Abstract
Objective
We aimed to evaluate the cost-effectiveness of voretigene neparvovec (VN) compared with standard of care (SoC) for patients with inherited retinal disease (IRD) caused by a biallelic RPE65-mutation. VN is a live, non-replicating adeno-associated virus serotype 2 (AAV2). SoC is best supportive care provided to patients with visual impairment. Patients under SoC may experience progressive vision loss leading to complete blindness.
Methods
We adapted a previously published Markov cohort model for IRD. An annual cycle length, life-long time horizon, discount rate of 3% for cost and health outcomes, and Swiss health system perspective were used. Data from a randomised controlled phase III trial of VN versus SoC (ClinicalTrials.gov: NCT00999609) were used to estimate transitions between health states in the first year, after which VN patients were assumed to remain for 39 subsequent years in the health state they were in at the end of the first year. After the 40th year for VN patients and 1st year for SoC patients, visual decline was modelled based on observational data on the natural progression of the disease. Quality-adjusted life years (QALYs) were calculated based on an external study which elicited clinicians’ EQ-5D-5L-based utility estimates for IRD patients with a RPE65-mutation. Costs (Swiss Francs (CHF), year 2018-2019) included drug acquisition/ administration, adverse events, testing for sufficient viable retinal cells, and healthcare-related costs of blindness. Societal costs of blindness were added in a complementary analysis. Robustness of the model results were tested in sensitivity and scenario analyses.
Results
For the base-case, VN resulted in incremental costs per patient of CHF 764’402 (VN: CHF 901’654, SoC: CHF 137’252), incremental blindness-free years of 7.67 (VN: 28.32, SoC: 20.65) and incremental QALYs of 6.73 (VN: 18.35, SoC: 11.62), leading to an incremental cost-effectiveness ratio of CHF 113’526 per QALY gained. In probabilistic sensitivity analysis, the cost-effectiveness of VN was better than CHF 100,000 per QALY gained in 41% of iterations. For the scenario analysis in which a societal perspective was adopted and for which a 50% work-related productivity loss from blindness was assumed, incremental costs of CHF 423,837 and an ICER of CHF 62’947 per QALY gained were produced. The scenario assuming VN treatment effect lasts for 20 years produced an ICER of CHF 156’171 per QALY gained, whereas assuming a life-long VN treatment effect resulted in an ICER of CHF 96’384 per QALY gained.
Conclusion
The incremental cost-effectiveness ratio of VN compared to the SoC was estimated to be CHF 113’526 and CHF 62’947 per QALY gained, respectively, from a Swiss healthcare system, and societal perspective assuming a 50% productivity loss.
Similar content being viewed by others
Introduction
The term inherited retinal dystrophies (IRD) relates to a group of diseases caused by genetic defects that lead to blindness [1]. Retinitis pigmentosa (RP) is the most common IRD [2], while Leber congenital amaurosis (LCA) is another type of IRD which is characterised as one of the most severe IRD forms [1]. The occurrence of mutations in the retinal pigment epithelium-specific 65 kDa protein (RPE65) gene (henceforward, RPE65 mutations) within retinal dystrophies is rare by Swiss standards [3], and these mutations may cause RP or LCA. Before VN, there was so far no treatment available for patients diagnosed with RP or LCA caused by RPE65 mutations.
IRDs impose significant costs, particularly in relation to wellbeing costs, productivity costs and informal carer costs. In a recent study, total costs attributable to IRDs were estimated at GBP 523.3 million per year in the United Kingdom, with this estimate comprising both economic costs (GBP 327.2 million per year) and wellbeing costs (GBP 196.1 million per year) [4]. Individuals with RPE65-mediated IRDs are visually impaired at low levels of lighting from infancy and the majority become fully blind in adulthood [5].
Voretigene neparvovec (VN, Luxturna®), a gene therapy using an adeno-associated viral vector, aims to restore or maintain patients’ vision and delay progression by inserting a functioning copy of RPE65 into the cell [6]. VN is a one-time subretinal injection per each eye. It has demonstrated long-term improvements in visual function (i.e. visual acuity, visual field, full-field light sensitivity threshold) and functional vision (the ability to navigate in daily life) [7, 8]. This was shown in an open-label, randomised, controlled phase 3 trial of 31 patients performed at two sites in the USA, with an intervention arm in which 20 patients received bilateral VN treatment and 1 patient withdrew after randomisation, and a control arm consisting of 9 patients who participated and 1 patient who withdrew after randomisation (Study 301) [7,8,9]. After one year, the 9 participating patients in the control group were eligible to receive VN treatment (Study 302) [10]. The treatment regimen of VN for one eye begins with a course of oral prednisone, followed by an outpatient subretinal VN injection, followed by another course of oral prednisone; the process is then repeated for the other eye.
