Abstract
Background
Several classification systems for medication errors (MEs) have been established over time, but none of them apply optimally for classifying severe MEs. In severe MEs, recognizing the causes of the error is essential for error prevention and risk management. Therefore, this study focuses on exploring the applicability of a cause-based DRP classification system for classifying severe MEs and their causes.
Methods
This was a retrospective document analysis study on medication-related complaints and authoritative statements investigated by the Finnish National Supervisory Authority for Welfare and Health (Valvira) in 2013–2017. The data was classified by applying a previously developed aggregated DRP classification system by Basger et al. Error setting and harm to the patient were identified using qualitative content analysis to describe the characteristics of the MEs in the data. The systems approach to human error, error prevention, and risk management was used as a theoretical framework.
Results
Fifty-eight of the complaints and authoritative statements concerned MEs, which had occurred in a wide range of social and healthcare settings. More than half of the ME cases (52%, n = 30) had caused the patient’s death or severe harm. In total, 100 MEs were identified from the ME case reports. In 53% (n = 31) of the cases, more than one ME was identified, and the mean number of MEs identified was 1.7 per case. It was possible to classify all MEs according to aggregated DRP system, and only a small proportion (8%, n = 8) were classified in the category “Other,” indicating that the cause of the ME could not be classified to specific cause-based category. MEs in the “Other” category included dispensing errors, documenting errors, prescribing error, and a near miss.
Conclusions
Our study provides promising preliminary results for using DRP classification system for classifying and analyzing especially severe MEs. With Basger et al.’s aggregated DRP classification system, we were able to categorize both the ME and its cause. More research is encouraged with other ME incident data from different reporting systems to confirm our results.
Similar content being viewed by others
Background
Medication errors (MEs) are one of the major concerns in patient safety [1,2,3]. A medication error is any preventable event that may cause or lead to inappropriate medication use or patient harm [4]. There is a need to learn effectively from errors that have already happened using error reporting systems and other available data sources to improve medication safety [1, 5,6,7,8]. Especially the reoccurrence of MEs causing severe harm or even the death of the patient should be prevented, which is also promoted by global actions [2].
In ME reporting systems and studies on medication safety, data have been typically analyzed using error classification systems and taxonomies. Theoretical or conceptual frameworks have usually guided the development of error classification systems, or the process has been data-driven [9]. Depending on the aim of the classification, the system can be contextual (e.g., the place or the medicine involved), modal (how the error happened, e.g., omission), or psychological (why the error occurred, e.g., skill-based error) [10]. Classification systems and taxonomies need to relate to similar events, describe essential clinical and systemic factors, and support analysis purposes [11].
Although there are multiple taxonomies for MEs, they often share the limitation of being in too general level and describing only outcomes, not the causes of the errors [12]. This deficiency has been noted in many widely internationally used classification systems (e.g., World Health Organization, National Coordinating Council for Medication Error Reporting and Prevention) [13, 14], but the challenges to describing MEs accurately enough and with the causes and contributing factors are still partly unresolved. Without knowing the causes and contributing factors of MEs, essential information for error prevention and risk management are lacking [12, 15]. Adequate information on what happened and why is crucial, especially when preventing severe MEs [16,17,18]. A classification system that gives enough information for medication error prevention and risk management, is desirable in the system-based approach to medication safety [11, 12]. Consequently, there is a need to develop ME classification systems further to support system-based medication safety.
Exploring the utility of existing classification systems, including also causes, can provide an option for acquiring more information about MEs to find novel and robust methods for describing ME data. Previous research has described the application of adverse drug event (ADE) classification systems to MEs, but the evidence is still limited [15]. According to our knowledge, none of the studies has evaluated how classification systems for drug-related problems (DRPs) could be applied to ME data, even though many of them also include causes for the problems [19].
