Abstract
Background
Although chronic kidney disease (CKD) is highly prevalent in the general population, little research has been conducted on CKD management in ambulatory care.
Objective was to assess management and quality of care by evaluating CKD coding in ambulatory care, patient diagnosis awareness, frequency of monitoring and whether appropriate patients are referred to nephrology.
Methods
Clinical data from the population-based cohort Study of Health in Pomerania (SHIP-START) were matched with claims data of the Association of Statutory Health Insurance Physicians. Quality of care was evaluated according international and German recommendations.
Results
Data from 1778 participants (56% female, mean age 59 years) were analysed. 10% had eGFR < 60 ml/min/1.73m2 (mean age 74 years), 15% had albuminuria. 21% had CKD as defined by KDIGO. 20% of these were coded and 7% self-reported having CKD. Coding increased with GFR stage (G3a 20%, G3b 61%, G4 75%, G5 100%). Serum creatinine and urinary dip stick testing were billed in the majority of all participants regardless of renal function. Testing frequency partially surpassed recommendations. Nephrology consultation was billed in few cases with stage G3b-G4.
Conclusion
CKD coding increased with stage and was performed reliably in stages ≥ G4, while CKD awareness was low. Adherence to monitoring and referral criteria varied, depending on the applicability of monitoring criteria. For assessing quality of care, consent on monitoring, patient education, referral criteria and coordination of care needs to be established, accounting for patient related factors, including age and comorbidity.
Trial registration
This study was prospectively registered as DRKS00009812 in the German Clinical Trials Register (DRKS).
Similar content being viewed by others
Introduction
Chronic Kidney Disease (CKD) has a prevalence of approximately 10% in adults in Germany and comparable industrialised countries and is an important public health issue [1, 2]. CKD prevalence is estimated to be 27% in German general practice (GP) settings and most of these patients have early-stage CKD [3]. CKD is usually asymptomatic in the early stages and the risk of progression to end stage renal disease (ESRD) is low. Still, approximately 15,000 new patients yearly require long-term renal replacement therapy in Germany [4]. Optimal management of risk factors like hypertension and diabetes and timely referral to specialist nephrology services are deemed important to prevent progression to ERSD [2].
Although the majority of patients with CKD are cared for in ambulatory care, most research is conducted in clinical settings and there is little data on the actual management of patients with CKD in ambulatory care, regarding monitoring and management of risk factors and nephrologist referral. It is adamant to evaluate existing care, to identify processes and healthcare gaps on which to focus improvement efforts when developing and implementing healthcare policy and guidelines on CKD. Also, the collection and analysis of such data allows for the comparison of CKD management at different points in time, to evaluate the implementation of improvement measures. Clinical practice guidelines, including recommendations for the stage-appropriate management of CKD in primary care, have been developed internationally[5]. The recommendations of the Kidney Disease Improving Global Outcomes (KDIGO) guideline serve as a blueprint for such national adaptations, the British NICE guideline being a prominent example [2, 6]. In Germany, national guidelines on management of patients with non-dialysis CKD were established in 2019 for patients with CKD in ambulatory care and for the subgroup of patients with diabetes and CKD in 2010 [7, 8].
This population-based study is the first to examine real-world CKD management in the ambulatory care setting in Germany by combining claims data with clinical data including objective measures of kidney function from a cohort study in Germany. Using data from a population-based cohort with matched claims data, this study investigated how CKD is coded in ambulatory care, whether patients are aware of the diagnosis, how often and which monitoring tests are performed and whether appropriate patients are referred to nephrology. Quality of care was assessed according to recommendations from national and international clinical practice guidelines.
Methods
Data sources and study design
The "Study of Health in Pomerania” (SHIP) is a population-based cohort study in Northern Germany [9, 10]. It consists of three independent cohorts. For this analysis, data from the SHIP-START cohort were matched with claims data of the Association of Statutory Health Insurance Physicians using a unique random linkage number, allowing the pseudonymised claims data to be analysed together with clinical data. Declarations of consent are available for all participants as well as positive decisions from the Ethics Committee of the University of Greifswald and the regional commissioner for data protection.
