear hormone receptors (AR, ERS1, ERS2) or indirectly by binding to the host gene promoters in intragenic miRNAs or through protein–protein interactions with other TFs [38]. There were no significant correlations between circulating estradiol or total testosterone levels and the expression of miRNAs within each sex. In line with previous literature, sex hormone receptors (AR, ERS1, ERS2) are not the sole regulators of sex-biased programming, which can also explain the lack of association [16]. Our putative analysis indeed revealed an enrichment of 23 TFs at baseline and 11 TFs in response to exercise that were not main sex hormone receptors. [16]. There was enrichment for TFs playing a role in determining the skeletal muscle phenotype. Specifically, TGFβ and its downstream mediator SMAD3 are involved in the skeletal muscle development [39, 40] and associated with altered substrate oxidation in the skeletal muscle [41,42,43]. TGFβ expression is influenced by sex in a range of tissues [44,45,46] and is known to regulate miRNA biogenesis [39]. At baseline, TGFβ putatively targeted 14 sex-biased miRNAs and 13 sex-biased miRNAs in response to a high-intensity interval exercise bout. One of these targets, miR-24, which displayed a sex bias at baseline and in response to exercise was repressed by TGFβ in C2C12 cells, leading to reduced expression of myogenic differentiation markers [47]. Further, TGFβ displays a sex-specific response to an acute bout of resistance exercise [48, 49]. Whether aerobic exercise elicits a similar sex-specific TGFβ response has not been investigated. Altogether, our results suggest that TFs that are not sex hormone receptors have sex-divergent targeting microRNA patterns in the skeletal muscle.
The miR-30 family plays a regulatory role in the differentiation of human skeletal muscle cells by targeting genes associated with cell cycle, proliferation and differentiation processes [50, 51]. We sought to investigate the effects of the miR-30 family on the myocyte transcriptome and to explore putative pathways in a sex-specific manner. We first wanted to establish whether the cell transcriptome exhibited sex differences in muscle primary cell lines. We therefore analysed the baseline differences in the transcriptome between male and female donor muscle cells and found that 26 genes were differentially expressed between the male and female donor cell lines. These included up-regulation of many Y-encoded (male-specific) genes that are considered to drive sex differences at a molecular level and included Ddx3y (DEAD box helicase 3), Uty (ubiquitously transcribed tetratricopeptide repeat gene on Y chromosome) and KDM5D (Lysine-specific demethylase 5D) amongst others [52, 53]. Interestingly, the X-encoded paralogs (Ddx3x, and KDM5C) were not different between the sexes, which may be due to X chromosome inactivation ensuring dosage compensation across the sexes [54]. The sex-biased genes were enriched for GO categories including immune system processes (antigen processing and presentation), gonad development (oocyte development and differentiation), DNA methylation and phenotypic switching which are known to display sex differences and sexual dimorphism (Additional file 2: Fig. S3). This finding adds to the existing evidence [55, 56] that sex differences originating from the sex chromosome complement may be conserved in primary cell lines [56]. However, the extent of the maintenance of sex differences mediated by lifelong exposure to different levels of gonadal sex hormones is still to be elucidated [52]. These data provide novel evidence that some sex differences in gene expression may be maintained in primary cell lines [57,58,59,60,61,62].
Conclusion
In conclusion, there were sex differences in the expression profile of skeletal muscle miRNAs both at baseline and in response to high-intensity interval exercise. These sex-biased miRNAs had regulatory roles in the skeletal muscle development but also displayed sex differences in their gene targeting patterns. Our findings highlight the important role that miRNAs play in programming sex differences in the skeletal muscle phenotype.
Methods
Participants
Samples were from the Gene and Skeletal Muscle Adaptive Response to Training (Gene SMART) cohort [63]. The detailed methodology has been previously published [64,65,66]. Briefly, 24 healthy males (age = 32 ± 8.1 years old; BMI = 25.0 ± 3.4 kg/m2) and 20 healthy pre-menopausal females (age = 34.6 ± 7.4 years old; BMI = 23.4 ± 2.9 kg/m2) participated in the study. Females were not taking any hormonal contraceptives and were tested in the early follicular phase (2–7 days post menses). This study was approved by the Human Ethics Research Committee at Victoria University (HRE13-223 and HRE 21–122), and all participants provided written informed consent.