Because patients with biallelic RPE65-mediated IRD and with sufficient viable cells for VN treatment is rare (there are only approximately 13 patients in Switzerland currently eligible for VN treatment [11,12,13,14,15,16]), the absolute costs and benefits to society from VN treatment may be considered to be small, even though VN treatment is important to the individual. However, high-cost treatments are currently becoming available for various ultra-orphan diseases. Therefore, the overall economic impact of these treatments may be of interest to decision makers in the health care system. Comparisons with other treatments for an ultra-rare disease are rarely feasible. Additionally, cost-effectiveness analyses constitute an appropriate approach to put high one-time costs in relation to expected long-term benefits. If incremental cost-effectiveness ratios (ICERs) are too high for a certain treatment, money may be better put to use for treatments in another area of the health care system. By 2024, orphan drugs are forecast to represent 20.3% of worldwide prescription sales, and orphan drug sales are expected to grow at approximately double the rate foreseen for the non-orphan drugs market [17].
VN has been licensed by Swissmedic [18], by European Medicines Agency [19] and the US Food and Drug Administration [20]. Further, the National Institute for Health and Care Excellence (NICE) recommended VN for use in the National Health Service in England and Wales [21]. Its cost-effectiveness has not yet been assessed for Switzerland.
Objective
The objective of this study was to assess the cost-effectiveness of VN in the Swiss setting, from a healthcare system and a societal perspective. As IRD is considered a birth defect in Switzerland, the initial decision on the reimbursement of VN for patients below 20 years is expected to be taken by the Swiss Federal Social Insurance Office, not by the Swiss Federal Office of Public Health, which is responsible for the compulsory health insurance system.
Methods
The cost-effectiveness analysis for Switzerland was carried out by adapting (i.e. validating and re-parametrising) a cost-effectiveness model previously developed and implemented for the UK [22], to inform a Highly Specialised Technology Appraisal by NICE [23]. VN treatment was compared against the current standard of care (SoC). SoC involves patients not receiving VN treatment and instead receiving best supportive care (such as visual aids, technical aids, support and care in daily life). The primary health economic outcome was the incremental cost-effectiveness ratio (ICER), expressed as cost per quality-adjusted life year (QALY) gained. Additional outcomes were life years, blindness-free life years, and total and disaggregated costs. After the first year, costs and QALYs were discounted by 3% per year. The time horizon was 85 years to represent the entire lifetime of patients, and the cycle length was one year.
Model structure and population
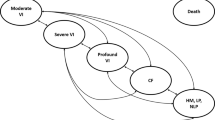
The model’s Markov structure considers five health states (HS) related to visual acuity (VA)/ visual field (VF), and a death state. The best vision-related health state patients could be in was 'moderate visual impairment' (i.e. VA>1 on LogMAR (Logarithm of the Minimum Angle of Resolution) scale as the average of both eyes and/or VF>240 degrees as the average of both eyes) and the worst was 'hand motion, light perception or no light perception' (i.e. VA≥3 on LogMAR scale as the average of both eyes, or indications of hand motion, light perception or no light perception across both eyes). Criteria used for categorising patients into a health state were derived from American Medical Association guidelines and are provided in appendix Table 1. Transitions between health states are presented in Fig. 1. During each model cycle, patients were simulated to either remain in the same health state, transition to a better (only possible in the first year) or worse vision-related health state, or die.
Markov model structure, taken from National Institute of Health and Care Excellence report [23]. KEY: CF, count fingers; HM, hand motion; LP, light perception; NLP, no light perception; VA, visual acuity; VF, visual field; VI, visual impairment
The population of interest were IRD patients with a biallelic RPE65-mutation and sufficient viable retinal cells. Patient characteristics were assumed to reflect those of the Study 301/302 (see below) trial population [8], with a mean age of 15.1 years at baseline, 23% of patients in HS1, 32% in HS2, 23% in HS3, 19% in HS4 and 3% in HS5.
Clinical effectiveness parameters
The clinical effectiveness of VN treatment was based on Study 301/302 trial data (ClinicalTrials.gov: NCT00999609) [7, 8, 10]. Study 301/302 is the only Phase 3 randomised controlled trial of VN for IRD patients with a biallelic RPE65-mutation. The study found a statistically significant improvement in multi-luminance mobility testing (MLMT) performance (primary outcome) at 1-year follow-up (MLMT improvement=1·6; 95% CI: 0·72 to 2·41). The MLMT measures functional vision, by assessing the ability of an individual to navigate an obstacle course accurately and at a reasonable pace at different levels of illumination.