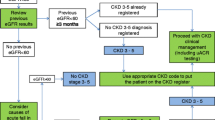
We had access to socio-demographic data, clinical parameters, laboratory data from urine and non-fasting blood samples and data from a computer-assisted personal interview from the second follow-up examination (SHIP-START-2, 2008–2012, n = 2333). Data of 1778 (76%) participants with available eGFR-values could be matched with claims data from the corresponding period. No claims data were available for participants with private or other, non-statutory health insurance, or for participants who did not consent to linkage with claims data (Fig. 1). The study relies on a single measurement of kidney function for classification of CKD, assuming that kidney functioning remains relatively constant over time. A detailed description of the SHIP study and data sources is provided in supplement 1.
Flow chart of the study population. Data from the second follow-up of the Study of Health in Pomerania (SHIP-START-2) were analyzed. From the 2333 participants in SHIP-START-2, 1778 data sets were available for the analysis. *Of the excluded participants, some participants fulfilled more than one of the exclusion criteria
Clinical data
The glomerular filtration rate (eGFR) was estimated using the CKD-EPI Eq [11]. The urinary albumin-creatinine ratio (ACR) was calculated for all participants with available urinary albumin and creatinine measurements in SHIP-START-2 (n = 1321). Participants were classified according to the KDIGO albuminuria categorisation [2]. Diabetes was coded when participants reported diabetes in the questionnaire, or had HbA1c ≥ 6.5%, or serum glucose ≥ 11.1 mmol/L in any of the SHIP-START surveys. Hypertension was defined as blood pressure > 140/90 mmHg, or taking antihypertensive medication. Further details concerning measurements and testing methods are provided in supplement 1.
Claims data
ICD-10 codes and billing codes (GOPs) were available for SHIP-START participants’ statutory health insurance during the observation period (supplement 1). Coding of CKD with ICD codes N18 and N19 (suspected or confirmed) and billing codes for laboratory tests of creatinine (32,066, 32,067), haemoglobin (32,038, 32,120), albumin (urine or serum) (32,435), calcium (32,082), parathyroid hormone (32,411), Vitamin D (32,413), phosphate (32,086) and urinary dip stick testing (32,030), urine microscopy (32,031) and microalbuminuria (32,135) were analysed. Sonographic examinations were represented by GOPs 33,042 and 33,043, nephrological co-treatment by the billing codes for nephrological care (13,591, 13,592, 13,600, 13,602, 13,610).
Assessment of quality of care
Assessment of quality of care is based on the recommendations of the NICE and KDIGO guidelines for the management of chronic kidney disease, including the KDIGO guideline on CKD-Mineral and Bone Disorder (CKD-MBD), as well as the German guideline on kidney disease in diabetes and the recommendations for referral of the German Society of Nephrology [2, 6, 7, 12,13,14].
Data analysis
Descriptive statistics of the study data and comparison of participants with and without available claims data were performed using R version 4.2.0 with the package kidney.epi. Correlation between eGFR and age was assessed using Spearman’s rank correlation [15, 16]. Statistical analysis for categorical variables when comparing baseline characteristics of participants with and without linked claims data consisted of Pearson's Chi-squared tests with Yates' continuity correction and effect size Cramér’s V. For continuous variables, we reported p-values derived from Mann–Whitney-U tests and the absolute value of Cliff’s delta for effect sizes.
Results
Sample description
Data from 1778 participants (age: mean 59.3 years, standard deviation ± 13.1, 56% female) were included in the analysis. 307 (17%) participants had diabetes, 1040 (58%) were hypertensive (Table 1). Figure 2 shows the classification of study participants according to KDIGO [2].
Distribution of SHIP-START-2 participants according to KDIGO prognostic categorization [2]. Categories show the adjusted relative risk relating to 5 outcomes (all-cause mortality, cardiovascular mortality, kidney failure with dialysis, acute renal failure and CKD progression) based on a meta-analysis in the general population [25]. Green: low risk, yellow: moderate risk; orange: high risk; red: very high risk. Participants in the „low risk “ group can be classified as having normal kidney function. For 457 of the 1778 SHIP-START-2 participants in this study, albumin-creatinine ratios were not available. Thus, these participants were excluded for this visualization. Percentages indicate the respective fraction of the 1321 participants with available ACR included in this figure. ACR: albumin-creatinine ratio, GFR: Glomerular Filtration Rate, SHIP-START-2: second follow-up from the START cohort of the Study of Health In Pomerania
Laboratory values
Overall, 179 (10%) participants in SHIP-START-2 had a decreased eGFR (< 60 ml/min/1.73m2). Most participants with reduced eGFR were in stage G3a (Fig. 2) and eGFR was negatively associated with age (r = -0.66, p < 0.001). 262 participants (15%) had albuminuria and 377 (21%) were classified as having CKD because of decreased eGFR or albuminuria according to the KDIGO classification (Fig. 2).