Material and methods
Definitions of the key concepts used in this study
Drug-related problem (DRP) is an event or circumstance involving drug therapy that actually or potentially interferes with desired outcomes (e.g., the effect of the drug treatment is not optimal, Table 1) [20]. DRPs occur or have the potential to happen, because of a prior event or events that perform as a cause or causes of the DRP (e.g., failures associated with the medication use process) [20, 21]. As MEs can cause DRPs (e.g., error in prescribing, Table 1 and Fig. 1), this connection supports using existing DRP classification systems for classifying MEs. Table 1 and Fig. 1 present the relationship between the definitions of ADEs, DRPs and MEs as used in this study.
Aim of the study
This study is to explore the applicability of a cause-based DRP classification system in classifying severe MEs and their causes.
Study design and setting
This was a retrospective document analysis study [27] on medication-related complaints and authoritative statements investigated by the Finnish National Supervisory Authority for Welfare and Health (Valvira). Valvira is a national authority investigating patient safety incidents that have led to the severe harm or death of a patient due to inappropriate care [28]. Thus, Valvira has unique and extensive data emphasized on severe MEs that may rarely be captured by medication error reporting systems [29]. Contrary majority of the typical ME register data that mainly consist of structured data, Valvira´ s investigational documentation is based on qualitative descriptions of the incidents. The investigations are based on complaints made by patients and/or their relatives, notifications by healthcare organizations, or statement requests made by other Finnish authorities (e.g., the police, the parliamentary ombudsman, and the courts). The annual number of all new complaints and statement requests from social and healthcare was 632 in 2022 [30].
The theoretical framework for the study was the systems approach to human error, error prevention, and risk management through understanding the processes and causes leading to severe MEs [31, 32].
Material
The research data consisted of all medication-related complaints and authoritative statements that Valvira had investigated and concluded from 2013–2017. The following inclusion criteria were used for getting the research data: 1) the primary cause of the complaint or statement was classified as “pharmacotherapy” by Valvira; 2) Valvira assessed the case to involve inappropriate patient care (error in medication), and 3) the case was closed (Fig. 2). The search of the document material was first performed as an automatic search in Valvira´s electronic database (Qlikview) and then finalized by a manual search by one of the researchers (AT) to ensure that all the automatically searched cases fulfilled the study inclusion criteria.
Valvira´s documentation for the complaints and authoritative statements typically contained: 1) a copy of the patient records and other documents needed for incident evaluation; 2) responses from the professionals involved in the case and/or the managers of the healthcare organization; 3) an external expert (physician or another specialist) statement; and 4) the incident report by Valvira’s Senior Medical or Legal Officer. All the incident documentation was qualitative data in nature. Some of the incident documentation was in narrative form, and it described the incident and its circumstances, as well as the conclusion of the case. The total written material per case varied between 20–150 pages.
Research method
Several DRP classification systems have been established over time [19]. One important feature of the DRP classification systems is that they should be able to differentiate DRPs and their causes [21]. The present study applied the newest comprehensive DRP classification system, which was aggregated by Basger et al. based on a systematic inventory of existing DRP classification systems [19, 21]. Their classification system is comprehensive, easy to use, and separates DRPs and their causes. It forms a hierarchical classification system consisting of nine cause-of-DRP categories, including 33 subcategories and 58 sub-subcategories (Table 2). Basger et al.’s [21] aggregated classification system was adopted as such for the present study.
MEs were identified and classified from Valvira’s data by the main researcher (AT). First, the qualitative and partly narrative data in Valvira’s documentation was carefully read case by case. Identified ME process and contributing factors were summarized as a brief anonymized case description for each case. MEs were categorized from these brief case descriptions using Basger et al.’s. aggregated DRP classification system [21]. In cases where any difficulties were encountered in the categorization, another researcher was consulted (CLL), and the final classification was decided by a consensus from two researchers. In cases where no suitable category was found for the ME, the error was classified to the "Other” category (a cause that cannot be classified into any other categories, Category 9 in Table 2). The main researcher made notes of those MEs for detecting possible missing categories in the aggregated DRP classification system. Because severe MEs are often complex processes including several errors [16, 17, 31, 32], all identified MEs were categorized from description of each case.