The Markov model consisted of two phases to encompass the lifetime horizon of the analysis: an initial phase for the first year, and a long-term phase for the subsequent 84 years. Transition probabilities for the initial phase were calculated by assessing VA/VF outcomes in the first year of the Study 301/302 trial, with a further assumption made for patients in HS5 at baseline (Table 1). This assumption was made because no Study 301/302 patients in the VN or SoC strategies who were in HS5 at baseline were observed at 12 months (due to participant withdrawal). Therefore, it was assumed that for the initial phase, transition probabilities for HS5 patients at baseline would move in the same direction and same number of steps as the HS4 patients in each treatment strategy (e.g. as 100% of SoC patients in HS4 at baseline were in HS3 at 12 months, it was assumed 100% of SoC patients in HS5 at baseline were in HS4 at 12 months).
After year 1, for VN patients the VN treatment effect was assumed to be retained for the next 39 years. This meant that apart from the VN patients projected to transition to death, for 39 years VN patients were assumed to remain in the same health state that they were in at the end of year 1. A treatment effect duration of 40 years was assumed as it represents the midpoint between a minimum of 7.5 years (based on available Phase 1 trial follow-up data for VN [24]), and a maximum of 70 years (assuming the treatment effect lasts for the entire lifetime of patients [23]). 40 years was accepted to be the most plausible treatment effect duration by the NICE committee for the UK VN submission [23]. Transition probabilities representing visual decline during the long-term phase were based on a Weibull survival regression derived from a retrospective dataset of 70 IRD patients with RPE65-mutation (Appendix Table 2) [25, 26]. The Weibull model was chosen as it exhibited best statistical fit (assessed by Akaike and Bayesian information criterions) out of the different distributions tested. The Weibull regression was applied after the 1st year of the model for SoC patients and applied after the 40th year for VN patients. The multistate Weibull regression model used provides estimates of the transition intensities (which are converted to transition probabilities) at each cycle in the economic model. This effectively means that in each cycle a different transition probability matrix is used. The transition probabilities are therefore time-dependent, however this ‘time’ is time from baseline (and not time since entering a health state). The assumption made is the classical Markovian assumption of memorylessness and therefore this does not require knowledge of time spent in each health states. All transition data was pooled to inform a single multistate survival model which allowed the calculation of transition probabilities in each cycle based on time from the start of the model. An overview of the use of multi-state models for the analysis of time-to-event data is provided by Meira-Machado et al [27]. A reference transition from HS1 to HS2 was used, and hazard ratios representing other transitions (e.g. from HS1 to HS3) applied to calculate the probability of making each transition. Mortality was modelled based on Swiss lifetables [28].
Utility inputs
Health state utilities were derived from a study where EQ-5D-5L estimates for IRD patients with biallelic RPE65-mutation were elicited from clinicians (it was not considered feasible to collect EQ-5D-5L data directly from a representative sample of patients due to the rare nature of the disease) (Table 2) [5]. Utility decrements resulting from the adverse events in Study 301/302 were included for the adverse events which occurred in at least 2 patients, could be attributed to VN treatment/administration procedure, and are associated with a utility reduction [22]. These adverse events were cataracts, eye inflammation, and increased intraocular pressure (IOP), and were incorporated into the analysis in the form of a one-off QALY loss for VN patients (Table 3). The proportion of VN patients affected by these adverse events were taken from Study 301/302. The utility decrements of cataracts and eye inflammation and assumed duration of these adverse events, were obtained from a NICE report estimating these decrements for age-related macular degeneration patients [29]. Due to the absence of a study estimating the utility decrement of increased IOP, it was assumed to be the same as the utility decrement of uncontrolled/severe glaucoma which was obtained from a study by Pershing et al [30]. Duration of increased IOP was assumed to be one month as all increased IOP events in study 301/302 were fully resolved by 1 month.
Costs
In the base-case analysis, for the VN strategy health-care costs included eligibility testing, VN acquisition, administration of VN and treatment of adverse. For both treatment strategies, costs included those generated due to impaired vision, in the form of technical assistance including vision aids, community and residential care services for visually impaired patients over 65 years of age. All resource use parameters were taken from published studies, and – if required – verified to be applicable to Switzerland by Swiss clinical experts (Table 4). Unit costs for outpatient and inpatient services in Switzerland were derived from national sources [28, 31,32,33,34,35,36,37] (Table 4). The planned public price of VN (Swiss francs (CHF) 759'968) was used in the analysis. In a complementary analysis, a societal perspective was adopted. In this, indirect costs in the form of productivity losses from impaired vision, in the workplace for patients and caregivers, as well as increased education costs for patients aged below 18 years were additionally included. These were estimated from Swiss national statistical data [31, 35, 36].