Self-reporting of CKD
Out of 179 participants with an eGFR < 60 ml/min/1.73m2, 16 (9%) reported having a CKD, of which 11 participants (69%) reported having been treated for CKD within the last 12 months. 7% (19/262) of participants with albuminuria KDIGO stage A2 or A3 reported having CKD, of which 68% (13/19) reported having been treated for CKD within the last 12 months.
CKD coding in ambulatory care
Coding of CKD was retrieved in claims data of 5% (91/1778) of all participants during the year following the SHIP-START-2 examination, corresponding to 20% (75/377) of participants with CKD according to eGFR or albuminuria. Overall CKD was coded in 32% (57/179) of participants with eGFR < 60 ml/min/1.73m2, 20% (26/131) of participants in stage G3a, 61% (23/38) of participants in stage G3b, 75% (6/8) of participants in stage G4 and all participants in stage G5 (2/2) were coded (Table 2). For 18% (48/262) of participants with albuminuria, CKD was coded in the year following the SHIP-START-2 examination (Table 3).
Management of CKD in ambulatory care
The proportion of patients receiving laboratory and ultrasound examinations increased with higher CKD stage (Tables 2 and 3). Serum creatinine and urinary dip stick testing were billed in the majority of all participants during the year after the SHIP-START-2 examination. Testing for microalbuminuria was performed only in the lower CKD stages. Quantitative albuminuria was rarely measured (3% of all participants).
In 3% (46/1778) of all participants and 6% (10/179) of participants with reduced eGFR, nephrological care was billed at least once during the year following the SHIP-START-2 examination (Table 2). 2% (36/1599) of participants with normal eGFR were seen by a nephrologist. In 7% (18/262) of participants with albuminuria, nephrological care was billed, as well as in 2% (19/1059) of participants without albuminuria (Table 3).
Discussion
Summary of the main results
Overall, 21% of the sample had CKD according to KDIGO (10% reduced GFR and 15% albuminuria). Of those, CKD was coded in 32% with reduced GFR and in 18% with albuminuria. 9% of participants with reduced GFR reported having kidney disease. Regardless of renal function, tests like serum creatinine and urinalysis were frequently billed in the majority of participants during the study period. Markers for CKD-MBD were rarely completely evaluated. For only 11% of the participants in stages G3b and G4, a nephrologist consultation was billed.
Meaning of the results and comparison with scientific literature
CKD prevalence is generally estimated to be 10% in adults worldwide, with 3–17% in European populations [1]. In a sample of the German general population (2008–2011) of comparable age range, CKD prevalence for reduced GFR and albuminuria combined was reported to be 13%, which is significantly lower than in our analysis [17]. A comparison between SHIP-START-1 (2002–2006) and a Southern German sample (2006–2008) showed a significantly higher prevalence in Northern Germany, which may be partly explained by a different distribution of risk factors [1, 18].
Coding and awareness of CKD
To date, no German data were available for CKD coding and monitoring. Coding is a surrogate parameter for physicians having diagnosed the patient with CKD and incorporating this knowledge into patient management. International studies show that only 15–38% of patients with CKD are coded [19,20,21,22]. The proportion of coded CKD in our analysis was within this range (20%) and increased with diminishing kidney function. This reflects the limited clinical significance of decreased kidney function in older adults and is congruent with the findings in literature. The importance of coding for quality assessment is that when using claims data this has a large impact on the denominator for quality assessment, since only coded patients will be included. Our data suggest that claims data do not capture many patients in stage G3a.
The awareness of kidney damage was 28% in the German DEGS study (DEGS1, 2008–2011) [17]. In our analysis, only 9% with reduced GFR reported having CKD and less than half of those with stage G4 (3/8). This is in line with data from the National Health and Nutrition Examination Survey of U.S. Center of Disease Control Surveillance program for the years 2008–2012, suggesting overall CKD awareness of 10–13% in stages 3–4 with awareness increasing with stage from 3–5% in stage G3a to 38–51% in stage G4 and being negatively correlated with age[23]. The lower diagnosis awareness found in our study can be partly explained by the significantly higher age and higher rate of comorbidities of the SHIP-START population [9, 10, 24].