Aerobic capacity (graded exercise test)
Aerobic capacity was assessed by a graded exercise test (GXT) performed on an electronically braked cycle-ergometer (Lode-Excalibur sport, Groningen, the Netherlands) to measure maximal oxygen uptake (V̇O2peak) and peak power output (Wpeak). The V̇O2peak was determined using a calibrated Quark CPET metabolic system (COSMED, Rome, Italy). The GXT was performed at a minimum of 48 h before the acute exercise session and biopsy collection. It consisted of 4-min stages separated by 30-s rest periods until voluntary exhaustion with incremental increases in resistance at each stage. Capillary blood samples were collected at the end of each 4-min stage and immediately after exhaustion and were analysed by the YSI 2300 STAT Plus system (Ohio, USA) to establish lactate concentration. Lactate threshold (LT) was calculated by the modified DMAX method as previously described [64]. The GXT was performed in duplicate at both baseline and after the intervention and the average was calculated for all parameters between the two tests. In addition, at baseline, participants performed a familiarisation test of the GXT.
Diet control (48 h prior to testing)
To standardise diet across the participants and minimise the effects of this confounding factor, each participant was provided with an individualised pre-packaged diet 48 h prior to providing the blood samples [67, 68]. The energy content of the provided meals was calculated using the Mifflin-St Jeor equation using the participant’s body mass, height and age [69]. The content of the diets was based on the current Australian National Health and Medical Research Council (NHMRC) guidelines. Participants were asked to abstain from caffeine and alcohol throughout the 48-h diet as well as food consumption 12 h prior to blood and muscle collection.
Blood collection
Venous blood samples were collected at rest via venepuncture or cannulation in BD SST Vacutainers (Becton and Dickson Company, USA). They were left at room temperature (10 min) before being centrifuged at 3500 rpm for 10 min at 4 °C. Serum was collected and stored at − 80 °C.
Muscle collection
Muscle biopsies were taken from the participants vastus lateralis muscle under local anaesthesia (1% xylocaine) using a Bergström needle modified to include suction [70] at baseline and 3 h after the acute exercise bout. Muscle was obtained from the same leg at both timepoints. The muscle was immediately frozen in liquid nitrogen and stored at − 80 °C for subsequent analyses. A small piece of the muscle (~ 10–15 mg) was placed in serum-free Ham’s F-10 medium (Life Technologies) for primary muscle cell culture.
Hormone analysis
As the age of our cohort was 18–45 years, we measured the two major circulating sex hormones for this life stage, testosterone, and E2 at resting baseline [71, 72]. The assays were completed in the accredited NATA and Royal College of Pathologists of Australasia (RCPA) pathology laboratory at Monash Health, Clayton, Australia. Testosterone was measured using high performance liquid chromatography–mass spectrometry (HPLCMS/MS) method using a liquid sample extraction (AB Sciex Triple Quad 5500 LC/MS/MS system). Estradiol (E2) was measured using a competitive binding immunoenzymatic assay performed on a Beckman Coulter Unicel DXI 800 analyser (Beckman Coulter, Lane Cove, NSW).
Acute HIIE bout
Male and female participants completed HIIE on an electronically braked cycle ergometer (Velotron, Racer Mate Inc, Seattle, USA). Participants completed approximately 5 min of warm up at an intensity of their own choosing [range 25–60 W] and then cycled for 6 × 2-min intervals and this was interspersed with 1-min recovery periods at a power of 60 W (work-to-rest ratio of 2:1). Intensity was individually determined based on baseline GXT results and calculated as power at lactate threshold (LT) + 40% of the difference between peak aerobic power (Wpeak) and power at LT.