In order to assess the robustness of the results, a univariate sensitivity analysis and a probabilistic sensitivity analysis (PSA) of the base case results for the healthcare system perspective were performed in addition to a range of scenario analyses.
Sensitivity and scenario analyses
In the univariate sensitivity analyses, parameters were varied by their 95% confidence limits if available, and otherwise by ±20%. The following groups of parameters were included: Weibull regression parameters representing visual decline in the long-term phase, parameters representing health state utilities or adverse event related utility decrements, parameters representing duration and probability of AEs, resource use parameters, and cost parameters (excluding the acquisition cost of VN, which was treated as fixed). A Tornado diagram was produced; the presentation of results were restricted to the 10 most influential parameters.
A probabilistic sensitivity analysis using 10’000 iterations was performed. Normal distributions were used for the Weibull regression parameters representing visual decline in the long-term phase. Beta distributions were used for parameters representing probabilities and absolute utilities. Gamma distributions were used for parameters representing utility decrements, resource use and costs.
In addition, several scenario analyses were conducted. The univariate sensitivity analysis did not cover the transitions between health states occurring in the first year of the model [7, 8]. To account for uncertainty in treatment effectiveness, the assumption on the duration of the VN treatment effect was varied. In the base-case analysis the treatment effect was assumed to last for 40 years; in scenario analyses alternative durations of 7.5 years (which is the currently available Phase 1 trial follow-up data for VN), 20 years and life-long have been adopted. In another set of scenario analyses, alternative distributions have been used to model the visual decline of IRD patients with RPE65 mutation during the long-term phase of the model. Also, alternative health state utilities (Table 2) were implemented in a scenario analysis; using Health Utilities Index Mark 3 (HUI3) estimates obtained from the same clinicians from which EQ-5D-5L estimates were obtained for the base-case [5]. In another scenario, health states were defined according to visual function only (in the base-case, health states were defined according to the worst of visual acuity or visual function). In further scenarios, alternative discount rates of 0% to 5% were applied. In another scenario analysis, we assigned health states according to the best-seeing eye, rather than the average eye. In an additional scenario analysis, for health state transitions with no data (from HS5), we assume patients remain in the same health state instead of assuming the movement is the same as the previous state.
We carried out two complementary analyses adopting a societal perspective. In the first, we included productivity losses of patients, by assuming patients of working age with severe visual impairment are 80% less productive than those without. This assumption was made based on a study from India [45]. In the second, we more conservatively assumed patients are 50% less productive as estimated from a Royal National Institute of Blind People (RNIB) study in the UK [23].
The original model that was adapted, was validated using standard procedures including tests to check model outputs were logical, and cell-by-cell checks of consistency and logic [23]. The model was implemented in Microsoft Excel 2010.
Results
From the Swiss healthcare system perspective, the implementation of VN resulted in discounted strategy costs per patient of CHF 901’654 versus CHF 137’252 in the SoC strategy. The resulting incremental costs were CHF 764’402 per patient. Discounted QALYs per patient were 18.35 in the VN strategy and 11.62 in the SoC strategy, resulting in an increment of 6.73 QALYs per patient. This resulted in an ICER of CHF 113’526 per QALY gained for the VN strategy versus the SoC strategy (Table 5)
From the societal perspective, the VN and SoC strategies cost CHF 1’213’557 and CHF 977’006, respectively, yielding an increment of CHF 236’551 per patient. The ICER for the societal perspective was CHF 35’132 per QALY gained.
In the univariate sensitivity analysis for the healthcare system perspective, variation of the constant and ancillary parameters of the regression-based Weibull distribution reflecting the natural history model of long-term disease progression were the most influential (Fig. 2).
At an assumed cost-effectiveness threshold of CHF 100,000 per QALY gained, the PSA-based probability of being cost-effective was 41%, for the healthcare system perspective (Figs. 3 and 4).
Results from analyses from a societal perspective and scenario analyses are presented in Table 6. The analyses from a societal perspective resulted in ICERs of CHF 35’132 per QALY gained when assuming an 80% productivity loss, and CHF 62’947 per QALY gained when assuming a 50% productivity loss. Assuming a duration of the treatment effect of VN of 7.5 years, 20 years or life-long, compared to 40 years in the base case, resulted in ICERs of CHF 275`213, CHF 156'171 and CHF 96'384 per QALY gained, respectively. Applying a discount rate of 0% generated an ICER of CHF 39'767 per QALY gained, and a discount rate of 5% generated an ICER of CHF 193'548 per QALY gained.
