Abstract
Background
Newborn screening (NBS) can prevent inborn errors of metabolism (IEMs), which may cause long-term disability and even death in newborns. However, in China, tandem mass spectrometry (MS/MS) screening has just started. This study aimed to assess the cost-effectiveness of NBS using MS/MS in Shenzhen under the nationally recommended program, as well as evaluate the value and affordability of introducing this new screening technology.
Methods
A Markov model was built to estimate the cost and quality-adjusted life-years (QALYs) of different screening programs. We compared PKU screening using traditional immunofluorescence (IF) with the other 11 IEMs not screened and all 12 IEMs screened using MS/MS, and the programs detecting different numbers of IEMs chosen from the national recommended program were also compared. A sensitivity analysis and budget impact analysis (BIA) were performed.
Results
The incremental cost-effectiveness ratio (ICER) of detecting all 12 IEMs in the national program is 277,823 RMB per QALY, below three times per capita GDP in Shenzhen. MS/MS screening in Shenzhen can be cost-effective only if at least three diseases (PKU, PCD and MMA) are covered and when the screening program covers five diseases (PKU, PCD, MMA, MSUD, IVA), the ICER closely approaches its critical threshold. The BIA indicated the implementation cost of the national program to be around 490 million RMB over 10 years and showed no difference in budget between programs detecting different numbers of IEMs.
Conclusions
We conclude that the newborn screening using MS/MS in Shenzhen is cost-effective, and the budget affordable for the Shenzhen government. Two concepts for selecting the IEMs to be detected are also presented. One is to choose the most cost-effective screening programs detecting highest number of IEMs to achieve a minimal ICER. The other considers the curability and affordability of the disease as the basis of healthcare decisions to screen suitable IEMs, achieving an ICER under the threshold and close to the minimum value.
Similar content being viewed by others
Background
Inborn errors of metabolism
Inborn errors of metabolism (IEMs) are a group of diseases caused by abnormal biochemical metabolic indicators which block the metabolic pathways in our bodies [1, 2]. While most published studies have numbered IEMs in the 500–700 range [3,4,5], a recent article estimates the number to be more than 1015 [6], which suggests a relatively higher cumulative incidence of IEMs, compared to a simple disease. There are many discrepancies in the prevalence of IEMs reported in these studies. Donald Waters et al. [7] estimated the global birth prevalence to be 50.9 per 100,000 live births based on a systematic literature review of birth prevalence and case fatality of IEMs globally. Other research indicates prevalence between 40 per 100,000 and 125 per 100,000 [8, 9]. In China, although epidemiological statistics are absent at the country level, regional data reveals an incidence of IEMs ranging from 35.3 per 100,000 to 136.4 per 100,000 [10,11,12]. Notwithstanding the actual incidence, it is now clear that IEMs affect a multitude of newborns and families all over the world.
Most IEMs are curable given early detection, but without prompt recognition can give rise to long-term disability and even death [13]. Since IEMs are ingravescent, and overlap in clinical manifestations, early symptoms may not be apparent or are challenging for differential diagnosis, if any [14]. The development of newborn screening (NBS) is now a critical tool in the prevention of primary diseases. IEMs can be detected via NBS in asymptomatic patients. In other words, medical intervention can be made quickly in the early stage to control disease progression [15]. Thus, morbidity and mortality associated with IEMs can be effectively reduced [16], and life-threatening or long-term sequelae prevented [17].
Newborn screening
Neonatal screening began in the 1970s when Dr. Robert Guthrie developed the dried blood spot (DBS) analysis to measure metabolites in the diagnosis of phenylalanine (PKU) [18]. From there, NBS gradually evolved from a relatively simple test detecting a single congenital condition to a more comprehensive and complex screening system covering over 50 different diseases [19]. Screening methods advanced with the successive application of Gas chromatography/mass spectrometry (GC/MS), liquid chromatography/mass spectrometry (LC/MS), and tandem mass spectrometry (MS/MS) in NBS. Now, MS/MS is the mainstay of NBS given its high specificity and sensitivity [9, 20]. Different conditions, from the most common disease categories to rarer diseases can be simultaneously detected using filter paper spots or directly, in biological fluids [14, 21]. Following Millington et al. [22] first putting MS/MS into practice in 1990 to identify metabolic disorders in NBS, its use became widespread across countries [23, 24]. By 2010, the MS/MS newborn screening coverage in the US had reached 100% with 20–40 diseases being detected among different states [25]. Moreover, screening rates in other countries, such as Germany, the UK and Japan, also reached 90% [26].
The number of IEMs screened varies from country to country. In the US and Canada, NBS covers the largest number, 42 IEMs [27, 28]. By contrast, other countries have set the number within the range of 20–30, for example, Australia (21 IEMs), New Zealand (23), and Japan (24) [29,30,31], which is similar to that set in some provinces of China, such as Taiwan (24), Zhejiang (28) and Hefei (29) [32,33,34,35,36]. In other provinces of Mainland China, the number of IEMs screened for is very limited, only three in Beijing [37, 38] and four in Shenzhen [38]. Additionally, screening programs in South Korea, Germany, and the UK cover less than 20 IEMs, respectively 18, 16, and 9 [31, 39, 40].
The newborn screening program in China was first launched in the 1980s [41]. Since then, significant success has been achieved, with the screening rate increasing from 2% in 1995 to 97.5% in 2017 [42]. The application of the MS/MS method to newborn screening in China began relatively recently, in 2002 [43]. A study revealed that more than 60 laboratories throughout the country had performed MS/MS analysis, and about 40 newborn screening centers had developed the MS/MS screening by 2016 [44]. In China, an increasing number of NBS have adopted using MS/MS rather than the traditional immunofluorescence (IF), which is still the primary means in Shenzhen. Thus, there is an urgent need for the Shenzhen government to implement MS/MS screening throughout the city, especially in the context of “Newborn Screening Management Measures”. In February 2019, the Health Commission of Guangdong province revised its “Newborn Screening Management Measures”, highlighting the potential application of advanced technologies, such as MS/MS, to NBS in the province.
