Abstract
Background
Clinical practice guidelines assist health professionals’ (HPs) decisions. Costly to develop, many guidelines are not implemented in clinical settings. This paper describes an evaluation of contextual factors to inform clinical guideline implementation strategies for the common and distressing problem of cancer-related fatigue (CRF) at an Australian cancer hospital.
Methods
A qualitative inquiry involving interviews and focus groups with consumers and multidisciplinary HPs explored key Canadian CRF guideline recommendations. Four HP focus groups examined the feasibility of a specific recommendation, while a consumer focus group examined experiences and preferences for managing CRF. Audio recordings were analysed using a rapid method of content analysis designed to accelerate implementation research. Strategies for implementation were guided by the Consolidated Framework for Implementation Research.
Results
Five consumers and 31 multidisciplinary HPs participated in eight interviews and five focus groups. Key HP barriers to fatigue management were insufficient knowledge and time; and lack of accessible screening and management tools or referral pathways. Consumer barriers included priority for cancer control during short health consultations, limited stamina for extended or extra visits addressing fatigue, and HP attitudes towards fatigue. Enablers of optimal fatigue management were alignment with existing healthcare practices, increased HP knowledge of CRF guidelines and tools, and improved referral pathways. Consumers valued their HPs addressing fatigue as part of treatment, with a personal fatigue prevention or management plan including self-monitoring. Consumers preferred fatigue management outside clinic appointments and use of telehealth consultations.
Conclusions
Strategies that reduce barriers and leverage enablers to guideline use should be trialled. Approaches should include (1) accessible knowledge and practice resources for busy HPs, (2) time efficient processes for patients and their HPs and (3) alignment of processes with existing practice. Funding for cancer care must enable best practice supportive care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction / Aim
Clinical practice guidelines are designed to assist health care professionals (HPs) to provide the most appropriate care for their patients [1]. Using best available evidence, guideline development is slow and expensive [2]. Yet, many guidelines are not implemented [3], with potential benefits for patients lost. Reasons why effective interventions recommended in guidelines are not used include lack of leadership on treatment policy, lack of awareness of or trust in the guideline and complexity of guideline recommendations [4]. The feasibility of guideline use in real clinical settings has rarely been explored in any condition. Guidelines often include several recommendations that were developed by stakeholders (including content experts, health professionals, consumers) based on evidence, but their practicability is not commonly tested [5]. Strategic approaches are needed to overcome barriers and facilitate enablers to guideline use [6, 7]. According to the Consolidated Framework for Implementation Research (CFIR), characteristics of the guideline (intervention), health practitioners, organisations (internal setting), external context (e.g. consumer needs and policy) and implementation strategies should all be considered [8].
Fatigue is recognised as a common, distressing symptom during and after cancer treatment [9, 10]. Approximately 50% of patients during treatment, and 30% of survivors after treatment experience prolonged and debilitating fatigue [11]. Persistent fatigue at moderate to severe levels is associated with disability, psychological disorders [12, 13] and poor performance status [11, 14]. International guidelines for cancer-related fatigue (CRF) recommend systematic screening for fatigue, assessment of contributing factors, and interventions including symptom management, exercise, fatigue education and sleep enhancement [15]. Many Australian cancer care professionals do not routinely use CRF guidelines [9, 16], and the feasibility of their use is not known. A study of clinician and consumer perceptions about applying a Canadian CRF guideline [13] highlighted insufficient detail to perform guideline recommendations, and a lack of clinical tools including assessments and consumer education [9]. A Canadian study reported lack of HP knowledge, resources and system barriers, together with inconsistent provider-patient communication of fatigue accounted for their CRF practice gap [17]. These and other barriers need to be addressed to enable CRF guideline use and optimal care for cancer survivors [15]. Currently, the best approaches to implementing CRF guidelines in complex “real-world” cancer practices are unknown [15], with scant data related to patient outcomes.
This paper describes the development of strategies for implementing a clinical guideline for CRF at an Australian specialist cancer hospital, using the lens of the CFIR [8]. The research question was ‘What strategies could increase the feasibility of CRF guideline use?’ Study aims were (1) to assess barriers and enablers to implementing a fatigue guideline at a Comprehensive Cancer Centre, and (2) to identify strategies to support guideline implementation at the Centre.