Error setting and harm to the patient were identified and documented for the data analysis to explain the essential characteristics of the MEs in the data. Four categories were used to classify the severity of harm to the patient: death, severe harm, non-severe harm, and no harm. Harm to the patient was defined as severe when the error had been life-threatening, led to hospitalization or prolonged hospitalization, or caused permanent or significant injury with incapacity [33]. The data categorized in this study were quantitatively analyzed in Microsoft Excel for descriptive statistics (frequencies and percentages).
Results
Valvira received a total of 1654 complaints and statement requests from 2013–2017. Of these cases, 58 cases were medication-related and included in this study. The identified MEs had occurred in a wide range of social and healthcare settings, most commonly in assisted living facilities (n = 16, 25% of all settings, Table 3). There were 6 cases (10%) where more than one care setting was involved in the error process. In more than half of the cases (52%, n = 30), the ME caused the patient’s death or severe harm. Other characteristics of the data have been published elsewhere [17].
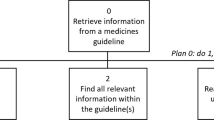
It was possible to classify all MEs according to Basger et al. [21]. In total, 100 MEs were identified from the cases (n = 58) (Table 2). In 53% (n = 31) of the cases, more than one ME was identified (mean number of MEs, 1.7 per case). Figure 3 presents an example of how MEs were identified and categorized from the case descriptions. A small proportion (8%, n = 8) of the MEs identified from the reports were classified in the “Other” category (a cause that cannot be classified into one of Basger’s et al.’s eight categories, Category 9, Table 2). The”Other” category included the following MEs (n = 8):
-
1)
dispensing errors in a community pharmacy (n = 1)
-
2)
documenting errors related to medication administration (e.g., no information available if the medication had been administered or in which dose, n = 5)
-
3)
prescribing errors in which cessation of the medication was carried out inappropriately (n = 1)
-
4)
dispensing error in the care unit detected before reaching the patient (near miss, n = 1).
An example of how MEs were identified from the case descriptions (n = 58) and categorized with Basger et al.’s aggregated DRP classification system [21]
The identified MEs (n = 100) fell into all nine main categories of Basger et al.'s classification, 21/33 (64%) of the subcategories and 21/58 (36%) of the sub-subcategories (Table 2). Most of the MEs (n = 89, 89%) were categorized into the following six main categories: “Drug selection” (n = 22, 22%), “Drug use process” (n = 18, 18%), “Monitoring” (n = 14, 14%), “Dose selection” (n = 13, 13%), “Treatment duration” (n = 11, 11%) and “Logistics” (n = 11, 11%). Subcategory and sub-subcategories were used for all MEs if there were such defined in Basger et al.´s classification system.
Discussion
The present study suggests that the aggregated DRP classification system by Basger et al. [21] can be applied to categorizing severe MEs. To our knowledge, this was the first international study to pilot test the applicability of a DRP classification system for this purpose. Further studies are needed to confirm our findings. Because of its authoritative nature, Valvira’s data had comprehensive qualitative and narrative documentation on the ME cases that enabled us to identify reliably multiple MEs and their causes in each case.
As Valvira’s data included both severe and non-severe MEs in different social and healthcare settings, our study indicated that the DRP classification might be applied to MEs with different levels of harm occurring in different care settings. Still, there were some challenges in using aggregated DRP classification system for all MEs included in our data. This concerns especially the potential limitation of not being able to categorize all MEs prevented before they reached the patient (i.e., near misses). With severe MEs, this situation is not usually an issue as they typically reach the patient and cause some problems or harm to the patient. However, this limitation of DRP classification can become a major challenge when classifying MEs that are potential (Fig. 1.). It would be problematic if the same classification system would not apply to near miss events as usually the ME data consists of all kinds of ME, ranging from near misses to most fatal ones. As there was only one near miss case in our study material, there is a need for further research with more extensive data acquired from other ME sources, such as ME reporting systems commonly used in healthcare organizations.