MicroRNA profiling of the skeletal muscle
RNA was extracted with Tri-Reagent Solution (Ambion) as per manufacturer’s protocol. RNA concentrations (243.3 ng/μl ± 81.7 ng/μl) and quality (A260/280 ratio = 1.97 ± 0.07) were determined by using the Nanodrop 1000 Spectrophotometer (Thermo Fisher Scientific). A total of 360 ng of RNA was reverse transcribed as using Taqman microRNA RT kit and Megaplex RT primers, Human Pool A and B v3.0 (Life Technologies, Australia) as per manufacturer’s protocol. MiRNA expression was measured using the TaqMan array, Human microRNA A and B (Life Technologies, Australia). Collectively, these cards allow for the accurate quantitation of 758 human miRNAs. The data was first normalised using global normalisation in cloud-based ThermoFisher software which applies a constant scaling factor to every miRNA, so they all have similar median intensity minimising batch effect. The following conditions were used to remove miRNAs that were not expressed in the skeletal muscle for analysis: miRNAs with an average Cq greater than 35 across all samples were excluded (n = 105), miRNAs where more than 20% of the samples (18/88) had a Cq of 35 were excluded (n = 345). The remaining 308 miRNAs were used in the analysis. The Cq values were then transformed into arbitrary units (AU) using the following equation: AU = 0.5Ct1010. The cut-off for the relevant level of expression of each miRNA was set at a mean (Ct) < 35, as recommended by the manufacturer. Out of the 758 miRNAs measured, 450 (58%) were not expressed in the skeletal muscle and were excluded, leaving 308 miRNAs for further analyses.
Human primary cells
Experiment overview
Human primary cells were established from muscle biopsies of three females (age: 35.3 ± 8.6 years) and three males (32.3 ± 11.6 years) from the Gene SMART cohort (see Additional file 2: Table S10). A small piece of the muscle biopsy (please see above for detailed biopsy method) was collected. Satellite cells were isolated by dissociation with 0.05% trypsin/EDTA (Life Technologies). Cells were plated on a flask coated with extracellular matrix (ECM; Sigma-Aldrich, Castle Hill, Australia) and allowed to proliferate to 70% confluence before passaging. Myoblasts were maintained in proliferation media [Ham’s F-10 medium (Life Technologies) containing 20% FBS, 25 ng/mL fibroblast growth factor (bFGF; Promega), 1% penicillin streptomycin and 0.5% amphoteromycin (Life Technologies)] at 37 °C and 5% CO2. Cells were passaged by mechanical disturbance twice and then frozen down in proliferation media and 10% DMSO. Upon thawing, cells were passaged once before myoblasts were purified using CD56 + Microbeads (Miltenyi Biotec) to eliminate fibroblasts and other cell populations as per manufacturer instructions. At 70–80% confluence, cells were seeded in triplicate into 6-well plates and were allowed to differentiate by replacing proliferation media to differentiation media [DMEM (Life Technologies) containing 2% HS (New Zealand origin; Gibco, Life Technologies) and 1% penicillin/streptomycin. Medium was replaced every 48 h. On day 5 of differentiation, myotubes were transfected with either 20 nM of miR-30a or miR-30c mimics or 20 nM of a mimic scramble (mirVana™ miRNA mimics, ThermoFisher) using Lipofectamine® RNAiMAX Transfection Reagent (Ambion, Life Technologies) diluted 16.7X in Opti-MEM I reduced serum medium (Life Technologies). This solution was added to differentiation medium at a 10 × dilution for 24 h. After 24 h transfection, cells were harvested for RNA [73].
RNA extraction and RNA sequencing
RNA was extracted from human primary cells using AllPrep RNA/RNA/miRNA universal kit (Qiagen) according to manufacturer instructions. Prior to adding the extraction buffer, the media was removed, and wells were washed with PBS to remove any floating dead cells. RNA quantity and quality was established using the Agilent Tape Station (Agilent); the average sample yield was 167 ng/μL ± 76.8 ng/μL and the RIN average was 9.6 ± 0.2. Overexpression was confirmed by quantifying transcript levels via qPCR using TaqMan Advanced miRNA probes and was normalised by loading a consistent amount of total RNA in the RT reaction. Each donor cell line was pooled into a single sample for library preparation. The RNAseq libraries were prepared using the Illumina TruSeq Stranded Total RNA with Ribo-Zero Gold protocol and sequenced with 150-bp paired-end reads on the Illumina Novaseq6000 (Macrogen Oceania Platform). Reads underwent quality check with FastQC (v0.11.9); Kallisto (v0.46.1) was used to map reads to the human reference genome (HomoSapien GRCh38) and to generate transcript counts. Differential gene analysis was conducted using the R package DeSeq2 [74] using the model: Genes ~ Sex * Condition. Pre-filtering was undertaken to remove genes with less than 10 reads per million (RPM) in less than two samples from further analysis [74].