Our data do not allow drawing conclusions about whether the diagnosis was not made, not communicated or not remembered by the patient. From the nephrologist perspective, it is desirable for patients to be aware of their CKD [24, 25]. From a primary care perspective, other health issues are often more prominent and information about CKD does not always appear to be crucial for older people and might lead to unnecessary worries [26, 27]. On the other hand, being aware of decreased kidney function may be useful for dose-adjustment of kidney-excreted drugs and avoiding nephrotoxic substances [28]. Patients who are aware of their CKD can inform other physicians and pharmacists about their condition. However, to date, there is no evidence for a benefit of specific patient education in reducing CKD progression [29, 30]. Awareness of CKD cannot be measured with claims data.
Monitoring for kidney function
Serum creatinine measurements and urinary dip stick testing, which are prerequisites for staging of CKD, were performed predominantly in participants with impaired kidney function, but also very frequently in patients without renal impairment (Table 2). The frequency of measurements increased with increasing CKD stage and partially exceeded the monitoring recommendations of the KDIGO and NICE guidelines [2, 6]. These recommendations are based on expert consensus, because there are no empirical studies on monitoring intervals. The high frequency of measurements in many patients may be caused by the fact that anaemia and creatinine measurement are often ordered as part of routine laboratory testing and because creatinine used to be part of the 2-yearly preventive check-up examination (from the age of 35) and many physicians still tend to include it [31]. Additionally, it indicates a lack of coordination and communication in ambulatory care. Albuminuria was measured by urinary dip stick testing in the majority of participants. This might be due to the fact that dip stick testing is part of the preventive check-up examination. Quantitative measurement of albuminuria by ACR, as recommended by guidelines, is necessary at least once for the classification of CKD according to KDIGO [2]. The claims data indicate that quantitative albuminuria is rarely assessed. It must be kept in mind that data were collected prior to the release of the KDIGO guideline in 2012 and measurement of ACR was not formally required during the study period. Future assessment of quality of care for CKD should include at least one measurement of ACR.
Monitoring for CKD-MBD
The KDIGO guideline on mineral and bone disorder in chronic kidney disease (CKD-MBD) recommends with limited evidence to monitor for CKD-MBD every 6–12 months from stage G3 onwards [2, 12]. However, phosphate, calcium and parathyroid hormone assessments were significantly less frequently performed than recommended. NICE recommends monitoring only from stage G4 [6]. Awareness of monitoring for CKD-MBD seems to be low, but so is the evidence for the benefits of monitoring on clinically relevant endpoints. Therefore, measuring markers for CKD-MBD should not be part of a quality indicator set unless there is better evidence.
Monitoring for Anaemia
The KDIGO guideline recommends annual monitoring of haemoglobin values from CKD G3a and every six months from stage G4 [2]. NICE recommends testing patients with symptoms indicative of anaemia and recommends annual monitoring only from stage G3b onwards [6]. Actual haemoglobin testing was slightly higher than recommended by KDIGO in stage G3b and well above the recommended number of tests in stage G5. Given the high frequency of haemoglobin measurements this is a dispensable quality indicator in the German health care setting.
Referral to nephrology
KDIGO and NICE recommend referral to a nephrologist from stage G4, or earlier in the presence of e.g. albuminuria, persistent haematuria or progression [6, 2, 8]. The German Societies for Nephrology (DGfN) and Internal Medicine (DGIM) recommend referral from G3b, or from G3a, if additional criteria such as proteinuria or refractory hypertension are met [14]. This recommendation was not consented and is not agreed upon by German Society for General Practice and Family Medicine (DEGAM). The German guideline for Diabetes and CKD (NVL) recommends referral for persons ≤ 65 years from G3a and > 65 years from G3b [7]. International clinical practice guidelines do not distinguish between a one-time consultation to exclude specific kidney disease needing specific treatment and continuing co-treatment of patients [2, 8]. The observed nephrology consultation rate was 25% for stage G4, well below the recommendations. However, in our analysis, only 10 out of 1778 participants were in stages G4-5. A retrospective German study reports that 58% of patients with CKD in stage G1-4 had been seen by a nephrologist [32]. A similar rate was observed in an Italian study where co-treatment was reported in 56% of patients in stages G4 and above [20, 33]. From a nephrological point of view, many patients are referred too late [27]. From the primary care point of view, the progression to ESRD is a rare event and there is often no specific therapy for CKD that cannot be provided in general practice [31, 34]. The conflict in risk perception between general practitioners and nephrologists is evident when the KDIGO risk model is applied to the SHIP-START cohort (Fig. 2) [2]. This table categorises patients with warning colours in 4 risk categories and is displayed in many guidelines. Unlike one might assume this is not the risk of progressing to ESRD, but combined risk for five events (acute renal failure, CKD progression, ESRD, all-cause mortality, cardiovascular mortality) [2]. It is not quantified what is assumed to be a moderate or high risk. In fact, the risk is calculated for 1000 observed patients’ years, not adjusted for patient’s age. In this model, a patient with stage G3a and proteinuria A3 carries a risk of 5/1000 for ESRD and is classified as high risk, independent of age [2]. This is overstating the perceived and real risk of CKD and has been criticised [35].