Methods
This qualitative study applied a content analysis approach to key informant interviews, focus groups and field notes to explore the context for implementing the Canadian Association for Psychosocial Oncology (CAPO) fatigue guideline [13]. A previous systematic guideline search and appraisal using the AGREE-II instrument identified the CAPO guideline as suitable for CRF assessment and management worldwide [18]. Our study was conducted during 2018–19 and procedures were approved by the Peter MacCallum Cancer Centre Human Research Ethics Committee (LNR/18/PMCC/205). A project steering committee including the authors, a consumer, a senior oncologist, director of allied health, physiotherapy department head and an implementation researcher provided advice and oversight to the study.
Participants and recruitment
Multidisciplinary HPs in relevant senior organisational roles with knowledge of current health care delivery, systems and processes at an Australian cancer centre were invited purposively by email to participate in key informant interviews. Focus group participants were invited via emails to discipline heads, presentations at team meetings and advertising in internal communications i.e., convenience sampling. Interested participants were sent an online poll to indicate their availability. Many HPs at the cancer centre were former colleagues of the lead researcher (EP), with knowledge of their CRF research and oncology practice background.
Consumers with experience of CRF were invited to participate in a focus group or interview via information leaflets in outpatient waiting areas, and e-newsletter to a consumer register. Specific culturally and linguistically diverse groups were also approached by a cancer centre consumer liaison officer.
Inclusion criteria
1. Health professionals
Registered medical, nursing or allied HPs at Peter MacCallum Cancer Centre (Peter Mac) with skills and experience in delivery of cancer care.
2. Health administrators
Professionals in administration, management or policy roles within Peter Mac.
3. Consumers
Aged 18 + , with any cancer diagnosis, treatment stage or comorbidities, who identified as having experienced CRF. Exclusion criterion: Inability to complete study tasks including consent due to cognitive barriers.
Interview and focus group participants signed informed consent before data was collected. Participants completed demographic details on a study registration form. This included phone or email, year of birth, sex, education, current occupation, cancer diagnosis, treatments, experience with CRF, professional discipline and practice experience, as relevant. The HPs could attend more than one session and were allocated to sessions according to their availability and topic relevance. See Additional Material 1 for optimal group membership.
Additionally, individuals and teams from occupational therapy, clinical psychology, speech pathology, nutrition, day chemotherapy, radiotherapy nursing, haematology and palliative care were consulted informally about their current practice and ways to implement the fatigue guideline. Project team members recorded field notes from these informal meetings or observation. Existing documents related to internal systems and processes, or screening, assessment and interventions for CRF were included as field notes.
In this qualitative study, our pragmatic target sample size was approximately 50 diverse participants including consumers, due to project funding and time constraints. With purposeful sampling and iterative processes, this number was considered sufficient to identify critical issues [19]. An ideal focus group size is six to eight participants [20]. To allow for dropouts, eight to 10 participants from relevant disciplines and services were recruited for each focus group. Formal individual interviews and informal discussions at team meetings provided additional perspectives and increased data richness [21].
Interview and focus group procedures
Four HP focus groups explored existing practice, barriers and facilitators for different key CRF guideline recommendations: fatigue screening, patient education, fatigue assessment and physical activity. A consumer focus group discussed their experience of, and preferences for CRF management. Interviews and focus groups were led by one female post-doctoral researcher (EP), previously employed as a senior occupational therapist at Peter Mac for over 15 years, with extensive interview experience. This prior relationship facilitated participant trust and engagement with the project. The lead researcher was aware of potential biases due to familiarity with the work setting. She checked assumptions and interpretation routinely with the research team and other stakeholders. A female allied health-qualified research assistant co-facilitated the focus groups and recorded field notes. One research assistant was a doctoral candidate and experienced physiotherapist at Peter Mac and the second, a recently graduated nutritionist. Interviews and focus groups were up to 60 and 75 min in duration respectively and were audio-recorded. A core semi-structured interview schedule was developed, guided by the CFIR domains: characteristics of the intervention (guideline), the inner (organisation) and outer (consumers, society, health policy) settings, individual health professionals’ characteristics and implementation strategies [8]. The schedule was adjusted to match each interviewee’s practice area, and focus group topics. Background information including the CAPO fatigue guideline algorithm and recommendations was provided and used during the sessions. See Additional file 1 for the interview guides, including development information.