Statistical analysis
All data were analysed using Rstudio 4.1.3 [75]. Missing data were imputed using the kNN function in the VIM package [76]. To identify differences in miRNA expression between sexes at baseline and after exercise, linear mixed models were used and implemented in the limma package in R [77]. Participant ID was used as a blocking variable to account for repeated measures using the DuplicateCorrelation function. The main sources of variability as determined by principal component analysis were included in the models (Additional file 2: Fig. S1). Sex and time were grouped to create means model of the form \(\mathrm{miRNA }\sim 0+\mathrm{ Sex}.\mathrm{Timepoint}+\mathrm{BMI}+\mathrm{Wpeak}\) and a contrast matrix was constructed. Four contrasts were examined and included the difference between males and female at baseline, what miRNAs changed 3 h after an acute bout of exercise separately in each sex and finally to determine the differential response of miRNAs to an acute bout of exercise between sexes.
We then ran putative analysis to understand whether sex chromosomes or sex hormones were potential upstream regulators of miRNAs displaying sex differences. We tested associations between expression of miRNAs and hormone levels within each sex to assess whether hormone levels could explain some of the variability in miRNA expression. This analysis was performed in males and females separately, as they show very different ranges of hormones (thus avoiding collinearity). The model was of the form \(\mathrm{miRNA }\sim \mathrm{ Hormone}+\left(1|\mathrm{ID}\right)\). A Fisher test was performed to investigate if sex-biased miRNA genes were more likely to be encoded on the sex chromosomes or the autosomes. Putative gene targets of the miRNAs were detected with the R package multi-MiR [78], using only gene targets from the experimentally validated databases (miRecords, miRTarBase and TarBase). Overrepresentation analysis (ORA) was used for gene target enrichment and implemented by the R package clusterProfiler [79]. The background gene list used for the ORA analysis comprised all experimentally validated targets of the miRNAs detected in the microarray. Tissue enrichment of the validated gene targets was conducted in the R package TissueEnrich [20]. Finally, transcription factor-microRNA interactions were explored using TransmiR v2.0 using the default methods. The putative transcriptional regulatory region was considered 300 bp upstream and 100 bp downstream of each miR TSS or was based on published chip-seq data [80].
We considered significant miRNAs, pathways, tissues and transcription factors significant with an FDR adjusted p value < 0.05. The following packages were also used in our analysis: lme4 [81], lmerTest [82] and tidyverse [83]. The full R code can be found at https://doi.org/10.5281/zenodo.8412095.
Availability of data and materials
All data generated or analysed during this study are included in this published article, its supplementary information files and publicly available repositories (GEO: GSE242861 and https://doi.org/10.5281/zenodo.8412095).
Abbreviations
- BMI:
-
Body mass index
- Ddx3y:
-
DEAD box helicase 3
- E2:
-
Estradiol
- fT:
-
Free testosterone
- GO:
-
Gene Ontology
- GXT:
-
Graded exercise test
- HIIE:
-
High-intensity interval exercise
- KDM5D:
-
Lysine-specific demethylase 5D
- LT:
-
Lactate threshold
- miRNA:
-
MicroRNAs
- myo-miRNAs:
-
Muscle-enriched miRNAs
- SHBG:
-
Sex hormone binding globulin
- T2DM:
-
Type 2 diabetes mellitus
- TFs:
-
Transcription factors
- TSS:
-
Transcription start site
- TT:
-
Total testosterone
- Uty:
-
Ubiquitously transcribed tetratricopeptide repeat gene on Y chromosome
- W:
-
Watts
References
Khramtsova EA, Davis LK, Stranger BE. The role of sex in the genomics of human complex traits. Nat Rev Genet. 2019;20(3):173–90.
Oliva M, Munoz-Aguirre M, Kim-Hellmuth S, Wucher V, Gewirtz ADH, Cotter DJ, et al. The impact of sex on gene expression across human tissues. Science. 2020;369(6509):eaba3066.
Cui C, Yang W, Shi J, Zhou Y, Yang J, Cui Q, et al. Identification and analysis of human sex-biased MicroRNAs. Genomics Proteomics Bioinformatics. 2018;16(3):200–11.