In the base case, health state membership was assigned based on the worst of VA/VF in the patient, using the average of both eyes. When health state membership was alternatively assigned using VF only, the ICER increased to CHF 157'157 per QALY gained. Using alternative distributional assumptions for modelling visual decline in the long-term did not change the base case ICER substantially. Use of alternative health state utility values estimated using the HUI3 instead of the EQ-5D-5L resulted in an ICER of CHF 104,413 per QALY gained.
Discussion
The cost-effectiveness analysis of VN, a novel treatment for IRD patients with biallelic RPE65-mutation, resulted in an ICER of CHF 113’526 per QALY gained from the healthcare system perspective. Calculating costs from a societal perspective changed the cost-effectiveness substantially, mainly due to the avoidance of loss of patient productivity during adulthood. When assuming that the productivity of a person with at least severe visual impairment is 20% or 50% of that of a person without or with moderate visual impairment, the resulting ICERs were CHF 35’132 and 62’947 per QALY gained respectively. There are approximately 13 patients in Switzerland currently eligible for VN treatment, and the net undiscounted incremental cost to the Swiss health care system of treating these 13 patients over their lifetime is estimated to be CHF 9,654,567 (mean undiscounted incremental costs from this study multiplied by 13 patients).
In terms of cost-effectiveness thresholds adopted in Switzerland, for some interventions a threshold of CHF 100,000 per QALY, from the perspective of the statutory health insurance, has been tentatively assumed in previous analyses [47,48,49]. However, for ultra-orphan diseases, it may be appropriate to consider a higher threshold; for instance NICE in the United Kingdom recognises thresholds ranging from GBP 100,000 to 300,000 (CHF 122,000 to 366,000) depending on the size of treatment effect, for ultra-orphan diseases [50]. These thresholds are much higher than the GBP 20,000 to 30,000 threshold range that NICE typically adopts for diseases which are not very rare.
These results were affected by a substantial degree of uncertainty. This is unavoidable given small patient numbers, the rareness of the disease, and still limited observation periods. In terms of costs, the treatment costs of VN and, from the societal perspective, the avoided loss of patient productivity, were the dominant drivers of the difference between the VN and SoC strategies. The impact of all other cost elements was relatively marginal. In terms of effectiveness, the key assumption underlying the base case ICER result from the healthcare system perspective, and also of the ICER from the societal perspective, was the assumption of a duration of VN treatment effect of 40 years. The uncertainty around the long-term effect of the treatment is a limitation of this analysis. If the duration of the treatment effect was substantially shorter (20 years), the ICER would increase substantially (e.g. to CHF 156,171 per QALY gained from the healthcare system perspective). On the other hand, if the durability of the treatment is life-long, the ICER would decrease substantially. In a phase 1 trial of VN in 12 patients who were followed up for 7.5 years after VN treatment, improvements in visual function were observed to be sustained throughout the period [24]. Another limitation of this study is that due to the wide scope of costs generated from visual impairment not all have been captured; for instance the cost of treating depression resulting from blindness or visual impairment. In the scenario analyses for the healthcare perspective, influential parameters included the alternative assumptions on utility estimates and choice of distributional assumption for the estimation of transition probabilities representing natural history of disease in the Markov part of the cost-effectiveness model. The Weibull distribution assumed in the base case generated more favourable results than other assumptions, but was also statistically most plausible [25].
A further limitation was that age-dependent utility was not accounted for. Reliance on international parameters for estimation of productivity losses in patients of working age with severe visual impairment [23, 45], was another limitation of this analysis, but was necessary due to an absence of Swiss sources, and that no better sources were identified. It may be considered that the UK study provides a better approximation of the productivity losses in Switzerland compared with the Indian study, as the UK study is more recent and the UK has a more similar economic and social structure to Switzerland. Working age was assumed to begin at age 18 in the UK study, and at age 15 in the Indian study. We would propose undertaking a future study in Switzerland (e.g. survey-based) to provide more recent and relevant data for Switzerland on productivity losses in patients resulting from blindness/visual impairment.