Data analysis
Descriptive statistics were used to summarise participant demographics. To accelerate the progress of the project, a rapid content analysis approach was used for interview and focus group data. Rapid analysis is a method of qualitative investigation that is particularly suited to designing implementation strategies when there is a time constraint [22]. Three concepts characterise rapid analysis: (1) a system perspective, (2) triangulation of data, and (3) iterative data collection and analysis [22]. The rapid analyses used methods described by McNall and Foster-Fishman [23]. To reduce potential researcher bias and increase the study’s validity, two researchers made detailed notes from recordings of each focus group and interview. Concurrently, the notes were coded as direct quotes, paraphrasing or researcher hypothesis. Three researchers cross-checked notes and resolved discrepancies through discussion [23]. Findings were discussed in depth within the team and with some individual stakeholders. To balance the research team’s interpretation, other steering committee members provided practical and theoretical appraisal of results. Summary statements for each question or topic were created i.e., deductive content analysis. These were triangulated with field notes to increase validity and identify barriers, facilitators and potential strategies for CRF guideline implementation. Data saturation was tested via continual feedback and discussion with key participants and teams throughout the project to identify critical factors that would support or prevent guideline use. Reporting adheres to the COREQ checklist [24].
Results
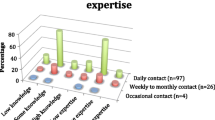
Five consumers and 31 multidisciplinary HPs participated in eight individual key informant interviews and five focus groups. One HP and one consumer participated via telephone; all others participated in person at the cancer centre. Two students observed one HP interview. Seven HPs and one consumer expressed interest in attending a focus group but were unavailable for a scheduled session. The HP participants were predominantly female (84%), had a median age 43 years (range 24–68) and 10.5 years cancer services experience (range 1.5–32). Most were nurses (55%). Two male and three female consumers participated, having a median age of 59 years (range 43–65) and 5 years (range 2–9) since their diagnosis of four cancer types. Table 1 shows further demographic details.
Composition of the interviews and focus groups is detailed in Additional file 1. Nurses included chemotherapy, specialist, palliative care and research nurses. Doctors included a pain specialist, one medical and two radiation oncologists. Four HPs participated in more than one session.
Barriers and enablers to implementing the CAPO fatigue guideline in ambulatory care
Key barriers to address, and enablers to optimise use of the CAPO fatigue guideline are depicted in Fig. 1. A critical barrier to consistent and comprehensive fatigue management for all HPs was limited time. Most HPs other than occupational therapists felt they lacked adequate fatigue knowledge and resources to support practice. Enablers were alignment of fatigue management with current care processes such as symptom screening, accessible education and simplified practice tools. A further practice barrier was fatigue management not being considered part of the care pathway: ‘It’s not usual care, it’s Unusual care, while it should be usual care’ (Nurse, interview). For consumers, reduced stamina and cognitive abilities, coupled with a perception that HPs did not recognise or prioritise their fatigue were barriers to self-advocacy. Addressing fatigue prevention and management by integrating with treatment, tailoring to individuals’ needs, involving caregivers and using telehealth were consumer-enabling approaches. Table 2 summarises the barriers and enablers to implementing the CAPO fatigue guideline reported by study participants, aligned to four CFIR domains [8]. The fifth CFIR domain—Implementation strategies – includes potential actions to reduce barriers and harness enablers to implementation.
Staff perceptions of fatigue management in practice
Application of the CAPO recommendations for cancer fatigue screening, assessment and management was inconsistent across Peter Mac, with a lack of clarity around whose role it was. Occupational therapists provided fatigue management in their routine practice, and received most referrals from palliative care staff. Fatigue was recognised by HPs as a problem, but during medical encounters there was barely sufficient time to address key disease concerns; while nurses were screening and managing multiple physical and psychosocial issues. Despite recognising fatigue as a key issue, doctors and nurses were often hesitant to screen or ask about fatigue, due to lack of time to follow up or because they were uncertain what to do next e.g., where to refer. This was explained in the fatigue education focus group: ‘The thing is, if you ask the question and then you get the answer – you’ve gotta do something about it. So sometimes … I won’t ask the question because I don’t know if I can do anything about it’ (Nurse, focus group).