Economic evaluation of MS/MS newborn screening
Developments in economic evaluations of MS/MS newborn screening in different countries have aimed to provide scientific and reasoned evidence to support and improve newborn screening programs, including Canada [45], Germany [16, 46], the US [47,48,49,50], the UK [51, 52], Thailand [53] and other countries [54, 55]. Most of these analyses focus on cost-effectiveness (cost-utility analysis). A smaller proportion of the articles are cost–benefit analyses [56,57,58]. The vast majority of published articles, excepting a study from Thailand, clearly demonstrate that MS/MS screening in their specific country setting is likely a cost-effective healthcare intervention. However, for lack of essential data about IEMs, the number of diseases incorporated in these analyses is usually less than 10. Further, the studies' results are not comparable given the varying regions, time frames, incremental cost-effectiveness ratio (ICER) thresholds, etc. employed in studies. Nevertheless, what cannot be ignored is that economic evaluations prove MS/MS newborn screening to be economically efficient, and offer policymakers helpful scientific evidence for the benefits of NBS programs.
In China, to say nothing of Shenzhen province, any economic evaluation of MS/MS neonatal screening is yet to be carried out, except by one research group [59]. However, the focus of that published article was the whole screening program rather than a specific single disease; prioritizing different IEMs for inclusion in the regional screening program remained undiscussed. Also, as the authors of the study themselves acknowledged, its major limitation was its not applying a Markov model-based analysis to evaluate the costs and health benefits of MS/MS screening in China. The Markov model can simulate the natural progression of the IEMs, providing more rigorous results. Endeavoring to correct this situation, we have conducted this study to determine the cost-effectiveness of MS/MS screening in the social and economic context of Shenzhen based on the diseases nominated by the national program. In doing so, we take into account the government budget and explore how to select the appropriate number of IEMs for detection.
Methods
Selection of IEMs
To guide applicants preparing the material of registration and application for amino acid, carnitine, and succinylacetone detection reagent (MS/MS), the Center for Medical Device Evaluation of National Medical Products Administration of China (NMPA) enacted “Guiding Principles for Amino Acid, Carnitine and Succinylacetone Detection Reagent Registration” in 2019 [60]. The guideline, based on the criteria for disease screening set by the WHO [61], recommends screening for 12 types of IEMs that are relatively common in China and suitable for screening by MS/MS. The twelve IEMs include Phenylketonuria (PKU), Methylmalonic acidemia (MMA), Primary carnitine deficiency (PCD), Medium-chain acyl-CoA dehydrogenase deficiency (MCAD), Very long-chain acyl-CoA dehydrogenase deficiency (VLCAD), Isovaleric acidemia (IVA), Glutaric acidemia type I (GAI), Maple syrup urine disease (MSUD), Citrullinemia type II (CIT-II), Citrullinemia type I (CIT-I), Propionic acidemia (PA) and Homocystinuria (HCY). Compared with the numbers of IEMs detected in other countries, it is a conservative recommendation based on China’s actual situation, thus suiting a referable and applicable pilot program for Shenzhen.
Perspective, discount and comparators
This study measured the inputs and outputs of MS/MS newborn screening from a societal perspective. We then estimated both cost and effectiveness to calculate the ICER of expanded screening, with the cost and effectiveness discounted at an annual rate of 3%.
For the moment, PKU, CH (congenital hypothyroidism), CAH (congenital adrenal hyperplasia) and G6PD deficiency (Glucose-6-phosphate Dehydrogenase deficiency) – the four diseases included in the current screening program – are compulsorily detected by IF independently in Shenzhen.
The expanded screening program, as nationally recommended, is “12 IEMs detected by tandem mass spectrometry (MS/MS), including PKU, MMA, PCD, MCAD, VLCAD, IVA, GA-I, MSUD, CIT-II, CIT-I, PA, HCY”. Only PKU is covered in both the current program and expanded screening. Should the expanded program be implemented, PKU will be detected by MS/MS, while CH, CAH and G6PD will still invariably be detected by IF. Using MS/MS to screen the 12 IEMs does not mean that CH, CAH and G6PD would not be screened.
Since the screening program for CH, CAH and G6PD are unchanged and independent and don’t influence each other, we didn’t take them into consideration. All in all, this study compared PKU screened using traditional immunofluorescence (IF) with the remaining 11 IEMs not screened to all 12 IEMs screened using MS/MS.
In order to determine an adequate and reasonable number of IEMs, we compared the results of economic evaluations and budget impact analyses of MS/MS screening programs with different disease combinations. First, we conducted a cost-effectiveness analysis on the program detecting every disease to obtain an ICER ranking of various diseases. Since PKU is already covered in the current NBS program, we needed to concentrate only on the other 11 IEMs. We could then finally determine 11 screening strategies according to the ranking. The first strategy includes PKU and the disease with the smallest ICER, and the second includes PKU and the top two diseases. By parity of reasoning, the 11th strategy is the nationally recommended program covering all 12 diseases.
Model structure
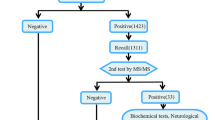
Screening methods in Shenzhen include immunofluorescence for the current program with only the expanded screening program using MS/MS. Positive cases, detected in both the current program and expanded program, will be compulsorily screened in the positive test. We developed a decision-tree and Markov model to conduct the cost-effectiveness analysis of expanded newborn screening using the following assumptions:
-
(i)
A child can have only one kind of disease.
-
(ii)
The progress of IEMs is divided into several independent Markov states (health states) according to the main sequelae. And in each cycle, a child can be in only one of the Markov states.
-
(iii)
The states’ future distribution depends only on current events, and not on those that occurred before.
A half-cycle correction was made to the model. Based on the field investigation, the numbers of people participating in NBS from 2016 to 2018 in Shenzhen were 209,443, 228,206 and 206,670, respectively. Also, there were 194,393 puerperae throughout 2019, so we set the cohort at 200,000 newborns with a cycle-length of one year. The Markov model was terminated at the 82nd cycle since the average life expectancy of Shenzhen residents was 82 years in 2018. States transition of IEMs is shown in Fig. 1, and the Markov model structure in Fig. 2.
Sensitivity and specificity
We retrieved research conducted in China and other countries and undertook a literature review to confirm the sensitivity and specificity of the two screening technologies. We assumed that, as shown in Table 1, the sensitivity and specificity of immunofluorescence are respectively 100% and 78.6%, and their counterparts are both 100%.
The sensitivity and specificity of MS/MS screening is 100%, meaning that the MS/MS screening accuracy is 100%, which implies that no IEM cases were missed by the MS/MS screening and similarly, no healthy cases were misdiagnosed using MS/MS. However, 100% accuracy does not imply there is no positive test. As noted earlier, positive cases detected in both the current and expanded programs, will be detected again in the positive test.