Wide variation in the size of estimated VN treatment effect was observed in cost-effectiveness analyses published in other countries. These estimates range from 2.7 discounted incremental QALYs in a US Institute for Clinical and Economic Review report (leading to an ICER of 287’915 United States Dollars per QALY gained) [6], 4.0 discounted incremental QALYs in the preferred analysis of the evidence review group for the NICE VN assessment for the UK (leading to an ICER of Great British Pounds 155’750 per QALY gained) [23], 6.4 discounted incremental QALYs in the UK economic evaluation based on the same model as the present analysis (leading to an ICER of Great British Pounds 95’072 per QALY gained) [22], to 9.5 discounted incremental QALYs in an US economic evaluation published by Johnson et al (leading to an ICER of 79’618 United States Dollars per QALY gained from a health care perspective) [51]. The estimated discounted incremental QALYs in analyses performed for Canada [52], Denmark [53], Germany [54], and the Netherlands [55], also fall within this range.
There were several methodological differences between these studies, including the discount rates used and the assumed duration of VN treatment effect [56]. Importantly, compared with this study which assumed a duration of VN treatment effect of 40 years, in the Institute for Clinical and Economic Review report, a maximum duration of treatment effect of 20 years was assumed [6] and a lifelong duration of treatment effect was assumed by Johnson et al [51]. A 40 year duration of VN treatment effect was assumed by the evidence review group for the NICE VN assessment.
Conclusion
Assuming a public price of VN of CHF 759’968, the incremental cost-effectiveness ratio of the VN strategy compared to the SoC strategy is estimated to be CHF 113’526 per QALY gained from a Swiss healthcare system perspective. Furthermore, ICERs of CHF 35'132 and 62'947 per QALY gained were estimated from the societal perspective assuming a 80% and 50% productivity loss from visual impairment in working-age patients, respectively. There is substantial uncertainty present in these estimates, most importantly driven by the clinical assumptions related to the lasting effects of treatment. This analysis aims to provide information to Swiss decision makers on whether or not VN is cost-effective at a public price of CHF 759'968.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to copyright, but are available from the corresponding author on reasonable request. Acceptance of the request is subject to approval from Novartis Pharma AG.
References
Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A. 2013;110(6):E517–25.
Verbakel SK, van Huet RAC, Boon CJF, den Hollander AI, Collin RWJ, Klaver CCW, et al. Non-syndromic retinitis pigmentosa. Prog Retin Eye Res. 2018;66:157–86.
Tiwari A, Bahr A, Bähr L, Fleischhauer J, Zinkernagel MS, Winkler N, et al. Next generation sequencing based identification of disease-associated mutations in Swiss patients with retinal dystrophies. Sci Rep. 2016;6:28755.
Economics DA. The socioeconomic impact of Inherited Retinal Dystrophies (IRDs) in the United Kingdom. Retina Int. 2019. https://www2.deloitte.com/au/en/pages/economics/articles/socioeconomic-impact-inherited-retinaldystrophies.html
Lloyd A, Piglowska N, Ciulla T, Pitluck S, Johnson S, Buessing M, et al. Estimation of impact of RPE65-mediated inherited retinal disease on quality of life and the potential benefits of gene therapy. Br J Ophthalmol. 2019;103(11):1610–4.
Institute for Clinical and Economic Review. Voretigene Neparvovec for Biallelic RPE65-mediated retinal disease: effectiveness and value. 2018.
Spark Therapeutics. Clinical Study Report (Study 301): A safety and efficacy study in subjects with Leber Congenital Amaurosis (LCA) using adeno-associated viral vector to deliver the gene for human RPE65 to the Retinal Pigment Epithelium (RPE). [Internal document]. 2017.
Russell S, Bennett J, Wellman JA, Chung DC, Yu Z-F, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65 -mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–60.
MiraldiUtz V, Coussa RG, Antaki F, Traboulsi EI. Gene therapy for RPE65-related retinal disease. Ophthalmic Genet. 2018;39(6):671–7.
Spark Therapeutics. Study 302. Data on file. [Internal document]. 2018.
Ammann F, Klein D, Franceschetti A. Genetic and epidemiological investigations on pigmentary degeneration of the retina and allied disorders in Switzerland. J Neurol Sci. 1965;2(2):183–96.
Bertelsen M, Jensen H, Bregnhøj JF, Rosenberg T. Prevalence of generalized retinal dystrophy in Denmark. Ophthalmic Epidemiol. 2014;21(4):217–23.
Bocquet B, Marzouka NA, Hebrard M, Manes G, Sénéchal A, Meunier I, et al. Homozygosity mapping in autosomal recessive retinitis pigmentosa families detects novel mutations. Mol Vis. 2013;19:2487–500.
Henderson RH, Waseem N, Searle R, van der Spuy J, Russell-Eggitt I, Bhattacharya SS, et al. An assessment of the apex microarray technology in genotyping patients with Leber congenital amaurosis and early-onset severe retinal dystrophy. Invest Ophthalmol Vis Sci. 2007;48(12):5684–9.
Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc Natl Acad Sci U S A. 1998;95(6):3088–93.
Stone EM. Leber congenital amaurosis - a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144(6):791–811.
EvaluatePharma. Orphan Drug Report 2019. https://www.evaluate.com/sites/default/files/media/download-files/EvaluatePharma_Orphan_Drug_Report_2019.pdf 2019.
Swissmedic Fachinformation. Luxturna 2020 [Available from: www.swissmedicinfo.ch.
European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/luxturna. Accessed 2019-11-10. 2018.
Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/luxturna. Accessed 2019-11-10. 2017.
National Institute for Health and Care Excellence. Voretigene neparvovec for treating inherited retinal dystrophies caused by RPE65 gene mutations. https://www.nice.org.uk/news/article/nice-recommends-novel-gene-therapy-treatment-for-rare-inherited-eye-disorder. Accessed 2019-11-10. 2019.
Viriato D, Bennett N, Sidhu R, Hancock E, Lomax H, Trueman D, et al. An Economic Evaluation of Voretigene Neparvovec for the Treatment of Biallelic RPE65-Mediated Inherited Retinal Dystrophies in the UK. Adv Ther. 2020;37(3):1233–47.
National Institute for Health and Care Excellence. Voretigene neparvovec for treating inherited retinal dystrophies caused by RPE65 gene mutations. Committee papers. https://www.nice.org.uk/guidance/hst11/evidence/committee-papers-pdf-6908685661. Accessed 2019-11-10. 2019.
Chung DC, Lee K, Reape KZ, High KA, Lacey S, Viriato D. Long-term effect of Voretigene Neparvovec on the full-field light sensitivity threshold test of patients with RPE65 mutation-associated inherited retinal dystrophy-post Hoc analysis of phase I trial data. Invest Ophthalmol Visual Sci. 2019;60(9):3398.
Novartis. Voretigene neparvovec in the treatment of RPE65 mediated IRD, Cost-effectiveness model report. Version 12.0. Date of preparation: 20th March 2019. [Internal document]. 2019.
Spark Therapeutics. Natural history of individuals with retinal degeneration due to autosomal recessive mutations in the RPE65 gene. [Internal document]. 2017.
Meira-Machado L, de Uña-Alvarez J, Cadarso-Suárez C, Andersen PK. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res. 2009;18(2):195–222.
Swiss Federal Statistical Office. Mortality, causes of death. https://www.bfs.admin.ch/bfs/en/home/statistics/health/state-health/mortality-causes-death.html. Accessed 2019-09-07. 2019 [
National Institute for Health and Care Excellence. Macular degeneration. Appendix J: Health Econ. 2018. https://www.nice.org.uk/guidance/ng82/evidence/appendix-j-health-economics-pdf-170036251093.
Pershing S, Enns EA, Matesic B, Owens DK, Goldhaber-Fiebert JD. Cost-effectiveness of treatment of diabetic macular edema. Ann Intern Med. 2014;160(1):18–29.
Swiss Federal Statistical Office. Costs of education. https://www.bfs.admin.ch/bfs/de/home/statistiken/bildung-wissenschaft/bildungsindikatoren/themen/ressourcen-betreuung/bildungsausgaben-kopf.assetdetail.7046156.html. Accessed 2019-10-12 2018 [
Swiss Federal Office of Public Health. Analysis list. https://www.bag.admin.ch/bag/de/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/Analysenliste.html. Accessed 2019-10-12 2019
Swiss Federal Social Insurance Office. Personal communication. 2019.
Swiss Federal Statistical Office. Statistik diagnosebezogener Fallkosten, Personen ab 65 Jahre. https://www.bfs.admin.ch/bfsstatic/dam/assets/2422598/master. Accessed 2019-11-05. 2014
Swiss Federal Statistical Office. Inflation rates. https://www.bfs.admin.ch/bfs/de/home/statistiken/preise/landesindex-konsumentenpreise/lik-resultate.assetdetail.10527650.html. Accessed 2019-11-05. 2018
Swiss Federal Statistical Office. Residential care. https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitswesen/alters-pflegeheime.html. Accessed 2019-11-05 2019
SwissDRG AG. https://www.swissdrg.org/de. Accessed 2019-10-03
FMH Swiss Medical Association. TARMED. https://www.fmh.ch/themen/ambulante-tarife/tarmed.cfm. Accessed 2019-10-16 2018
Crewe JM, Spilsbury K, Morlet N, Morgan WH, Mukhtar A, Clark A, et al. Health service use and mortality of the elderly blind. Ophthalmology. 2015;122(11):2344–50.