Fatigue management education available for HPs comprised a little known 3-page fatigue management guide ‘Follow up of survivors with cancer-related fatigue’ on the Peter Mac external website, and bespoke online training for occupational therapists. Policy and procedure documents were limited to a precinct document that included management of fatigue in terminal care. Consumers and HPs stated existing patient information was often too long or detailed for people with significant fatigue. Details of how current fatigue management practice aligned with the CAPO fatigue recommendations are shown Additional file 2.
Consumer experience of fatigue management
Consumers with fatigue had low stamina for travel, waiting and lengthy consultations. When fatigue is a problem, extra questions or tests could be overwhelming due to cognitive changes and exhaustion. ‘When fatigue was high it caused an inability to multitask and follow long discussions – caused me a lot of distress. What is wrong with me? … A lot of my cognitive skills just shut down’ (consumer #5, female age 43).
Screening and acknowledgement of fatigue was welcomed by consumers, but it was unusual: ‘Fatigue isn’t usually mentioned, it’s like it’s a given and that’s that.’ (consumer #1, female age 59); ‘this is not imaginary, this is debilitating, and it needs attention.’ (consumer #3, male age 65). However, screening could also be distressing, with some people under-rating their fatigue. One participant reported under-rating her fatigue due to feeling inadequate in self-management. ‘Whenever I’ve got a zero to ten, I tend to under rate. I don’t ever want to be a 10. Cause that means to me that I’m sort of not coping and I don’t want to admit that.’ (consumer #1, female age 59). Another consumer lowered her fatigue severity rating due to perceptions of staff time pressure: ‘I was asked to fill out a scale and … I circled a 6 [out of 10]. And [the HP] said ‘if it’s more than a 4 then you have to do the other side’ – and there was a few things on the other side. So I changed it to a 4…. She kind of was indicating she didn’t want to take any more time, so change it to a 4…’ (consumer #2, male age 60).
Discussion
Implementing guidelines for fatigue screening, assessment and management needs careful planning with consideration of consumer, practitioner and system/organisational perspectives [15]. Our investigation identified a range of barriers and enablers to implementing the CAPO fatigue guideline at an Australian comprehensive cancer centre. Predominant HP barriers to fatigue management related to lack of knowledge, time and practice resources, such as standard screening methods. These caused HPs to avoid asking about fatigue. Surprisingly, 87% of HPs rated their CRF expertise as ‘limited’ or ‘moderate’—despite having a median of 10.5 years’ oncology experience. This suggests access to fatigue training or opportunity to use the knowledge are lacking. When consumers did experience fatigue screening, lack of follow up discouraged them from raising the topic again, or to downgrade their fatigue severity rating. These findings are not unique to Australia, with knowledge and system barriers coupled with poor fatigue communication previously reported [9, 16, 17, 25,26,27,28]. A recent Canadian study found major themes which accounted for the CRF knowledge-practice gap were “a perfect storm” and “a breakdown in communication” [17], both themes strikingly congruent with our findings. “A perfect storm” characterised inadequate HP knowledge of CRF guidelines in a setting of system barriers and limited funding; while “a breakdown in communication” involved HPs avoiding or normalising fatigue, leaving patients feeling helpless and dismissed [17].
The interrelationship of the barriers identified highlights the complexity of implementation. For the HP, it begins with awareness and knowledge of the guideline [29]. Clearly HP education is required, but acquisition of knowledge and expertise takes time, a limited commodity in today’s health care context. Adding new tasks for managing fatigue on top of education in an already time-poor context increases pressure on HPs. Further, guidelines are often sparse in detail and notoriously lacking in practice resources to assist delivery to the particular patient [30], leaving interpretation and decisions to the HP – who may lack adequate knowledge [31]. The current and earlier studies [9, 18] reveal the CAPO fatigue algorithm has limited clinical utility in its current format and practice resources such as screening tools, assessment and management guides are needed. Lack of both time and knowledge about CRF then results in HPs avoiding the issue and leaving patients to manage on their own [17]. Multiple strategies are needed to overcome these barriers.