Florijn BW, Bijkerk R, van der Veer EP, van Zonneveld AJ. Gender and cardiovascular disease: are sex-biased microRNA networks a driving force behind heart failure with preserved ejection fraction in women? Cardiovasc Res. 2017;114(2):210–25.
Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–40.
Hall E, Volkov P, Dayeh T, Esguerra JL, Salö S, Eliasson L, et al. Sex differences in the genome-wide DNA methylation pattern and impact on gene expression, microRNA levels and insulin secretion in human pancreatic islets. Genome Biol. 2014;15(12):522.
Gershoni M, Pietrokovski S. The landscape of sex-differential transcriptome and its consequent selection in human adults. BMC Biol. 2017;15(1):7.
Oliva M, Muñoz-Aguirre M, Kim-Hellmuth S, Wucher V, Gewirtz ADH, Cotter DJ, et al. The impact of sex on gene expression across human tissues. Science (New York, NY). 2020;369(6509):eaba3066.
Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985). 2000;89(1):81–8.
Maughan R, Watson JS, Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983;338(1):37–49.
Rosa-Caldwell ME, Greene NP. Muscle metabolism and atrophy: let’s talk about sex. Biol Sex Differ. 2019;10(1):43.
Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016;23(6):1034–47.
Landen S, Hiam D, Voisin S, Jacques M, Lamon S, Eynon N. Physiological and molecular sex differences in human skeletal muscle in response to exercise training. J Physiol. 2021;601(3)419–34.
Mayne BT, Bianco-Miotto T, Buckberry S, Breen J, Clifton V, Shoubridge C, et al. Large scale gene expression meta-analysis reveals tissue-specific, sex-biased gene expression in humans. Front Genet. 2016;7:183.
Brown WM. Exercise-associated DNA methylation change in skeletal muscle and the importance of imprinted genes: a bioinformatics meta-analysis. Br J Sports Med. 2015;49(24):1567–78.
Lopes-Ramos CM, Chen C-Y, Kuijjer ML, Paulson JN, Sonawane AR, Fagny M, et al. Sex differences in gene expression and regulatory networks across 29 human tissues. Cell Rep. 2020;31(12): 107795.
Lindholm ME, Marabita F, Gomez-Cabrero D, Rundqvist H, Ekstrom TJ, Tegner J, et al. An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics. 2014;9(12):1557–69.
Landen S, Jacques M, Hiam D, Alvarez-Romero J, Harvey NR, Haupt LM, et al. Skeletal muscle methylome and transcriptome integration reveals profound sex differences related to muscle function and substrate metabolism. Clin Epigenetics. 2021;13(1):202.
Russell AP, Lamon S, Boon H, Wada S, Guller I, Brown EL, et al. Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short-term endurance training. J Physiol. 2013;591(18):4637–53.
Jain A, Tuteja G. TissueEnrich: tissue-specific gene enrichment analysis. Bioinformatics (Oxford, England). 2018;35(11):1966–7.
Delić D, Grosser C, Dkhil M, Al-Quraishy S, Wunderlich F. Testosterone-induced upregulation of miRNAs in the female mouse liver. Steroids. 2010;75(12):998–1004.
Bhat-Nakshatri P, Wang G, Collins NR, Thomson MJ, Geistlinger TR, Carroll JS, et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009;37(14):4850–61.
Horak M, Novak J, Bienertova-Vasku J. Muscle-specific microRNAs in skeletal muscle development. Dev Biol. 2016;410(1):1–13.
McCarthy JJ. The MyomiR network in skeletal muscle plasticity. Exerc Sport Sci Rev. 2011;39(3):150–4.
Diaz-Canestro C, Montero D. Sex Dimorphism of VO2max Trainability: a systematic review and meta-analysis. Sports Med. 2019;49(12):1949–56.
Landen S, Jacques M, Hiam D, Alvarez-Romero J, Schittenhelm RB, Shah AD, et al. Sex differences in muscle protein expression and DNA methylation in response to exercise training. Biol Sex Differ. 2023;14(1):56.
Amar D, Lindholm ME, Norrbom J, Wheeler MT, Rivas MA, Ashley EA. Time trajectories in the transcriptomic response to exercise-a meta-analysis. Nat Commun. 2021;12(1):1–12.
Nielsen S, Hvid T, Kelly M, Lindegaard B, Dethlefsen C, Winding K, et al. Muscle specific miRNAs are induced by testosterone and independently upregulated by age. Frontiers in Physiology. 2014;4(394).