The reasons for late or non-referral to nephrology of patients with CKD are complex as observed in our and many other studies. Causal factors that were identified were disease-specific, patient-related, as well as primary care physician related [31, 32]. Since monitoring of laboratory parameters can be carried out in general practice, patients with stable renal function and known underlying diseases may not have been referred. It is possible that some patients were seen by nephrologists before the observation period or as inpatients, which could not be recorded in our analysis. Another reason for the reluctance to refer is age-related decline of kidney function. A large proportion of people with CKD in our study were older than 75 years (Table 1). From a general practitioner's perspective, age, comorbidity, previous course and the need for a specific nephrological intervention should be incorporated for referral recommendations in addition to eGFR. Referral to nephrology co-supervision as a quality indicator for ambulatory care of patients with CKD is therefore only reasonable when a moderate performance target is set.
Strengths and limitations
This population-based study is the first to combine claims data with clinical data including objective measures of kidney function from a cohort study in Germany. The study design allowed including patients without coded CKD according to claims data in the analysis.
Since the SHIP-START-2 examination assessed kidney function at a single time point, potential biological variation should be kept in mind when interpreting the results and especially when classifying participants into risk categories. When interpreting claims data, it should be considered that the primary purpose of coding is billing, and therefore coding quality does not allow conclusions regarding the quality of services or the consequences of monitoring tests. As a rule, monitoring and referral recommendations for CKD are largely based on expert consensus. No official consensus had been established in Germany during the study period, which limits the evaluation of quality of care. In addition, individual factors that are not included in claims data, such as patient preferences, life expectancy and social factors, may cause deviations from expert recommendations. Certain aspects of quality of care, such as blood pressure measurement, drug adaptation or therapeutic goals (HbA1c, blood pressure, cholesterol management) could not be assessed in this study based on claims data. Changes in CKD stage could not be considered in the analysis, as laboratory measurements were available for one point in time only.
With regards to albumin and creatinine measurements, the billing codes correspond to lab measurements of certain substances, but do not differentiate between measurements in serum or urine. Because measurements of creatinine in urine and albumin in serum are uncommon in the German primary care setting, we assume that creatinine measurements pertain to serum and albumin to urine samples. In this population-based sample, only eight participants had CKD stage G4 and only two participants were categorised as stage G5. This should be considered when interpreting the data.
Claims data were only available for participants with statutory health insurance who consented to data linkage. 88.1% of the German population are covered by the statutory health insurance, while 10.5% have private insurance and 1.4% are otherwise insured, e.g. in the army [36]. Private health insurance is only possible for people with higher income or for those who are self-employed. As expected, participants without claims data tended to be younger and healthier (supplement Table 1), as this group is less likely to seek healthcare. We therefore do not assume that this has a relevant impact on the generalizability of our findings.
Conclusions
CKD coding in ambulatory care is performed more reliably in higher stages. Patient awareness of their kidney function is low and should be improved. In addition, albuminuria rarely leads to CKD coding. Assessment of renal function requires quantitative measurement of albuminuria by ACR and should be performed more frequently in patients with impaired GFR. Creatinine measurements and urinary dip stick testing are performed more often than recommended, indicating a lack of coordination of care. For measurements of quality of care regarding monitoring and referral recommendations, a consensus between general practitioners and nephrologists should be established, accounting for patient-related factors such as age.