Event probabilities
In Shenzhen, only the incidence of PKU can be collected. Therefore, this study referred to the MS/MS screening results in Zhejiang, covering 1,861,262 neonates and Nanjing, covering 850,486 neonates, as shown in Table 2. Because of the large numbers of people covered and the high maturity of widely used MS/MS, the incidence data is referable for Shenzhen.
Data for the age-specific mortality and incidence of sequelae of different IEMs between the screening and non-screening groups remain scarce in China, leaving us no alternative but to refer to studies from other countries. In order to simulate the natural progress of diseases more accurately, the rate of death due to other causes was also added to the model as a parameter. This part of event probabilities is detailed in Additional file 1.
Costs
The cost of MS/MS screening includes direct costs and indirect costs from a societal perspective. Direct costs include direct medical cost and direct non-medical cost. The former consists of expenditures for screening, confirmation, treatment (for the disease and its sequela), and follow-up, while the latter consists in family transportation costs and the program cost. Indirect costs are due to the lost productivity of families during the confirmation, treatment, and follow-up stages of NBS. Since detection in newborns occurs within six days in the hospital, families do not need to go to a specific hospital, so transportation and lost productivity costs during the screening stage are not counted.
Cost inputs used in the model are shown in Table 3. The data were derived mainly from the field investigation and estimation based on the Guidelines for the Treatment of Rare Diseases (2019) [64], drug prices at online pharmacies, and assumptions of this study. Also, we referred to published literature, government policies, and statistical yearbooks if parameters could not be directly obtained or estimated.
Effectiveness
Quality-adjusted life-years (QALYs) were estimated through the Markov model, multiplying the length of time in different health states by the utility value for states. We also calculated the ICER between current screening and expanded screening programs. An ICER threshold set at 568.704 RMB, three times per capita GDP in Shenzhen, was used in this study. The utility parameters of health states are listed in Table 4, estimated mainly from data in published articles. We presumed the utility of “alive state” to be 1, which means healthy.
Sensitivity analysis
We carried out one-way sensitivity analysis and constructed tornado diagrams to assess the uncertainty in the model and the robustness of the results. One-way sensitivity analysis in this study evaluated the influence of the discount rate in the range 0–10% (base value is 3%), with 1% as an interval of 10 categories. Tornado diagrams include factors like the incidence of IEMs, costs (e.g., the cost of screening, confirmation, transportation, etc.), and utility of health states. The incidence of IEMs was assumed to vary by 50% from their mean value, and costs were 10%. The value of utility being tested varied based on the upper/lower boundaries illustrated in published articles.
Budget impact analysis
Implementing MS/MS screening, the expansion of diseases screened, and the increase in costs will inevitably place a burden on health expenditure, making it necessary to conduct a budget impact analysis (BIA) of MS/MS screening from the standpoint of Shenzhen’s government.
Health expenditure entailed in the frame of this study included the cost of screening, treatment, and follow-up, as well as the program cost. We assumed that these program costs remain unchanged between the expanded screening and the status quo. What should be noted is that children must receive continuous medical treatment following a positive detection. Because of the increasing numbers of patients year by year, the cost of treatment is necessarily cumulative. The treatment cost of MS/MS screening is mainly that of the IEMs without sequelae, since early detection can sharply reduce the occurrence of sequelae, as indicated in the Markov model. By comparison, the treatment cost of current screening is mainly the treatment cost for the IEMs with sequelae. Due to the high mortality rates of some IEMs, we didn’t calculate the treatment cost of patients with diseases which can cause death within their first two years.
BIA was carried out under the assumption of 200,000 newborns screened annually for ten years (2019–2028). In China, the duration of a government project, program or policy is usually five years or ten years. When we began this study, the officials of Shenzhen Municipal Health Commission claimed the budget of the expanded screening program for the next ten years. Since the starting point of this study was 2019, utilizing data from 2018, we conducted the BIA from 2019 to 2028.
Nowadays, in Shenzhen, with relevant policies and regulations released [73] and a national medical insurance system established, the cost of screening and treatment is paid for by families and the government conjointly. The government undertakes 80% of the screening cost and 60% of the treatment cost. How the rate of the medical insurance reimbursement is calculated is shown in Additional file 3.
Results
Cost-effectiveness analysis and BIA
Detecting 12 IEMs in the nationally recommended program
Table 5 shows the results of the cost-effectiveness analysis of current screening and expanded screening. The ICER is 277823RMB per QALY, below the ICER threshold (568704RMB) according to the criteria set by the World Health Organization (WHO).
To specify the costs of the MS/MS newborn screening program, the results of BIA are detailed in Table 6.
The total cost of expanded screening is 47.63 million RMB in the first year of MS/MS implementation, increasing to 50.96 million RMB by 2028 at an average annual growth rate of 0.75%. The total health expenditure for the MS/MS screening program in Shenzhen will reach 493.26 million RMB in the next decade, costing a further 41.50 million RMB annually compared to the current NBS program. The cost of screening accounts for most of the expenditure over the whole decade (96.01%). Meanwhile, the cost of treatment increases as time goes by, accounting for only 0.56% of the total cost (0.27 million RMB) in 2019, rising to 7.06% (4.84 million RMB) by 2028.
Detecting some types of IEMs selected from national recommendations
The results of the cost-effectiveness analysis of screening a single disease are shown in Table 7. ICERs of all screening programs are higher than the threshold, and diseases are ranked by ICER (from minimum to maximum), i.e., PCD, MMA, MSUD, IVA, PA, MCAD, GA I, CIT I, CIT II, HCY, and VLCAD. The ICER of PCD is the minimum as 1,108,216 RMB per QALY, and the ICER of the VLCAD is maximum as 28,203,412 RMB per QALY.
According to the above ICER rankings of diseases, 11 screening strategies were finally examined. The ICER of each screening program is shown in Fig. 3, below. Only the ICER of the first strategy (PKU and PCD) is higher than the threshold, at 748,196 RMB per QALY. All other ICERs are below the threshold. As the number of diseases detected increases, the ICER gradually decreases and finally tends to stabilize.