Along with these barriers, we identified opportunities to implement CRF guidelines. It is noteworthy that both HPs and consumers in our study described time constraints in the clinic as barriers to fatigue management. Therefore, time-efficient strategies for fatigue screening and assessment should be prioritised. Documentation prompts such as an electronic medical record field for fatigue could contain a link to screening, assessment and management resources. Occupational therapists and other allied HPs with specialist knowledge and holistic assessment practices could lead comprehensive fatigue management. Because such cancer specialist HPs are limited in number, accessible education enabling local HPs to provide cancer fatigue management is essential for equitable cancer care.
Translation of CRF guidelines into practice has typically been slow globally [15]. This may be in part because prevention and management of fatigue is not commonly identified as a priority at organisational and policy levels, remaining forever in competition with other initiatives for cancer treatment [15]. Government supportive care policies without adequate funding or resources to provide care cannot be implemented equitably. We endorse Berger and colleagues’ proposition that innovative care models such as telehealth and harnessing e-health records are needed to adequately assess and manage cancer fatigue and other symptoms [15]. It is time to shift research focus to effectiveness-implementation hybrid trials [32] exploring novel ways to implement cancer fatigue guidelines that are sustainable for both provider and consumer. Approaches already being evaluated include stepped-care approaches [33,34,35], telehealth or online home based programs [36,37,38] and comprehensive assessment clinics [39].
This study had several strengths and limitations. Including a broad range of end-users in preliminary scoping work enabled a 360-degree approach to barriers and enablers to guideline implementation, and ‘ownership’ in the process of practice change [40]. However, some groups such as senior managers and doctors were under-represented and may have offered different views. Additional consumer input may have strengthened findings however our consumer findings were congruent with previous studies [9, 17]. Stakeholders’ insights into barriers and enablers in four CFIR domains point to strategies to enhance the success of guideline implementation efforts [41]. Rapid analysis of qualitative data is an established practice in crisis situations, that provided information in real-time [23] to accelerate the next phase of guideline implementation. Traditional or inductive qualitative analysis may have produced different results.
Only some of our findings such as documentation are site-specific. Lack of time is a global issue that needs to be addressed at national policy level. However, lack of practice resources and knowledge previously reported in local and international studies [9, 16, 17, 42] remain barriers to best practice fatigue management and these could be developed to be broadly applicable. The current study used the CFIR to enrich knowledge by identifying strategies to overcome implementation barriers related to the intervention (CRF guideline), individual, inner and outer contexts [8]. For successful implementation, a holistic approach is needed to tackle barriers in all CFIR domains within a local political context.
Conclusions
Our results underscore a critical need for practice tools and health professional education to support CRF guideline implementation. Cancer fatigue training and/or management should be accessible and time efficient for both HP and consumer and guideline processes should be integrated with existing processes. Strategies for guideline implementation focusing on sustainability should be trialled. To achieve adequate and equitable management of CRF, the scope of funding for cancer care must reflect best practice supportive care guidelines.
Availability of data and materials
De-identified focus group summaries are available upon reasonable request to Dr Pearson.
Abbreviations
- CAPO:
-
Canadian Association for Psychosocial Oncology
- CBT:
-
Cognitive behaviour therapy
- CFIR:
-
Consolidated Framework for Implementation Research
- CRF:
-
Cancer related fatigue
- HP:
-
Health professional
- NRS:
-
Numeric rating scale
References
IOM (Institute of Medicine). Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
Schrijvers D, Del Turco MR, Maddock C, Marotti L, Van Hemelryck F. Cancer guideline development in Europe: a survey among ECCO members. Eur J Cancer. 2012;48(9):1392–400.
Pronovost PJ. Enhancing physicians’ use of clinical guidelines. JAMA. 2013;310(23):2501–2.
Correa VC, Lugo-Agudelo LH, Aguirre-Acevedo DC, Contreras JAP, Borrero AMP, Patiño-Lugo DF, Valencia DAC. Individual, health system, and contextual barriers and facilitators for the implementation of clinical practice guidelines: a systematic metareview. Health ResPolicy Syst. 2020;18(1):74.