With Basger et al.’s aggregated DRP classification system, we were able to categorize both the problem (ME) and its cause. Subgroups and sub-subgroups of causes were seen to be useful in the categorization of the causes in a way that provides detailed information about the incident for ME prevention and risk management purposes from the systems approach, although further research is needed on ME categorization. Already existing ME classification systems could be further developed to include similar subgroups of error causes as the Basger et al.’s aggregated DRP classification system has. This kind of more detailed error cause categorization has been already experimented within e.g., a medical error study that used the WHO International Classification for Patient Safety [34]. Still, the strength of Basger et al.’s aggregated DRP classification system is that it is readily available and specifically designed for risk management in the medication use process. There is a need to further optimize ME classification so that it will provide enough information about error causes and contributing factors.
Having as much information as possible is a benefit when developing medication safety based on reported errors, especially severe ones [17, 18]. For example, prescribing error (as an outcome) can mean anything starting from the wrong drug or dose selection to duration of the medication treatment [35]. In our previous study with the same Valvira data as used in the present study, we were able to identify the most common ME types but were using an outcome-based ME taxonomy [17]. By this present analysis of the same data using Basger et al.’s aggregated DRP classification system, we were able to describe and understand the MEs and causes contributing to the incidents more in detail. Another strength of using Basger et al.’s aggregated DRP classification system is that it indicates whether the MEs were caused by healthcare professionals or by the patient.
Even though we found Basger et al.’s aggregated DRP classification system to be potential for categorizing severe and non-severe MEs, it could be further optimized for classifying MEs. Basger et al.’s aggregated DRP classification system had many subcategories that did not have any sub-subcategories to describe the cause for the problem (16/58). As we had rich qualitive data and were able to identify the cause(s) for the ME in most cases, we could have been able to add some sub-subcategories to the classification system (e.g., why too low medication dose was prescribed). Additional sub-subcategories could produce more information about the causes and contributing factors. On the other hand, they may increase the complexity of the classification system [21].
It became evident that we were not able to describe all contributing factors identified that caused the error by using the aggregated DRP classification system. For example, in a ME case presented in Fig. 3, we identified many more contributing factors than we were able to classify with the classification system (e.g., two different oxycodone concentrations in use, no double-checking procedures, an inexperienced physician who did not know the possibilities and resources for patient monitoring in assisted living facility unit). This result indicates that Basger et al.’s aggregated DRP classification system in its current form does not alone meet the need for comprehensively categorizing a wide range of factors contributing to severe MEs. Therefore, it is important to recognize the limitations of the DRP classification system and use other methods to supplement the understanding of the multiple causes and factors contributing to MEs.
Even though the aggregated DRP classification system helped to describe MEs and their causes in more detail, we are still lacking tools to describe complex medication errors. Severe errors often consist of multiple errors that are chained (e.g., Fig. 3) [16, 17, 31, 32, 36]. It is challenging to make these error chains visible using only structured classification, although there are some successful examples [16]. But in terms of learning in-depth from severe MEs and complex error cases, qualitative analysis (e.g., root cause analysis or causal tree analysis) can still be seen as more informative, because these tools enable the description of multiple error chains and the contributing factors in the medication use process [37, 38].