ICER of eleven screening strategiesa. aThe line L1 in the figure illustrates the ICER of 11 screening strategies. X-axis represents the screening strategy, y-axis represents the ICER of different screening strategies and the diseases detected in strategies are showed on the right. The first strategy covers PKU and PCD, the second covers PKU, PCD and MMA. By parity of reasoning, the 11th strategy is the nationally recommended program covering all 12 diseases. Different colored columns are used to distinguish different strategies
The results of the BIA of the 11 screening strategies above are shown in Fig. 4. Although the screening program’s budget grows with the increasing number of diseases detected, there is no meaningful difference between single program budgets. The budget for all screening programs holds steady, near 490million RMB.
Sensitivity analysis
We conducted a one-way sensitivity analysis based on the nationally recommended program of 12 IEMs (Fig. 5 and Fig. 6). We discussed the discount rate and other parameters separately, since the former is remarkably influential. It turns out that the ICER is less than three times per capita GDP when the discount rate is ≤ 7% and, particularly when the discount rate is equivalent to 7%, the ICER is very close to the threshold. Also, the ICER is lower than one times per capita GDP (189,568RMB) as the discount rate is ≤ 1.5%, which means the screening program is very cost-effective.
Tornado diagram of newborn screening parametersa, aThe parameters are sorted on the left according to their effect on incremental cost effectiveness ratio. The most influential parameter is on the top. EV: Expected value. P_X: The incidence of “X”, CT_X: The cost of treatment for “X”. “X” represents inborn errors of metabolism, including PCD, MMA, MSUD, IVA, PA, GA, MCAD, CIT-II (CIT 2), HCY, CIT I (CIT 1), VLCAD, PKU. U_Y: The utility of “Y”, CY: The cost of treatment for “Y”. “Y” represents states of the disease, including NS (No Sequela), DD (Development Delay), ND (Neurological Damage), MR (Mental Retardation), RD (Renal Damage). C_Pr: The program cost; C_MSScr: The cost of MS/MS screening test; C_FAScr: The cost of immunofluorescence screening test; C_FACom: The cost of MS/MS confirmation test; C_FACom: The cost of immunofluorescence confirmation test; CVisitMS: The cost of follow-up of the MS/MS screening program; CVisitFA: The cost of follow-up of the immunofluorescence screening program; CWage: The cost of lost productivity; Ctraffic: The cost of traffic
The top three influential parameters are the incidences of PCD (P_PCD), the incidence of MMA (P_MMA), and the cost of screening by MS/MS (C_MSScr). As we can see, the incidence of diseases accounts for a large proportion of top influential parameters, and the top three are the incidences of PCD, MMA, and MSUD. Apart from disease incidence, the cost of screening by MS/MS (C_MSScr), the utility of the state without sequelae (U_NS) and the cost of confirmation by IF (C_FACom) weigh heavily for the ICER. But, no matter how parameters change within the range, the ICER always remains below the threshold, affirming that the results are robust.
Discussion
This study considered the cost-effectiveness of MS/MS newborn screening in Shenzhen, China. From a societal perspective, we confirmed that it is cost-effective to implement the expanded screening, with some preconditions. First, it is not economically efficient to detect only one type of disease. Especially for PKU, the results illustrated IF to be a more cost-effective method currently. The factors contributing to this situation is that detecting a single disease cannot embody the advantages of MS/MS ‒ “one blood sampling for multiple diseases” [74] ‒ but reflects that MS/MS is more expensive than IF. Second, MS/MS screening can be cost-effective only if at least three diseases (PKU, PCD and MMA) are covered. The selection of IEMs for detection associates to the incidence of diseases. The higher the incidence is, the more QALYs can be saved, and the more diseases detected, and the smaller ICER of the program will be. Moreover, when the screening program covers five diseases (PKU, PCD, MMA, MSUD, IVA), the ICER closely approaches its critical value (since the ICER of the program covering six diseases accounts for more than 95% of the ICER of five diseases).
The above points can explain not only why the number of NBS programs varies from region to region but can also reveal two strategic policy ideas for selecting the number of diseases covered. One of these strategies is to screen as many IEMs as possible and get a most cost-effective screening program with a minimal ICER, like the 11th screening program mentioned above. “One blood sampling for multiple diseases” allows an MS/MS screening program to cover more IEMs without additional cost. Therefore, screening more diseases leads to more QALYs saved, making the screening program more economically efficient. The first policy idea finally can attain a minimal ICER as the decision-maker adds more and more IEMs into the NBS program, which is perhaps the reason for the US and Canada setting their number of IEMs at 42. Significantly, this strategy proposes the request to regions in terms of social, economic and medical science development. The other policy strategy is to get an almost best screening program detecting suitable IEMs, with an ICER under the cost-effectiveness threshold and close to its minimum value, like 5th screening program mentioned above. In fact, there exists the inevitable problem of how to treat these hard-to-cure diseases. Although various therapies have so far been developed [75], several factors, such as the cost of treatment, the complexity and difficulty of treatment, patient compliance, and so on [76, 77], nonetheless constitute a huge challenge for medical treatment. Without appropriate medical treatments being available, newborns detected positive can only cause families serious economic and emotional stress. It is therefore wise to include some IEMs with a high incidence and for which reasonable treatment is available in the screening program. And, in this case, the ICER of the program is already close to the minimum value. We believe that’s why some countries, such as the UK, South Korea, and Germany, have limited detectable diseases to less than 20. In brief, for the initial implementation of MS/MS screening in Shenzhen, we would suggest utilizing the second policy strategy.
In China, while economic evaluations of NBS have been made in many studies [78,79,80,81,82,83,84], only one has undertaken a cost-effectiveness analysis of MS/MS screening [59]. Most studies have concentrated on the traditional NBS for PKU and/or CH. The benefit–cost ratio ranges between 1:2.38 ~ 1:4.58 for PKU and between 1:3.60 ~ 1:19.94 for CH, demonstrating the economic benefit of traditional NBS. As for the results of the single study focusing on MS/MS screening, these showed the ICUR of MS/MS screening to be -768,429 RMB/QALY (MS/MS screened vs. non-screened) and the BCR was 6.09. That study’s finding of the cost effectiveness of MS/MS screening is consistent with the result of our study as well as the findings of those studies carried out in other countries [46, 48,49,50,51,52].
The results of this study are generally robust. One-way sensitivity analysis revealed that caution should be taken when the discount rate surpasses 7%. But, statistics indicate that China's annual inflation rate has remained at around 2% for the past decade (owing to COVID-19, the rate once approached 6%, but remained below 7%) [85]. That is to say, the implementation of MS/MS screening in Shenzhen will be cost-effective for some time to come and likely to be highly cost-effective, since inflation sometimes falls below 1.5%.