Telles GD, Libardi CA, Conceição MS, Vechin FC, Lixandrão ME, ALL DEA, et al. Time course of skeletal muscle miRNA expression after resistance, high-intensity interval, and concurrent exercise. Med Sci Sports Exerc. 2021;53(8):1708–18.
Nielsen S, Scheele C, Yfanti C, Akerström T, Nielsen AR, Pedersen BK, et al. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol. 2010;588(Pt 20):4029–37.
Silver JL, Alexander SE, Dillon HT, Lamon S, Wadley GD. Extracellular vesicular miRNA expression is not a proxy for skeletal muscle miRNA expression in males and females following acute, moderate intensity exercise. Physiol Rep. 2020;8(16):e14520.
Fyfe JJ, Bishop DJ, Zacharewicz E, Russell AP, Stepto NK. Concurrent exercise incorporating high-intensity interval or continuous training modulates mTORC1 signaling and microRNA expression in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2016;310(11):R1297–311.
Rapp D, Scharhag J, Wagenpfeil S, Scholl J. Reference values for peak oxygen uptake: cross-sectional analysis of cycle ergometry-based cardiopulmonary exercise tests of 10 090 adult German volunteers from the Prevention First Registry. BMJ Open. 2018;8(3):e018697.
Di Palo A, Siniscalchi C, Salerno M, Russo A, Gravholt CH, Potenza N. What microRNAs could tell us about the human X chromosome. Cell Mol Life Sci. 2020;77(20):4069–80.
Guo X, Su B, Zhou Z, Sha J. Rapid evolution of mammalian X-linked testis microRNAs. BMC Genomics. 2009;10(1):97.
Song R, Ro S, Michaels JD, Park C, McCarrey JR, Yan W. Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nat Genet. 2009;41(4):488–93.
Sosa E, Flores L, Yan W, McCarrey JR. Escape of X-linked miRNA genes from meiotic sex chromosome inactivation. Development. 2015;142(21):3791–800.
Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–42.
Butz H, Rácz K, Hunyady L, Patócs A. Crosstalk between TGF-β signaling and the microRNA machinery. Trends Pharmacol Sci. 2012;33(7):382–93.
Dreher SI, Höckele S, Huypens P, Irmler M, Hoffmann C, Jeske T, et al. TGF-β induction of miR-143/145 is associated to exercise response by influencing differentiation and insulin signaling molecules in human skeletal muscle. Cells. 2021;10(12):3443.
Dalgaard LT, Sørensen AE, Hardikar AA, Joglekar MV. The microRNA-29 family: role in metabolism and metabolic disease. Am J Physiol Cell Physiol. 2022;323(2):C367–77.
Droguett R, Cabello-Verrugio C, Santander C, Brandan E. TGF-beta receptors, in a Smad-independent manner, are required for terminal skeletal muscle differentiation. Exp Cell Res. 2010;316(15):2487–503.
Chen JL, Colgan TD, Walton KL, Gregorevic P, Harrison CA. The TGF-β Signalling network in muscle development, adaptation and disease. Adv Exp Med Biol. 2016;900:97–131.
Ziller N, Kotolloshi R, Esmaeili M, Liebisch M, Mrowka R, Baniahmad A, et al. Sex differences in diabetes- and TGF-β1-induced renal damage. Cells. 2020;9(10):2236.
Lin Y, Nakachi K, Ito Y, Kikuchi S, Tamakoshi A, Yagyu K, et al. Variations in serum transforming growth factor-<i>β</i>1 levels with gender, age and lifestyle factors of healthy Japanese adults. Dis Markers. 2009;27:529253.
Grilo GA, Shaver PR, Stoffel HJ, Morrow CA, Johnson OT, Iyer RP, et al. Age- and sex-dependent differences in extracellular matrix metabolism associate with cardiac functional and structural changes. J Mol Cell Cardiol. 2020;139:62–74.
Sun Q, Zhang Y, Yang G, Chen X, Zhang Y, Cao G, et al. Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res. 2008;36(8):2690–9.
Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation in women. J Appl Physiol (1985). 2020;129(6):1493–504.
Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985). 2020;128(1):87–99.