When claims data is used as a means to monitor quality of care for patients with CKD, careful consideration of the limitations is needed. Quality of care concerning monitoring of kidney function surpassed recommendations, while anaemia and CKD-MBD could be monitored more closely. Referral seems to be performed on a pragmatic basis, and more research is needed to establish if physicians in ambulatory care sufficiently consider patient-related factors such as age, comorbidities and prognosis.
Availability of data and materials
Data of the SHIP study are available on request and can be applied for at https://fvcm.med.uni-greifswald.de/dd_service/data_use_intro.php. The claims data analysed during the current study are not publicly available due to legal and privacy restrictions for the protection of personal data of research participants.
References
Bruck K, Stel VS, Gambaro G, Hallan S, Völzke H, Ärnlöv J, et al. CKD Prevalence Varies across the European General Population. J Am Soc Nephrol. 2016;27:2135–47.
KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. 2013a;3:1. Available from: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf.
Gergei I, Klotsche J, Woitas RP, Pieper L, Wittchen H-U, Krämer BK, et al. Chronic kidney disease in primary care in Germany. J Public Health. 2017;25:223–30.
Gemeinsame Berichterstattung des Bundes: [Initiation of kidney replacement therapy with haemodialysis, peritoneal dialysis or combined treatment in patients on kidney dialysis.] 2016. Gesundheitsberichterstattung des Bundes, www.gbe-bund.de. Last accessed July 20 2022.
Weckmann G, Stracke S, Haase A, et al. Diagnosis and management of non-dialysis chronic kidney disease in ambulatory care: a systematic review of clinical practice guidelines. BMC Nephrol. 2018;19:258. https://doi.org/10.1186/s12882-018-1048-5.
National Institute for Health and Clinical Excellence (Great Britain). Chronic kidney disease: Early identification and management of chronic kidney disease in adults in primary and secondary care. London: NICE; 2015.
Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF): [national guideline on renal disease in adults with diabetes – full guideline, 1st Edition]. Version 5. 2010, last update: Mai 2013. Available from: http://www.diabetes.versorgungsleitlinien.de [cited: 11.04.2020]; 10.6101/A, ZQ/000126.
Weckmann G, Chenot JF, Stracke S. Clinical practice guideline: the management of non–dialysis-dependent chronic kidney disease in primary care. Dtsch Arztebl Int. 2020;117:745–51. https://doi.org/10.3238/arztebl.2020.0745.
Völzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol. 2011;40:294–307.
Völzke H, Schössow J, Schmidt CO, et al. Cohort Profile Update: The Study of Health in Pomerania (SHIP). Int J Epidemiol 2022: dyac034. https://doi.org/10.1093/ije/dyac034
Levey AS, Stevens LA, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F. A new equation to estimate glomerular filtration rate. Ann Int Med. 2009;150:604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease – Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int. 2017;92:1558.
Ketteler M. Eckardt KU [KDIGO guidelines on mineral and bone disease in chronic kidney disease 2009.]. Nephrologe. 2009;4:437–40.
German Society for Nephrology, German Society for Internal Medicine. [Practice guide nephrology]; 2013. Available from: https://www.dgfn.eu/praxisratgeber.html.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from https://www.R-project.org/. (Last accessed: 20 Jul 2022)
Bikbov B. R Open source programming code for calculation of the kidney donor profile index and kidney donor risk index. Kidney Dis (Basel). 2018;4:269–2. https://doi.org/10.1159/000492427.
Girndt M, Trocci P, Scheidt-Nave C, Markau S, Stang A. The Prevalence of Renal Failure, results from the German health interview and examination survey for adults, 2008–2011 (DEGS1). Dtsch Arztebl Int. 2016;113:85–91. https://doi.org/10.3238/arztebl.2016.0085.
Aumann N, Baumeister SE, Rettig R, et al. Regional variation of chronic kidney disease in Germany: results from two population-based surveys. Kidney Blood Press Res. 2015;40:231–43.
Allen AS, Forman JP, Orav EJ, Bates DW, Denker BM, Sequist TD. Primary care management of chronic kidney disease. J Gen Intern Med. 2011;26:386–92.
Guessous I, McClellan W, Vupputuri S, Wasse H. Low documentation of chronic kidney disease among high-risk patients in a managed care population: a retrospective cohort study. BMC Nephrol. 2009;10:25.