The findings of the BIA performed demonstrate that no meaningful difference exists among different programs in the whole budget. Given we choose a cost-effective screening program, in every year over the next decade budgets will all reach about 49.33 million RMB. Combining this with the ICER of a program detecting five diseases which reaches the critical value, we can conclude that if the budget of the program covering five diseases is affordable for the Shenzhen administration, it is better to screen for all twelve diseases as per the nationally recommended program. According to the Guidelines for the Treatment of Rare Diseases (2019), there are specific and systematic treatments for these diseases.
Here, we also considered whether the Shenzhen government could bear the budget as proposed, and the answer is yes. According to the Department Final Report of the Health Commission of Shenzhen in 2018 [86], the budget for public health projects in NBS (including screening for hearing, for IEMs, and for trisomies 21, 18, and 13) was 289.21 million RMB at the beginning of 2018. However, the final expenditure was 123.73 million RMB, accounting for 42.7% of the budget. This indicates the sufficiency of Shenzhen government funds to finance an expanded program. Moreover, data from Health Statistics Summary of Shenzhen in 2019 [87] show that annual government expenditure on medical treatments and health services increased from 8.332 billion RMB in 2014 to 19.09 billion RMB in 2018, with an annual growth rate of 23.03%, which is much larger than the annual growth rate of the cost of MS/MS newborn screening program.
Another of our team’s studies[88], to investigate patients’ willingness to pay (WTP) for the MS/MS screening, revealed the average WTP value was 242 RMB, and that 68.71% (404/588) of families were willing to pay more than 200 RMB. So, the government pricing of the MS/MS screening is truly adjustable and flexible when the WTP value is compared with 20% of the screening cost (average 60.98RMB-79.57RMB per year) that a family should currently be able to afford. And if policymakers are concerned about the risks of health expenditure, it is opportune to increase the proportion of out-of-pocket payment to relieve the high-cost burdens of government. Such a policy change would, however, need to be properly considered since the aim of “gradually providing the NBS program free of charge” was proposed in a recent provincial policy. Briefly, the series of data presented above makes the claim that the MS/MS newborn screening program is affordable for the Shenzhen government.
Due to the lack of epidemiological investigation in China and Shenzhen, the integral local database of IEMs is still extraordinarily wanting. So, in order to solve this bottleneck problem, key recommendations are for research that outlines top priorities to enhance epidemiological studies of IEMs and advance the establishment of the IEMs database in China. Only in this way can we proceed with a cost-effectiveness analysis suiting China’s circumstances and provide further empirical evidence for the NBS program.
In this study, we assumed the sensitivity and specificity of MS/MS screening is 100%, implying that this method produces no false positive or false negative results. In fact, even if the accuracy of the technology is sufficiently high, false positives in the practical screening program can still occur. Such occurrences of false positive cases can incur additional costs, including for treatment, confirmation testing and follow-up while creating related stress for families. Taking the example of PKU, a false positive test can cause a loss of about 70,000 RMB every year, not accounting the latent costs of psychological stress. So, what we should concentrate on is screening accuracy and quality management of the whole screening program in the real world.
Conclusions
We have attempted to put forward policy suggestions for choosing diseases for MS/MS screening in Shenzhen, conducting a cost-effectiveness analysis based on the 12 IEMs recommended by NMPA. This study has confirmed that MS/MS screening covering at least three diseases is cost-effective. The cost of the MS/MS screening program detecting 12 IEMs is well within the budget constraints of the Shenzhen government. This study also discussed two policy concepts for selecting IEMs for detection. One represents the strategy of choosing the most cost-effective screening programs detecting highest number of IEMs. The other, considers the curability and affordability of the disease as the basis of healthcare decisions to screen suitable IEMs and achieves an ICER under the threshold and approaching the minimum value.
Availability of data and materials
All data generated or analysed during this study can be available publicly. Readers can contact the corresponding author if there are any questions about the data or requirements for more detailed data.
Abbreviations
- BIA:
-
Budget impact analysis
- CAH:
-
Congenital adrenal hyperplasia
- CH:
-
Congenital hypothyroidism
- CIT-I:
-
Citrullinemia type I
- CIT-II:
-
Citrullinemia type II
- DBS:
-
Dried blood spot
- DD:
-
Development Delay
- GAI:
-
Glutaric acidemia type I
- G6PD deficiency:
-
Glucose-6-phosphate Dehydrogenase deficiency
- GC/MS:
-
Gas chromatography/mass spectrometry
- HCY:
-
Homocystinuria
- ICER:
-
Incremental cost-effectiveness ratio
- IEMs:
-
Inborn errors of metabolism
- IF:
-
Immunofluorescence
- IVA:
-
Isovaleric acidemia
- LC/MS:
-
Liquid chromatography/mass spectrometry
- MCAD:
-
Medium-chain acyl-CoA dehydrogenase deficiency
- MMA:
-
Methylmalonic acidemia
- MR:
-
Mental Retardation
- MS/MS:
-
Tandem mass spectrometry
- MSUD:
-
Maple syrup urine disease
- NBS:
-
Newborn screening
- ND:
-
Neurological Damage
- NMPA:
-
National Medical Products Administration of China
- PA:
-
Propionic acidemia
- PCD:
-
Primary carnitine deficiency
- PKU:
-
Phenylalanine
- QALYs:
-
Quality-adjusted life-years
- RD:
-
Renal Damage
- VLCAD:
-
Very long-chain acyl-CoA dehydrogenase deficiency
- WHO:
-
World Health Organization
- WTP:
-
Willingness to pay
References
Pollitt RJ, et al. Neonatal screening for inborn errors of metabolism: cost, yield and outcome. Health Technol Assess. 1997;1(7):i–iv, 1–202.
Seymour CA, et al. Newborn screening for inborn errors of metabolism: a systematic review. Health Technol Assess. 1997;1(11):i-iv1-95.
Alfadhel M, et al. Thirteen year retrospective review of the spectrum of inborn errors of metabolism presenting in a tertiary center in Saudi Arabia. Orphanet J Rare Dis. 2016;11(1):126.
Illsinger S, Das AM. Impact of selected inborn errors of metabolism on prenatal and neonatal development. IUBMB Life. 2010;62(6):403–13.