All kinds of classification systems need to have an “other” category for miscellaneous issues because classification systems can rarely be completely comprehensive. The prevalence of using this category has been even higher in the studies using different DRP classification systems for DRPs than in our study with MEs [19]. Our data contained documenting errors at the administration phase that we were not able to categorize using Basger et al.’s aggregated DRP classification system. Documenting errors have potential to cause DRPs and thus harm the patient; therefore, they should be added to the classification system [39, 40]. They could be categorized under the main category “Logistics.” At all, the main category “Logistics” could be renamed because some of its sub- and sub-subcategories relate to other than purely logistic issues, such transcribing errors (Category 6.2) and errors in drug selection (Category 6.3) (Table 2). We also found that the classification system was missing an explicit DRP category for problems in medication cessation and implementing phased dose changes. Both these subcategories are important from the DRP and ME perspective.
Furthermore, we noticed that the classification system should be developed from the pharmacists’ perspective to better consider risks related to the preparation and dispensing phases of the medication use process, as well as counseling and educating medicine users. All these situations are potential causes for errors and causes for DRPs [41, 42]. The classification system should distinguish between transcribing errors that occur in medication reconciliation and the transcribing phase. Basger et al. [21] themselves noticed that DRPs associated with care transfers may require a taxonomy of its own, because these problems may be challenging to describe using only the DRP classification system. Indeed, the medication discrepancy taxonomy (MedTax) has been developed few years after Basger et al. published their aggregated DRP classification system [43].
Limitations
Basger et al.’s aggregated DRP classification system was seen potential for classifying the severe MEs in our study, but more studies are needed using other ME incident data from other reporting systems to confirm our findings. Although our data included all ME cases investigated by Valvira within a five-year period, the data had quite limited number of cases. This problem concerns particularly the number of severe MEs which were the special focus of this study. A larger number of severe ME cases in our data may have allowed us to generate more specific information about the applicability of the DRP classification system. The available data set was large enough to conclude whether DRP classification is in principle suitable for classifying MEs. Our data was rich and extensive, qualitative and narrative in nature. Thus, it was suitable for the qualitative analysis, even though primarily collected for authoritative purposes. The data enabled us to identify many MEs and their causes. Our rich data may also be considered as a limitation when comparing our results with the results of other studies that may have used data with less information about the ME incidents.
In our study, one researcher made the classification and only in cases where difficulties were encountered in the categorization, another researcher was consulted, and final classification decided as consensus. Although the aim of this study was not to validate the DRP classification system, the lack of more thorough validation of the classification process by two independent researchers can be seen as a limitation. Still, our study provides promising preliminary results for suitability of using DRP classification system for classifying and analyzing especially severe MEs. More research is needed to evaluate the applicability and utilization of DRP classification systems for classifying and analyzing ME data of different types and levels of harm, particularly data for understanding severe MEs and their contributing factors.
Conclusions
Our study suggests that the aggregated DRP classification system with some modifications could be used for analyzing and describing MEs and their causes, especially severe MEs. This finding aligns with the current definition framework in the study which defines that DRPs can be caused by MEs. Using a cause-based DRP classification system produces additional information which is essential for understanding why the MEs happened and how to prevent such MEs in future. This information is particularly valuable with severe MEs that are a priority to prevent. Because severe MEs are often complex processes including several errors and contributing factors, additional means to analyze the incidents are also needed. Further studies using different ME data are needed to validate our preliminary findings and learn more how DRP classification systems could be utilized in learning from errors and their contributing factors.
Availability of data and materials
The data that support the findings of this study are available from Valvira but restrictions apply to the availability of these data, and are not publicly available.
Abbreviations
- ME:
-
Medication error
- DRP:
-
Drug-related problem
- ADE:
-
Adverse drug event
- ADR:
-
Adverse drug reaction
References
Committee on Identifying and Preventing Medication Errors. Aspden P, Wolcott J, Bootman JL, Cronenwett LR (eds). Preventing medication errors: quality chasm series. Washington, DC: National Academies Press; 2006.
World Health Organization (WHO). The third WHO Global Patient Safety Challenge: Medication Without Harm. https://www.who.int/publications/i/item/WHO-HIS-SDS-2017.6. Accessed 6 March 2023.