Minutolo R, De Nicola L, Mazzaglia G, et al. Detection and awareness of moderate to advanced CKD by primary care practitioners: a cross-sectional study from Italy. Am J Kidney Dis. 2008;52:444–53.
Friedl C, Memetsberger M, Mader J, Fahrleitner-Pammer A, Pieber TR, Rosenkranz AR. Awareness of chronic kidney disease in Austria: a frequently under-recognized clinical picture. Wien Klin Wochenschr. 2013;13–14:362–7.
Centers for Disease Control and Prevention. Awareness of CKD among U.S. adults with CKD stage 3 or 4. Chronic Kidney Disease Surveillance System - United States. https://nccd.cdc.gov/CKD/detail.aspx?QNum=Q98 Last accessed: 05 Feb 2022
McIntyre NJ, Fluck R, McIntyre C, Taal M. Treatment needs and diagnosis awareness in primary care patients with chronic kidney disease. Br J Gen Pract. 2012;62:e227–32.
Levey AS, de Jong PE, Coresh J, El, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28.
Daker-White G, Rogers A, Kennedy A, Blakeman T, Blickem C, Chew-Graham C. Non-disclosure of chronic kidney disease in primary care and the limits of instrumental rationality in chronic illness self-management. Soc Sci Med. 2015;131:31–9.
Abdi Z, Gallagher H, O’Donoghue D. Telling the truth: why disclosure matters in chronic kidney disease. Br J Gen Pract. 2012;62:172–3.
Lopez-Vargas PA, Tong A, Howell M, Craig JC. Educational interventions for patients with CKD: a systematic review. Am J Kidney Dis 2016. pii: S0272–6386(16)00149–9.
Li T, Wu HM, Wang F, Huang CQ, Yang M, Dong BR, Liu GJ. Education programmes for people with diabetic kidney disease. Cochrane Database Syst Rev. 2011;(6):CD007374. Version 15 June 2011. https://doi.org/10.1002/14651858.CD007374.pub2.
Riegel W, Hahn K, Kreutz R, Weber M, Zidek W, Schmieder R. BENEFIT Niere–significance of a nephrology screening for start of intervention and therapy result. Deut Med Wochenschr. 2005;130:792–6.
Haase A, Stracke S, Chenot JF, Weckmann G. Perspective of General Practitioners on Management of Non-dialysis Chronic Kidney Disease - a Qualitative Study. Dtsch Med Wochenschr. 2021;146:e97–102. https://doi.org/10.1055/a-1582-0130.
Wauters JP, Lameire N, Davison A, Ritz E. Why patients with progressing kidney disease are referred late to the nephrologist: on causes and proposals for improvement. Nephrol Dial Transplant. 2005;20:490–6.
Ricardo AC, Roy JA, Tao K, Alper A, Chen J, Drawz PE, et al. Influence of Nephrologist Care on Management and Outcomes in Adults with Chronic Kidney Disease. J Gen Intern Med. 2016;31:22–9. https://doi.org/10.1007/s11606-015-3452-x.
Black C, Sharma P, Scotland G, et al. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess. 2010;14:1–184.
Onuigbo MAC, The CKD. Enigma with misleading statistics and myths about CKD, and conflicting ESRD and death rates in the literature: results of a 2008 US population-based cross-sectional CKD outcomes analysis. Renal Failure Ren Fail. 2013;35:338–43.
Daten zum Gesundheitswesen: Versicherte (available from https://www.vdek.com; last accessed: 19 July 2022.
Acknowledgements
We are grateful to all persons participating in the SHIP study.
Abstracts
Weckmann G, Kiel S, Chenot JF, Angelow A. Referral criteria for chronic kidney disease—implications for patient management and healthcare expenditure. (Vortrag) Conference of the World Organization of Family Doctors Europe 2020.