Tebani A, et al. Clinical Metabolomics: The New Metabolic Window for Inborn Errors of Metabolism Investigations in the Post-Genomic Era. Int J Mol Sci. 2016;17(7):1167.
Ferreira CR, et al. A proposed nosology of inborn errors of metabolism. Genet Med. 2019;21(1):102–6.
Waters D, et al. Global birth prevalence and mortality from inborn errors of metabolism: a systematic analysis of the evidence. J Glob Health. 2018;8(2):021102.
Ghosh A, et al. Diagnosing childhood-onset inborn errors of metabolism by next-generation sequencing. Arch Dis Child. 2017;102(11):1019–29.
Mussap M, Zaffanello M, Fanos V. Metabolomics: a challenge for detecting and monitoring inborn errors of metabolism. Ann Transl Med. 2018;6(17):338.
Li Q, et al. 1999–2018 Nian Zhe Jiang Sheng Xin Sheng Er Yi Chuan Dai Xie Bing Shai Cha Qing Kuang Fen Xi [Analysis on neonatal screening for inherited metabolic diseases in Zhejiang Province from 1999 to 2018]. Prev Med. 2019;31(11):1081–5.
Ma S, et al. He Nan Sheng 2013–2019 Nian Xin Sheng Er Yi Chuan Dai Xie Bing Shai Cha Hui Gu Xing Fen Xi [Retrospective analysis on screening of neonates inherited metabolic diseases from 2013 to 2019 in Henan province]. Laboratory Med Clin. 2020;17(14):1965–8.
Sun Y, et al. Nan Jing Di Qu 175 767 Li Chuan Lian Zhi Pu Ji Shu Xin Sheng Er Shai Cha Jie Guo Fen Xi [Newborn screening by tandem mass spectrometry in Nanjing: a retrospective analysis of 175 767 cases]. Chin J Perinat Med. 2020;23(04):224–31.
Wilcken B, et al. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003;348(23):2304–12.
Ismail IT, Showalter MR, Fiehn O. Inborn Errors of Metabolism in the Era of Untargeted Metabolomics and Lipidomics. Metabolites. 2019;9(10):242.
Vernon HJ. Inborn Errors of Metabolism: Advances in Diagnosis and Therapy. JAMA Pediatr. 2015;169(8):778–82.
Tran K, et al. Clinical efficacy and cost-effectiveness of newborn screening for medium chain acyl-CoA dehydrogenase deficiency using tandem mass spectrometry. Clin Biochem. 2007;40(3–4):235–41.
Almannai M, Marom R, Sutton VR. Newborn screening: a review of history, recent advancements, and future perspectives in the era of next generation sequencing. Curr Opin Pediatr. 2016;28(6):694–9.
Guthrie R, Susi A. A SIMPLE PHENYLALANINE METHOD FOR DETECTING PHENYLKETONURIA IN LARGE POPULATIONS OF NEWBORN INFANTS. Pediatrics. 1963;32:338–43.
Therrell BL, et al. Current status of newborn screening worldwide: 2015. Semin Perinatol. 2015;39(3):171–87.
Annesley T, et al. A Spectrum of Views on Clinical Mass Spectrometry. Clin Chem. 2016;62(1):30–6.
Pandor A, et al. Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review. Health Technol Assess. 2004;8(12):iii, 1–121.
Millington DS, et al. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13(3):321–4.
Kuehn BM. After 50 years, newborn screening continues to yield public health gains. JAMA. 2013;309(12):1215–7.
Mechtler TP, et al. Neonatal screening for lysosomal storage disorders: feasibility and incidence from a nationwide study in Austria. Lancet. 2012;379(9813):335–41.
Lehotay DC, et al. LC-MS/MS progress in newborn screening. Clin Biochem. 2011;44(1):21–31.
Wang X. Jiang Xi Sheng Xin Sheng Er Yi Chuan Dai Xie Bing Shai Cha 20 Nian Hui Gu Yu Zhan Wang [The retrospect and prospect of newborn screening for inherited metabolic disease in Jiangxi Province over two decades]. Jiangxi: Nan chang University; 2017. p. 35.
Health Resources & Services Administration. Administration. Recommended Uniform Screening Panel. 2020. Available from: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html. Cited 2021.
Canadian Organization for Rare Disorders. Newborn Screening in Canada Status Report. 2015. Available from: https://www.raredisorders.ca/content/uploads/Canada-NBS-status-updated-Sept.-3-2015.pdf. Cited 2021.
Human Genetics Society of Australasia. Recommendations for Screening for Specific Disorders. 2019. Available from: https://www.hgsa.org.au/resources/hgsa-policies-and-position-statements. Cited 2021.
National Screening Unit of NewZealand. Newborn Metabolic Screening Programme - heel prick test - Frequently asked questions : What are babies screened for? 2014. Available from: https://www.nsu.govt.nz/pregnancy-newborn-screening/newborn-metabolic-screening-programme-heel-prick-test/frequently-asked. Cited 2021.
Shibata N, et al. Diversity in the incidence and spectrum of organic acidemias, fatty acid oxidation disorders, and amino acid disorders in Asian countries: Selective screening vs expanded newborn screening. Mol Genet Metab Rep. 2018;16:5–10.
Niu DM, et al. Nationwide survey of extended newborn screening by tandem mass spectrometry in Taiwan. J Inherit Metab Dis. 2010;33(Suppl 2):S295-305.
Huang X, et al. Zhe Jiang Sheng Xin Sheng Er An Ji Suan Dai Xie Ji Bing Shai Cha Ji Sui Fang Fen Xi [Screening for amino acid metabolic disorders of newborns in Zhejiang province: prevalence, outcome, and follow-up]. J Zhejiang Univ (Med Sci). 2017;46(03):233–9.
Hong F, et al. Zhe Jiang Sheng Xin Sheng Er You Ji Suan Niao Zheng Shai Cha Ji Sui Fang Fen Xi [Screening for newborn organic aciduria in Zhejiang province: prevalence, outcome, and follow-up]. J Zhejiang Univ (Med Sci). 2017;46(03):240–7.
Zheng J, et al. Screening forfatty acid oxidation disorders of newborns in Zhejiang province:prevalence, outcome and follow-up. J Zhejiang Univ (Med Sci). 2017;46(03):248–55.
Hefei Municipal Health Commission. Hefei Newborn Screening Program Implementation Plan. 2020. Available from: http://zwgk.hefei.gov.cn/public/17771/105835811.html. Cited 2021.