Gagliardi AR. Translating knowledge to practice: optimizing the use of guidelines. Epidemiol Psychiatr Sci. 2012;21(3):231–6.
Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, Robinson N. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26(1):13–24.
Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):61–8.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):1–15.
Pearson EJM, Morris ME, McKinstry CE. Cancer related fatigue: implementing guidelines for optimal management. BMC Health Serv Res. 2017;17(1):496.
NCCN clinical practice guidelines in oncology: cancer-related fatigue version 2.2023 [https://www.nccn.org/professionals/physician_gls/default.aspx#supportive].
Minton O, Berger A, Barsevick A, Cramp F, Goedendorp M, Mitchell SA, Stone PC. Cancer-related fatigue and its impact on functioning. Cancer. 2013;119(11 suppl):2124–30.
Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116(24):5740–8.
A pan Canadian practice guideline for screening, assessment, and management of cancer-related fatigue in adults version 2–2015. [https://www.capo.ca/guidelines].
Wang XS, Zhao F, Fisch MJ, O’Mara AM, Cella D, Mendoza TR, Cleeland CS. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425–32.
Berger AM, Mitchell SA, Jacobsen PB, Pirl WF. Screening, evaluation, and management of cancer-related fatigue: ready for implementation to practice? CA Cancer J Clin. 2015;65(3):190–211.
Pearson EJM, Morris ME, McKinstry CE. Cancer-related fatigue: a survey of health practitioner knowledge and practice. Support Care Cancer. 2015;23(12):3521–9.
Jones G, Gollish M, Trudel G, Rutkowski N, Brunet J, Lebel S. A perfect storm and patient-provider breakdown in communication: two mechanisms underlying practice gaps in cancer-related fatigue guidelines implementation. Support Care Cancer. 2021;29:1873–81.
Pearson EJ, Morris ME, McKinstry CE. Cancer-related fatigue: appraising evidence-based guidelines for screening, assessment and management. Support Care Cancer. 2016;24(9):3935–42.
Busetto L, Wick W, Gumbinger C. How to use and assess qualitative research methods. Neurol Res Pract. 2020;2(1):14.
Gill P, Stewart K, Treasure E, Chadwick B. Methods of data collection in qualitative research: interviews and focus groups. Br Dent J. 2008;204(6):291–5.
Lambert SD, Loiselle CG. Combining individual interviews and focus groups to enhance data richness. J Adv Nurs. 2008;62(2):228–37.
Beebe J. Basic concepts and techniques of rapid appraisal. Hum Organ. 1995;54(1):42–51.
McNall M, Foster-Fishman PG. Methods of rapid evaluation, assessment, and appraisal. Am J Eval. 2007;28(2):151–68.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57.
Borneman T, Piper BF, Sun VC, Koczywas M, Uman G, Ferrell B. Implementing the fatigue guidelines at one NCCN member institution: process and outcomes. J Natl Compr Canc Netw. 2007;5(10):1092–101.
Passik SD, Kirsh KL, Donaghy K, Holtsclaw E, Theobald D, Cella D, Breitbart W. Patient-related barriers to fatigue communication: Initial validation of the fatigue management barriers questionnaire. J Pain Symptom Manage. 2002;24(5):481–93.
Pertl MM, Quigley J, Hevey D. ‘I’m not complaining because I’m alive’: Barriers to the emergence of a discourse of cancer-related fatigue. Psychol Health. 2014;29(2):141–61.
Shun SC, Lai YH, Hsiao FH. Patient-related barriers to fatigue communication in cancer patients receiving active treatment. Oncologist. 2009;14(9):936–43.
Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance: the case of pediatric vaccine recommendations. Med Care. 1996;34(9):873–89.
Gagliardi AR, Brouwers MC. Do guidelines offer implementation advice to target users? A systematic review of guideline applicability. BMJ Open. 2015;5:e007047.
Arts DL, Voncken AG, Medlock S, Abu-Hanna A, van Weert HCPM. Reasons for intentional guideline non-adherence: A systematic review. Int J Med Inform. 2016;89:55–62.