Sjögren RJO, Egan B, Katayama M, Zierath JR, Krook A. Temporal analysis of reciprocal miRNA-mRNA expression patterns predicts regulatory networks during differentiation in human skeletal muscle cells. Physiol Genomics. 2015;47(3):45–57.
Guess MG, Barthel KKB, Harrison BC, Leinwand LA. miR-30 Family microRNAs Regulate myogenic differentiation and provide negative feedback on the microRNA Pathway. PLoS ONE. 2015;10(2):e0118229.
Wijchers PJ, Yandim C, Panousopoulou E, Ahmad M, Harker N, Saveliev A, et al. Sexual dimorphism in mammalian autosomal gene regulation is determined not only by Sry but by sex chromosome complement as well. Dev Cell. 2010;19(3):477–84.
Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho T-J, et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014;508(7497):494–9.
Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550(7675):244–8.
Davegårdh C, Hall Wedin E, Broholm C, Henriksen TI, Pedersen M, Pedersen BK, et al. Sex influences DNA methylation and gene expression in human skeletal muscle myoblasts and myotubes. Stem Cell Res Ther. 2019;10(1):26.
O’Reilly J, Ono-Moore KD, Chintapalli SV, Rutkowsky JM, Tolentino T, Lloyd KCK, et al. Sex differences in skeletal muscle revealed through fiber type, capillarity, and transcriptomics profiling in mice. Physiol Rep. 2021;9(18):e15031.
Guo L, Zhang Q, Ma X, Wang J, Liang T. miRNA and mRNA expression analysis reveals potential sex-biased miRNA expression. Sci Rep. 2017;7:39812.
Simon LM, Edelstein LC, Nagalla S, Woodley AB, Chen ES, Kong X, et al. Human platelet microRNA-mRNA networks associated with age and gender revealed by integrated plateletomics. Blood. 2014;123(16):e37–45.
Chantranupong L, Scaria SM, Saxton RA, Gygi MP, Shen K, Wyant GA, et al. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell. 2016;165(1):153–64.
Kelly AC, Rosario FJ, Chan J, Cox LA, Powell TL, Jansson T. Transcriptomic responses are sex-dependent in the skeletal muscle and liver in offspring of obese mice. Am J Physiol Endocrinol Metab. 2022;323(4):E336–53.
Gürgen D, Kusch A, Klewitz R, Hoff U, Catar R, Hegner B, et al. Sex-specific mTOR signaling determines sexual dimorphism in myocardial adaptation in normotensive DOCA-salt model. Hypertension. 2013;61(3):730–6.
Chen Y, Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48(D1):D127–31.
Pitsiladis YP, Tanaka M, Eynon N, Bouchard C, North KN, Williams AG, et al. Athlome Project Consortium: a concerted effort to discover genomic and other “omic” markers of athletic performance. Physiol Genomics. 2016;48(3):183–90.
Yan X, Eynon N, Papadimitriou ID, Kuang J, Munson F, Tirosh O, et al. The gene SMART study: method, study design, and preliminary findings. BMC Genomics. 2017;18(Suppl 8):821.
Hiam D, Smith C, Voisin S, Denham J, Yan X, Landen S, et al. Aerobic capacity and telomere length in human skeletal muscle and leukocytes across the lifespan. Aging. 2020;12(1):359–69.
Hiam D, Voisin S, Yan X, Landen S, Jacques M, Papadimitriou ID, et al. The association between bone mineral density gene variants and osteocalcin at baseline, and in response to exercise: The Gene SMART study. Bone. 2019;123:23–7.
Willems HME, van den Heuvel EGHM, Schoemaker RJW, Klein-Nulend J, Bakker AD. Diet and exercise: a match made in bone. Curr Osteoporos Rep. 2017;15(6):555–63.
Starup-Linde J, Lykkeboe S, Gregersen S, Hauge EM, Langdahl BL, Handberg A, et al. Differences in biochemical bone markers by diabetes type and the impact of glucose. Bone. 2016;83:149–55.
Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–7.
Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14(1):101–2.
Huhtaniemi IT, Tajar A, Lee DM, O’Neill TW, Finn JD, Bartfai G, et al. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol. 2012;166(6):983–91.
Frenkel B, Hong A, Baniwal SK, Coetzee GA, Ohlsson C, Khalid O, et al. Regulation of adult bone turnover by sex steroids. J Cell Physiol. 2010;224(2):305–10.