Panagiotti M, Khan K, Keers NR, Abuzour A, Phipps D, Kontopantelos E, et al. Prevalence, severity, and nature of preventable patient harm across medical care settings: systematic review and meta-analysis. BMJ. 2019;366:l4185. https://doi.org/10.1136/bmj.l4185.
National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP). What is a Medication Error? https://www.nccmerp.org/about-medication-errors. Accessed 6 March 2023.
Institute for Safe Medication Practices. Error Reporting and Analysis. https://www.ismp.org/error-reporting-programs. Accessed 6 March 2023.
Holmström A-R, Airaksinen M, Weiss M, Wuliji T, Chan XH, Laaksonen R. National and local medication error reporting systems: a survey of practices in 16 countries. J Patient Saf. 2012;8:165–76. https://doi.org/10.1097/PTS.0b013e3182676cf3.
Holmström A-R, Laaksonen R, Airaksinen M. How to make medication error reporting systems work - Factors associated with their successful development and implementation. Health Policy. 2015;119:1046–54. https://doi.org/10.1016/j.healthpol.2015.03.002.
World Health Organization (WHO). Patient safety incident reporting and learning systems: technical report and guidance. Published 2020. https://www.who.int/publications/i/item/9789240010338. Accessed 6 March 2023.
Dovey S, Hickner J, Phillips B. Developing and using taxonomies of errors. In: Walshe K, Boaden R, editors. Patient Safety – Research into practice. Open University Press; 2006. p. 93–107.
Aronson JK. Medication errors: definitions and classification. Br J Clin Pharmacol. 2009;67:599–604. https://doi.org/10.1111/j.1365-2125.2009.03415.x.
Macrae C. The problem with incident reporting. BMJ Qual Saf. 2016;25:71–5. https://doi.org/10.1136/bmjqs-2015-004732.
Ferner RE, Aronson JK. Clarification of terminology in medication errors. Drug Saf. 2006;29:1011–22.
World Health Organization (WHO). Conceptual Framework for the International Classification for Patient Safety. Published January 2009. https://apps.who.int/iris/bitstream/handle/10665/70882/WHO_IER_PSP_2010.2_eng.pdf. Accessed 6 March 2023.
National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). NCC MERP Taxonomy of Medication Errors. https://www.nccmerp.org/sites/default/files/taxonomy2001-07-31.pdf. Accessed 6 March 2023.
Malpass A, Helps SC, Sexton EJ, Maiale D, Runciman WB. A classification for adverse drug events. J Qual Clin Practice. 1999;19:23–6. https://doi.org/10.1046/j.1440-1762.1999.00286.x.
Huckels-Baumgart S, Manser T. Identifying medication error chains from critical incident reports: a new analytic approach. J Clin Pharmacol. 2014;54:1188–97. https://doi.org/10.1002/jcph.319.
Linden-Lahti C, Takala A, Holmström A-R, Airaksinen M. What severe medication errors reported to health care supervisory authority tell about medication safety? J Patient Saf. 2021;17:e1179–85. https://doi.org/10.1097/PTS.0000000000000914.
Mulac A, Taxis K, Hagesaether E, Granas AG. Severe and fatal medication errors in hospitals: findings from the Norwegian Incident Reporting System. Eur J Hosp Pharm. 2021;28(Suppl 2):e56–61. https://doi.org/10.1136/ejhpharm-2020-002298.
Basger BJ, Moles RJ, Chen TF. Application of drug-related problem (DRP) classification systems: a review of the literature. Eur J Clin Pharmacol. 2014;70:799–815. https://doi.org/10.1007/s00228-014-1686-x.
Pharmaceutical Care Network Europe (PCNE). PCNE Classification for Drug-Related Problems V9.1. Published 2020. https://www.pcne.org/upload/files/417_PCNE_classification_V9-1_final.pdf. Accessed 6 March 2023.