Beijing Municipal Health Commission. Beijing newborn screening Management Measures. 2020. Available from: http://wjw.beijing.gov.cn/zwgk_20040/zxgk/202006/t20200619_1928560.html. Cited 2021.
Shenzhen Municipal Health Commission. Newborn screening for inborn errors of metabolism. 2019. Available from: http://wjw.sz.gov.cn/ztzl/jksc/dfggwsfwxm/content/post_3122209.html. Cited 2021.
Gemeinsamer Bundesausschuss. Richtlinie zur Früherkennung von Krankheiten bei Kindern in der Version vom 14.05.2020. 2020. Available from: https://www.g-ba.de/sys/suche/?suchbegriff=Neugeborenen-Screening&kategorie=richtlinien&sortierung=inkrafttretendatum. Cited 2021.
National Institute for Health Research. EXPANDED NEWBORN SCREENING. 2014. Available from: http://www.expandedscreening.org/site/home/start.asp. Cited 2021.
Chen R, et al. Xian tian xing jia zhuang xian ji neng di xia de xin sheng er shai cha [NEONATAL SCREENING FOR CONGENITAL HYPOTHYROIDISM]. Chin J Endocrinol Metab. 1985;1(02):28-30+64.
National Health Commission of the People’s Republic of China. Report on the Development of Maternal and Child Health in China (2019). 2019. Available from: http://www.nhc.gov.cn/fys/s7901/201905/bbd8e2134a7e47958c5c9ef032e1dfa2.shtml. Cited 2021.
Gu X, et al. Chuan Lian Zhi Pu Ji Shu Zai Yi Chuan Xing Dai Xie Bing Gao Wei Er Tong Shai Cha Zhong De Chu Bu Ying Yong [A pilot study of selective screening for high risk children with inborn error of metabolism using tandem mass spectrometry in China]. Chin J Pediatr. 2004;42(06):5-8+85.
Ye J. Xin Sheng Er Yi Chuan Dai Xie Bing Shai Cha Fa Zhan Ji Zhen Zhi Gui Fan [Development of newborn screening and diagnosis for inborn errors of metabolism]. Chinese J Fam Plann Gynecotokology. 2016;8(01):6–13.
Cipriano LE, Rupar CA, Zaric GS. The cost-effectiveness of expanding newborn screening for up to 21 inherited metabolic disorders using tandem mass spectrometry: results from a decision-analytic model. Value Health. 2007;10(2):83–97.
Pfeil J, et al. Newborn screening by tandem mass spectrometry for glutaric aciduria type 1: a cost-effectiveness analysis. Orphanet J Rare Dis. 2013;8:167.
Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics. 2006;117(5 Pt 2):S287–95.
Venditti LN, et al. Newborn screening by tandem mass spectrometry for medium-chain Acyl-CoA dehydrogenase deficiency: a cost-effectiveness analysis. Pediatrics. 2003;112(5):1005–15.
Tiwana SK, Rascati KL, Park H. Cost-effectiveness of expanded newborn screening in Texas. Value Health. 2012;15(5):613–21.
Feuchtbaum L, Cunningham G. Economic evaluation of tandem mass spectrometry screening in California. Pediatrics. 2006;117(5 Pt 2):S280–6.
Bessey A, et al. The Cost-Effectiveness of Expanding the UK Newborn Bloodspot Screening Programme to Include Five Additional Inborn Errors of Metabolism. Int J Neonatal Screen. 2020;6(4):93.
Insinga RP, Laessig RH, Hoffman GL. Newborn screening with tandem mass spectrometry: examining its cost-effectiveness in the Wisconsin Newborn Screening Panel. J Pediatr. 2002;141(4):524–31.
Thiboonboon K, et al. An Economic Evaluation of Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry in Thailand. PLoS ONE. 2015;10(8):e0134782.
Hamers FF, Rumeau-Pichon C. Cost-effectiveness analysis of universal newborn screening for medium chain acyl-CoA dehydrogenase deficiency in France. BMC Pediatr. 2012;12:60.
Autti-Rämö I, et al. Expanding screening for rare metabolic disease in the newborn: an analysis of costs, effect and ethical consequences for decision-making in Finland. Acta Paediatr. 2005;94(8):1126–36.
Norman R, et al. Economic evaluation of tandem mass spectrometry newborn screening in Australia. Pediatrics. 2009;123(2):451–7.
Schoen EJ, et al. Cost-benefit analysis of universal tandem mass spectrometry for newborn screening. Pediatrics. 2002;110(4):781–6.
Khneisser I, et al. Cost-benefit analysis: newborn screening for inborn errors of metabolism in Lebanon. J Med Screen. 2015;22(4):182–6.
Zhao Z, et al. Newborn screening for inherited metabolic diseases using tandem mass spectrometry in China: Outcome and cost-utility analysis. J Med Screen. 2022;29(1):12–20.
National Medical Products Administration. Guiding Principles for Amino Acid, Carnitine and Succinylacetone Detection Reagent Registration. 2019. Available from: https://www.nmpa.gov.cn/ylqx/ylqxggtg/ylqxzhdyz/20191115172001960.html. Cited 2021.
Wilson JMG, Jungner G. PRINCIPLES AND PRACTICE OF SCREENING FOR DISEASE. 1968. Available from: https://apps.who.int/iris/bitstream/handle/10665/37650/WHO_PHP_34.pdf?sequence=17. Cited 6 Feb 2022.
Liu W, et al. Chuan Lian Zhi Pu Fa He Ying Guang Fen Xi Fa Shai Cha Ben Bing Tong Niao Zheng De Dui Bi Fen Xi [Comparative analysis of screening for phenylketonuria by tandem mass spectrometry and fluorescence analysis]. Matern Child Health Care China. 2018;33(24):6020–2.
Huang X, et al. Chuan Lian Zhi Pu Ji Shu Dui Xin Sheng Er Yi Chuan Dai Xie Bing De Shai Cha Ji Sui Fang Yan Jiu [Screening for neonatal inborn errors of metabolism by electrospray ionization-tandem mass spectrometry and follow-up]. Chinese J Pediatrics. 2011;49(10):765–70.
National Health Commission of the People’s Republic of China. Guidelines for the Treatment of Rare Diseases (2019). 2019. Available from: http://www.nhc.gov.cn/yzygj/s7659/201902/61d06b4916c348e0810ce1fceb844333.shtml. Cited 2021.