Zacharewicz E, Kalanon M, Murphy RM, Russell AP, Lamon S. MicroRNA-99b-5p downregulates protein synthesis in human primary myotubes. Am J Physiol Cell Physiol. 2020;319(2):C432–40.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
R Core Team. R: a language and environment for statistical computing. 2021.
Kowarik A, Templ M. Imputation with the R Package VIM. J Stat Softw. 2016;74(7):1–16.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47-e.
Ru Y, Kechris KJ, Tabakoff B, Hoffman P, Radcliffe RA, Bowler R. The multiMiR R package and database: integration of microRNA–target interactions along with their disease and drug associations. Nucleic Acids Res. 2014;42(17):e133-e.
Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation. 2021;2(3):100141.
Tong Z, Cui Q, Wang J, Zhou Y. TransmiR v2.0: an updated transcription factor-microRNA regulation database. Nucleic Acids Res. 2019;47(D1):D253–8.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48.
Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: tests in linear mixed effects models. J Stat Softw. 2017;82(13):1–26.
Wickham H, Averick M, Bryan J, Chang W, McGowan LDA, François R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4(43):1686.
Acknowledgements
We thank the participants for partaking in this study.
Funding
Severine Lamon is supported by Australian Research Council (ARC) Future Fellowship (FT210100278). This work was supported by Nir Eynon’s NHMRC Career Development Fellowship (APP1140644) and NHMRC Investigator Grant (APP1194159). The Gene SMART study is also supported by an Australian Research Council (ARC) Discovery Project Grant for Nir Eynon, Sarah Voisin and Severine Lamon (DP200101830).
Author information
Authors and Affiliations
Contributions
Conceptualisation: DH, SV, SLamon and NE. Investigation (human exercise trials): DH, SLanden, MJ, SV and NE. Laboratory investigation (cell experiments and biochemical analyses): DH. Bioinformatics: DH and SV. Formal analysis and visualisation: DH, SLanden, SV and Slamon. Writing—original draft: DH, SLanden and SLamon. Writing—review and editing: all authors. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Human Ethics Research Committee at Victoria University (HRE13-223, and HRE 21–122). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The response of miRNAs expression following exercise in males, Table S2. The response of miRNAs expression following exercise in females, Table S3. Comparison of expression levels of miRNAs at baseline between males and females, Table S4. Comparison of expression levels of miRNAs post exercise bout between males and females, Table S5. Interaction analysis: miRNAs whose response to an acute bout of exercise differed between sexes, Table S6. miRNA gene targets and skeletal muscle enriched genes, Table S7. Over-representation analysis of sex-biased miRNA gene targets at baseline, Table S8. Over-representation analysis of sex-biased miRNA gene targets from interaction analysis, Table S9. Correlation analysis of circulating sex hormones and sex-biased miRNAs, Table S10. Transcription factor enrichment analysis of sex-biased miRNAs at baseline, Table S11. Transcription factor enrichment analysis of sex-biased miRNAs from interaction analysis.
Additional file 2: Figure S1.
Principal component analysis: A) Scree Plot, B) Eigencorplot: Figure S2. The proportion of the sex-biased microRNAs genes expressed across the autosomes compared to the sex chromosomes at A) baseline B) after an acute bout of exercise. Figure S3. Gene set enrichment analysis: Top enriched GO terms of baseline male and female donor primary cells.
Additional file 3: Table S1.
Comparison of male and female at baseline (scramble condition), Table S2. Comparison of male scramble vs overexpression of miR-30a, Table S3. Comparison of female scramble vs overexpression of miR-30a, Table S4. Comparison of male overexpression miR-30a and female overexpression miR30a, Table S5. Interaction Analysis of miR-30a, Table S6. Comparison of male scramble vs overexpression of miR-30c, Table S7. Comparison of female scramble vs overexpression of miR-30c, Table S8. Comparison of male overexpression miR-30c and female overexpression miR30c, Table S9. Interaction Analysis of 30c. Table S10. Donor Characteristics.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hiam, D., Landen, S., Jacques, M. et al. Muscle miRNAs are influenced by sex at baseline and in response to exercise. BMC Biol 21, 273 (2023). https://doi.org/10.1186/s12915-023-01755-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12915-023-01755-3