Landes SJ, McBain SA, Curran GM. An introduction to effectiveness-implementation hybrid designs. Psychiatry Res. 2019;280:112513.
Williams LK, Ftanou M, Pearson EJ. Stepped-care cognitive behaviour therapy program for treating cancer-related fatigue: protocol for a feasibility study. Pilot Feasibility Stud. 2022;8(1):112.
Khan MM, Manduchi B, Rodriguez V, Fitch MI, Barbon CEA, McMillan H, Hutcheson KA, Martino R. Exploring patient experiences with a telehealth approach for the PRO-ACTIVE trial intervention in head and neck cancer patients. BMC Health Serv Res. 2022;22(1):1218.
Canella C, Mikolasek M, Rostock M, Beyer J, Guckenberger M, Jenewein J, et al. Developing an integrative treatment program for cancer-related fatigue using stakeholder engagement - a qualitative study. Integr Cancer Ther. 2017;17(3):762–73.
Cheville AL, Kollasch J, Vandenberg J, Shen T, Grothey A, Gamble G, Basford JR. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45(5):811–21.
Ladwa R, Pinkham EP, Teleni L, Hanley B, Lock G, Nixon J, Agbejule OA, Crawford-Williams F, Jones L, Pinkham MB, et al. Telehealth cancer-related fatigue clinic model for cancer survivors: a pilot randomised controlled trial protocol (the T-CRF trial). BMJ Open. 2022;12(5):e059952.
Foster C, Grimmett C, May CM, Ewings S, Myall M, Hulme C, Smith PW, Powers C, Calman L, Armes J, et al. A web-based intervention (RESTORE) to support self-management of cancer-related fatigue following primary cancer treatment: a multi-centre proof of concept randomised controlled trial. Support Care Cancer. 2015;24(6):2445–53.
Fischer I, Riedner C, Bojko P, Heim ME, Rüffer JU, Besseler M, Heußner P, Milani V, Rinas N, Schlimok G, et al. Consultation program for patients with cancer-related fatigue: a systematic evaluation of the experiences of the Bavarian Cancer Society. Oncology Research and Treatment. 2016;39(10):646–51.
Geerligs L, Shepherd HL, Butow P, Shaw J, Masya L, Cuddy J, Andrews G, Baychek K, Beale P, Allison K, et al. What factors influence organisational readiness for change? Implementation of the Australian clinical pathway for the screening, assessment and management of anxiety and depression in adult cancer patients (ADAPT CP). Support Care Cancer. 2021;29(6):3235–44.
Geerligs L, Rankin NM, Shepherd HL, Butow P. Hospital-based interventions: a systematic review of staff-reported barriers and facilitators to implementation processes. Implement Sci. 2018;13(1):36.
Abdalrahim MS, Herzallah MS, Zeilani RS, Alhalaiqa F. Jordanian nurses’ knowledge and attitudes toward cancer-related fatigue as a barrier of fatigue management. J Am Sci. 2014;10(2):191–7.
Acknowledgements
The authors thank research assistants Amanda Appathurai and Jamie Waterland, for their part in recruitment, data collection and analysis. We are extremely grateful to Graeme Sissing for his dedicated involvement as consumer research partner and member of the project steering committee. Finally, we extend thanks to steering committee members.
Funding
The study was funded by Victorian Cancer Agency (Australia) via Dr Pearson’s Health Services Research Fellowship (2018–2020).
Author information
Authors and Affiliations
Contributions
EP conducted participant recruitment, data collection and analysis, and wrote the manuscript versions. All authors contributed to study design, data interpretation, review and editing of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study procedures were approved by the Peter MacCallum Cancer Centre Human Research Ethics Committee (LNR/18/PMCC/205). The study was conducted according to the NHMRC National Statement on Ethical Conduct in Human Research (2007 and updates) and the World Medical Association Declaration of Helsinki (2013 and updates). All participants provided signed informed consent to participate in interviews and/or focus groups, and for publication of results.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pearson, E.J., Denehy, L. & Edbrooke, L. Identifying strategies for implementing a clinical guideline for cancer-related fatigue: a qualitative study. BMC Health Serv Res 23, 395 (2023). https://doi.org/10.1186/s12913-023-09377-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-023-09377-9