Weckmann G, Haase A, Chenot JF, Stracke S, Spallek J, Angelow A. Public Health implications of different referral criteria for chronic kidney disease – analysis of a population based sample. European Public Health Conference (EUPH), Ljubljana, Slovenia 2018
Weckmann G, Haase A, Chenot JF, Stracke S, Spallek J, Angelow A. Symptomatic anemia in patients with chronic kidney disease – analysis of a population based sample (Poster). 51. Jahrestagung der Deutschen Gesellschaft für Transfusionsmedizin und Immunhämatologie e.V. (DGTI)19.–21. September 2018, Lübeck, Germany
Weckmann G, Chenot JF, Stracke S, Haase A, Spallek J, Angelow A. Auswirkung von Überweisungskriterien zum Nephrologen bei chronischer Niereninsuffizienz ‐ Analyse einer Populationsbasierten Stichprobe. Gemeinsame Jahrestagung der Deutschen Gesellschaft für Epidemiologie, Deutschen Gesellschaft für Medizinische Soziologie und Deutsche Gesellschaft für Sozialmedizin und Prävention. September 2017; Lübeck, Germany.
Ludwig F, Raus C, Mahner M, Haase A, Weckmann G, Chenot J , Stracke S. REFACE—REnal Function in Ambulatory CarE: Kodierung und Management der chronischen Niereninsuffizienz in Hausarztpraxen in Mecklenburg-Vorpommern. (Poster) Deutsche Gesellschaft für Nephrologie (DGfN); 2016 Sep 12; Berlin.
Ludwig F, Raus C, Wirkner J, Mahner M, Haase A, Weckmann G, et al. Diagnostik, Kodierung und Management der Chronischen Niereninsuffizienz (CKD) in der Hausarztpraxis: eine Querschnittsstudie. (Vortrag) 50. Kongress für Allgemeinmedizin und Familienmedizin (DEGAM); Frankfurt am Main. In: German Medical Science GMS Publishing House; 2016. Doc16degam075
Wirkner J, Ludwig F, Raus C, Weckmann G, Dabers T, Völzke H, Schmidt C, Chenot J, Stracke S. REFACE (REnal Function in Ambulatory CarE): Diagnose und Verlaufskontrolle der chronischen Niereninsuffizienz in der Hausarztpraxis ‐ eine Analyse von Primär‐ und Sekundärdaten in der bevölkerungsepidemiologischen "Study of Health in Pomerania" (Poster) Kongress für Nephrologie 2015 der Deutschen Gesellschaft für Nephrologie (DGfN); 2015 Sep 13;v Berlin.http://nephrologie2015.aey-congresse.de/programm/sitzung.html?id=2019
Ludwig F, Wirkner J, Raus C, Weckmann G, Stracke S, Chenot JF. Wird die chronische Niereninsuffizienz in der ambulanten Versorgung kodiert und kontrolliert? Eine Analyse von Primär- und Sekundärdaten (presentation). DEGAM. 49th Congress of General Practice and Family Medicine. Bolzano, Italy, 17.‐19.09.2015. Düsseldorf: German Medical Science GMS Publishing House. Doc15degam010, https://doi.org/10.3205/15degam010, urn:nbn:de:0183‐15degam2019
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was conducted as part of the REnal Function in Ambulatory CarE (REFACE) study, which was funded by the German foundation “KfH Stiftung Präventivmedizin”. The SHIP study was funded by the Federal Ministry of Education and Research [03ZIK012], the Ministry of Cultural Affairs; the Social Ministry of the Federal State of Mecklenburg-Vorpommern. The authors declare that the funding body had no role or any influence in the design of the study, in collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
SS, JFC und GW designed the study, JW, EK, CZ, GW and AH performed data analysis, SS, JFC, GW, JW and COS interpreted the results. All authors wrote the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from all study participants. Only data from participants with informed consent were analyzed. The data security administrator approved data linkage. Data are stored according to the data safety and management plan of the Institute for Community Medicine of the University of Greifswald. The study protocol was consistent with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the University of Greifswald and the regional commissioner for data protection.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
The results presented in this paper have not been published previously in whole or part, except in abstract format.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplement1.
SHIP study and detailed description of data sources.
Additional file 2: Supplemental Table 1.
Comparison of baseline characteristics of participants with and without linked claims data.
Additional file 3: Supplemental Table 2.
Billing and coding according to KDIGO glomerular filtration rate categorization for the observation period 2008 – 2012.
Additional file 4: Supplemental Table 3.
Billing and coding according to KDIGO albuminuria categorization* for the observation period 2008 – 2012.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Weckmann, G., Wirkner, J., Kasbohm, E. et al. Monitoring and management of chronic kidney disease in ambulatory care – analysis of clinical and claims data from a population-based study. BMC Health Serv Res 22, 1330 (2022). https://doi.org/10.1186/s12913-022-08691-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-022-08691-y