Basger BJ, Moles RJ, Chen TF. Development of an aggregated system for classifying causes of drug-related problems. Ann Pharmacother. 2015;49:405–18. https://doi.org/10.1177/1060028014568008.
Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse events. Implication for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34.
Morimoto T, Gandhi TK, Seger AC, Hsieh TC, Bates DW. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13:306–14. https://doi.org/10.1136/qshc.2004.010611.
Otero M, Schmitt E. Clarifying Terminology for Adverse Drug Events. Ann Intern Med. 2005;142:77–9. https://doi.org/10.7326/0003-4819-142-1-200501040-00016.
European Medicines Agency (EMA). Medication errors. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medication-errors. Accessed 6 March 2023.
The European Parliament and the Council of the European Union. Directive 2010/84/EU of the European Parliament and the Council of the European Union amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use. http://data.europa.eu/eli/dir/2010/84/oj. Accessed 6 March 2023.
Weinger MB, Slagle J, Jain S, et al. Retrospective data collection and analytical techniques for patient safety studies. J Biomed Inform. 2003;36:106–19.
The Finnish National Supervisory Authority for Welfare and Health (Valvira). Valvira promoting welfare and health trough effective supervision. https://www.valvira.fi/web/en/front-page. Accessed 6 March 2023.
Cheung K, van den Bemt PMLA, Bouvy ML, Wensing M, De Smet PAGM. A nationwide medication incidents reporting system in The Netherlands. J Am Med Inform Assoc. 2011;18:799–804. https://doi.org/10.1136/amiajnl-2011-000191.
The Finnish National Supervisory Authority for Welfare and Health (Valvira). Financial statement and action report 2022, Dnro V/2933/2023 (in Finnish). https://www.valvira.fi/documents/14444/398591/Valviran_tilinpaatos_ja_toimintakertomus_2022.pdf/52b2fd6b-7d1d-85e6-b84a-c2deee278632?t=1677590578170. Accessed 6 March 2023.
Reason J. Human error. Cambridge University Press. 1990.
Reason J. Human error: Models and management. BMJ. 2000;320:768–70.
Gates PJ, Baysari MT, Mumford V, Raban MZ, Westbrook JI. Standardising the Classifcation of Harm Associated with Medication Errors: The Harm Associated with Medication Error Classifcation (HAMEC). Drug Saf. 2019;42:931–9. https://doi.org/10.1007/s40264-019-00823-4.
Mitchell R, Faris M, Lystad R, Fajardo Pulido D, Norton G, Baysari M, et al. Using the WHO International Classification of patient safety framework to identify incident characteristics and contributing factors for medical or surgical complication deaths. Appl Ergon. 2020;82:102920. https://doi.org/10.1016/j.apergo.2019.102920.
Ashcroft DM, Lewis PJ, Tully MP, Farragher TM, Taylor D, Wass V, et al. Prevalence, Nature, Severity and Risk Factors for Prescribing Errors in Hospital Inpatients: Prospective Study in 20 UK Hospitals. Drug Saf. 2015;38:833–43. https://doi.org/10.1007/s40264-015-0320-x.
Thomas AN, MacDonald JJ. A review of patient safety incidents reported as ‘severe’ or ‘death’ from critical care units in England and Wales between 2004 and 2014. Anaesthesia. 2016;71:1013–23. https://doi.org/10.1111/anae.13547.
VHA National Center for Patient Safety (NCPS). Root Cause Analysis Tools. Revised October 10, 2020. https://www.patientsafety.va.gov/docs/RCA_Guidebook_10212020.pdf. Accessed 7 March 2023.
Smith M, Janssen J, de Vet R, Zwaan L, Timmermans D, Groenewegen P, Wagner C. Analysis of unintended events in hospitals: inter-rater reliability of constructing causal trees and classifying root causes. Int J Qual Health Care. 2009;21:292–300. https://doi.org/10.1093/intqhc/mzp023.