Zhang F. Bu Tong Gong Can Mo Shi Xue Sheng Can De Ying Yang Xue He Jing Ji Xue Ping Jia [The nutrition and economic evaluation of school meal with different catering modes]. Beijing: CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION; 2015. p. 153.
Cui W. He Bei Sheng Nao Tan Huan Er Sheng Cun Zhi Liang Zhuang Kuang Diao Cha Yu Jing Ji Fu Dan Ping Jia [Evaluation of Hebei Province to Investigate the Quality of Life and Economic Burden in Children with Cerebral Palsy]. Jilin: Jilin University; 2014. p. 63.
Shenzhen Disabled Persons Federation. Administrative Measures on Rehabilitation and Assistance Services for Disabled Children in Shenzhen. 2018. Available from: http://www.cjr.org.cn/info/laws/201805/t20180528_19086624.htm. Cited 2021.
Li X. Man Xing Shen Bing Zhi Liao De Wei Sheng Jing Ji Xue Ping Jia [Health Economic Evaluation For Chronic Kidney Disease Treatment]. Hubei: Huazhong University of Science and Technology; 2016. p. 76.
Shenzhen Bureau of Statistics. Shenzhen Statistical Yearbook 2019. 2020. Available from: http://tjj.sz.gov.cn/zwgk/zfxxgkml/tjsj/tjnj/content/post_7971762.html. Cited 2021.
Shi J. Wo Guo Nong Cun Di Qu Zi Gong Jing Ai Shai Cha Fang An De Wei Sheng Jing Ji Xue Ping Jia Yan Jiu [Cost-effectiveness on Various Modalities of Cervical Cancer Screening in Rural China]. Beijing: Peking Union Medical College; 2009. p. 107.
Carroll AE, Downs SM. Improving decision analyses: parent preferences (utility values) for pediatric health outcomes. J Pediatr. 2009;155(1):21–5, 25.e1-5.
Dionisi-Vici C, et al. “Classical” organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J Inherit Metab Dis. 2006;29(2–3):383–9.
Shenzhen Municipal Health Commission. Financial subsidies for neonatal inborn errors of metabolism screening costs. 2016. Available from: http://wjw.sz.gov.cn/xxgk/tzgg/content/post_3159718.html. Cited 2021.
Ombrone D, et al. Expanded newborn screening by mass spectrometry: New tests, future perspectives. Mass Spectrom Rev. 2016;35(1):71–84.
Gambello MJ, Li H. Current strategies for the treatment of inborn errors of metabolism. J Genet Genomics. 2018;45(2):61–70.
Evaluate. EvaluatePharma Orphan Drug Report 2019. 2019. Available from: https://www.evaluate.com/thought-leadership/pharma/evaluatepharma-orphan-drug-report-2019. Cited 2021.
Boyer SW, Barclay LJ, Burrage LC. Inherited Metabolic Disorders: Aspects of Chronic Nutrition Management. Nutr Clin Pract. 2015;30(4):502–10.
Jiajun W, et al. Cost benefit analysis of screening for neonatal diseases in Shanghai. Chinese Health Resources. 1999;04:11–3.
Ruixue H, et al. cost-effectiveness and cost-utility of neonatal congenital hypothyroidism screening in Foshan City. Journal of Chongqin Medicine. 2017;46(20):2820–2.
Wen-yan Z, et al. A cost-benefit evaluation of neonatal screening for congenital hypothyroidism. Chin J Ctrl Endem Dis. 2013;28(06):443–4.
Xiao-wei M, et al. Cost -benefit Analysis of Neonatal Screening Project. Chinese Health Econ. 2011;30(05):91–3.
Xuefan G, et al. A cost-benefit evaluation of neonatal screening for phenylketonuria and congenital hypothyroidism. Chin J Prev Med. 2000;03:20–2.
Yan L, et al. Cost-Benefit Analysis of Neonatal Screening Project in Shenzhen. Chinese J Social Med. 2012;29(03):214–6.
Yao C, et al. Cost-effectiveness analysis on neonatal screening in Fuzhou. MCH. 2015;30(13):1980–2.
TRADING ECONOMICS. China’s inflation rate. 2021. Available from: https://zh.tradingeconomics.com/china/inflation-cpi. Cited 2021.
Shenzhen Municipal Health Commission. Department Final Report of the Health Commission of Shenzhen in 2018. 2019. Available from: http://wjw.sz.gov.cn/attachment/0/600/600932/3133572.pdf. Cited 2021.
Shenzhen Municipal Health Commission. Health Statistics Summary of Shenzhen in 2019. 2020. Available from: http://wjw.sz.gov.cn/xxgk/tjsj/zxtjxx/content/post_7786068.html. Cited 2021.
Du J, et al. Yun Chan Fu Dui Xin Sheng Er Yi Chuan Dai Xie Bing Chuan Lian Zhi Pu Shai Cha Ren Zhi Ji Zhi Fu Yi Yuan Fen Xi [Analysis on cognition and willingness-to-pay for tandem mass spectrometry screening of neonatal genetic metabolic diseases among pregnant women]. MCH. 2020;35(08):1503–7.
Acknowledgements
Not applicable.
Funding
This work was supported by Shenzhen Health Development Research Center(023105160048). No additional funding was obtained.
Author information
Authors and Affiliations
Contributions
JX, JD and MY participated in the study design. JX and MY participated in theoretical parts of manuscript and drafted the manuscript. XS is responsible for collecting data. MY and JD is responsible for data management and statistical analysis. JX, MY, XS and JD revised the manuscript. All authors took part the interpretation of findings and have approved the revised manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted, according to the ethical guidelines of the Helsinki Declaration. The Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (IORG No: IORG0003571) give a final APPROVAL on 24/07/2019 for the study. Written informed consent was obtained from all subjects before the start of the study. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interest
No conflict of interest exists in submitting this manuscript, and all authors for publication approved the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
S1. Age-specific mortality and sequelae incidence of IEMs.
Additional file 2:
S2. How to calculate the treatment cost of IEMs.
Additional file 3:
S3. Actual rate of the medical insurance reimbursement in China.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, M., Xu, J., Song, X. et al. Cost-effectiveness analysis of newborn screening by tandem mass spectrometry in Shenzhen, China: value and affordability of new screening technology. BMC Health Serv Res 22, 1039 (2022). https://doi.org/10.1186/s12913-022-08394-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-022-08394-4