Roydhouse SA, Carland JE, Debono DS, Baysari MT, Reuter SE, Staciwa AJ, et al. Accuracy of documented administration times for intravenous antimicrobial drugs and impact on dosing decisions. Br J Clin Pharmacol. 2021;87:4273–82. https://doi.org/10.1111/bcp.14844.
Savva G, Merkourius A, Charalambous A, Papastavrou E. Omissions and deviations from safe drug administration guidelines in 2 medical wards and risk factors: findings from an observational study. J Patient Saf. 2022;18:e645–51. https://doi.org/10.1097/PTS.0000000000000913.
James KL, Barlow D, McArtney R, Hiom S, Roberts D, Whittlesea C. Incidence, type and causes of dispensing errors: a review of the literature. Int J Pharm Pract. 2009;17:9–30.
Hong K, Hong YD, Cooke CE. Medication errors in community pharmacies: The need for commitment, transparency, and research. Res Social Adm Pharm. 2019;15:823–6. https://doi.org/10.1016/j.sapharm.2018.11.014.
Almanasreh E, Moles R, Chen TF. The medication discrepancy taxonomy (MedTax): The development and validation of a classification system for medication discrepancies identified through medication reconciliation. Res Social Adm Pharm. 2020;16:142–8. https://doi.org/10.1016/j.sapharm.2019.04.005.
Finnish National Board on Research Integrity (TENK). Ethical review in human sciences. https://tenk.fi/en/ethical-review/ethical-review-human-sciences. Accessed 31 March 2023.
The European Parliament and the Council of the European Union. Regulation (EU) 2016/679 of the European Parliament and the Council of the European Union on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). https://eur-lex.europa.eu/eli/reg/2016/679/oj. Accessed 6 March 2023.
All European Academies (ALLEA). The European Code of Conduct for Research Integrity. Revised edition 2017. Available at: https://www.allea.org/wp-content/uploads/2017/05/ALLEA-European-Code-of-Conduct-for-Research-Integrity-2017.pdf. Accessed 7 March 2023.
Acknowledgements
We want to thank Valvira for their valuable contributions in the design and implementation of the study.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. This research was funded by the Helsinki University Pharmacy and Finnish Cultural Foundation.
Author information
Authors and Affiliations
Contributions
CLL, AT and MA structured this study. AT analyzed data and when needed, consensus in analysis was made together with CLL. AT and CLL drafted initial manuscript. CLL, AT, AH, and MA critically revised the manuscript and helped interpret data. All authors reviewed the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was retrospective register-based document analysis from authority data collected to supervisory purposes. According to Finnish National Board on Research Integrity, ethical approval is not needed for retrospective register-based study unless there is a special risk for information security in merging data or it is a medical study [44]. This study was not medical study that intervened to patient’s physical or mental integrity according to definition of Finnish Act on Medical Study (1999/488). According to Finnish Act on Secondary Use of Social and Health Information (552/2019), Valvira can give permission to use data including authoritative statements in research purposes that fulfils the legislative requirements. The study was granted a study approval from Valvira. The study was conducted under Valvira’s supervision and data has been pseudonymized for the purposes of this study analysis. Good research practice and data protection guidelines were followed throughout the research process [45, 46].
Consent for publication
Because of the nature of register-based data collected to supervisory purposes and the use of data according to Finnish Act on Secondary Use of Social and Health Information (552/2019), consent for publication was not applicable. Valvira´s study approval included a statement that the results of the study are allowed to be presented in a way that specific patients, professionals or organizations are not recognized. Figure 3, which includes the case description of ME is publicized under Valvira´s study approval and no patients, professionals or organizations included are recognizable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Linden-Lahti, C., Takala, A., Holmström, AR. et al. Applicability of drug-related problem (DRP) classification system for classifying severe medication errors. BMC Health Serv Res 23, 743 (2023). https://doi.org/10.1186/s12913-023-09763-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-023-09763-3