Lafuma A, Brezin A, Fagnani F, Mimaud V, Mesbah M, Berdeaux G. Nonmedical economic consequences attributable to visual impairment: a nation-wide approach in France. Eur J Health Econ. 2006;7(3):158–64.
Meads C, Hyde C. What is the cost of blindness? Br J Ophthalmol. 2003;87(10):1201–4.
Schweizerischer Zentralverein für das Blindenwesen. Sehbehinderung und Blindheit: Entwicklungin der Schweiz. https://www.szb.ch/fileadmin/user_upload/szb-factsheet_sehbehinderung_und_blindheit_entwicklung_in_der_schweiz_2012.pdf. Accessed 2019-06-30. 2012.
Schweizerischer Zentralverein für das Blindenwesen. Sehen und hören in Spitex- und Heimpflege. https://www.szb.ch/fileadmin/pdfs/forschung/SZB_2017_-_Sehen_und_horen_in_Spitex-_und_Heimpflege_bar.frei_o.QV.pdf. Accessed 2019-06-30. 2017.
Wittenborn JS, Zhang X, Feagan CW, Crouse WL, Shrestha S, Kemper AR, et al. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology. 2013;120(9):1728–35.
Shamanna BR, Dandona L, Rao GN. Economic burden of blindness in India. Indian J Ophthalmol. 1998;46(3):169–72.
Schmier JK, Halpern MT, Covert D, Delgado J, Sharma S. Impact of visual impairment on use of caregiving by individuals with age-related macular degeneration. Retina (Philadelphia, Pa). 2006;26(9):1056–62.
Bhadhuri A, Insinga R, Guggisberg P, Panje C, Schwenkglenks M. Cost effectiveness of pembrolizumab vs chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in Switzerland. Swiss Med Wkly. 2019;149:w20170.
Panje CM, Lupatsch JE, Barbier M, Pardo E, Lorez M, Dedes KJ, et al. A cost-effectiveness analysis of consolidation immunotherapy with durvalumab in stage III NSCLC responding to definitive radiochemotherapy in Switzerland. Ann Oncol. 2020;31(4):501–6.
Pavic M, Pfeil AM, Szucs TD. Estimating the potential annual welfare impact of innovative drugs in use in Switzerland. Front Public Health. 2014;2:48.
National Institute for Health and Care Excellence. NICE health technology evaluations: the manual. https://www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741. Accessed 16 Jan 2022. 2017.
Johnson S, Buessing M, O'Connell T, Pitluck S, Ciulla TA. Cost-effectiveness of Voretigene Neparvovec-rzyl vs Standard Care for RPE65-Mediated Inherited Retinal Disease. JAMA Ophthalmol. 2019;137(10):1115-1123. https://doi.org/10.1001/jamaophthalmol.2019.2512.
CADTH Common Drug Reviews. Pharmacoeconomic report: Voretigene Neparvovec (Luxturna): (Novartis Pharmaceuticals Canada Inc): indication: vision loss, inherited retinal dystrophy. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2021.
Morgan A, Zheng S. Cost-Utility Analysis of Luxturna versus the Standard of Care Treatment from a Narrow Danish Societal Perspective (thesis). 2020.
Uhrmann MF, Lorenz B, Gissel C. Cost Effectiveness of Voretigene Neparvovec for RPE65-Mediated Inherited Retinal Degeneration in Germany. Transl Vis Sci Technol. 2020;9(9):17.
Zorginstituut Nederland. Farmacotherapeutisch rapport voretigene neparvovec (Luxturna®) bij de behandeling van visusverlies door erfelijke retinale dystrofie met bi-allelische RPE65-mutaties. 2020.
Huygens SA, Versteegh MM, Vegter S, Schouten LJ, Kanters TA. Methodological Challenges in the Economic Evaluation of a Gene Therapy for RPE65-Mediated Inherited Retinal Disease: The Value of Vision. Pharmacoeconomics. 2021;39(4):383–97.
Acknowledgements
Input on Swiss-specific aspects was provided by two experts in the area of ophthalmology, rare retinal disorders and genetics, Prof Dr Pascal Escher, Department of Ophthalmology, Inselspital, Bern, and Prof Dr Hendrik Scholl, Institute of Molecular and Clinical Ophthalmology, Basel. We thank both experts for their valuable input. We also thank the Swiss Federal Social Insurance Office for providing cost data. These parties are not in any way responsible for the content of this article. The responsibility for the content is with the authors.
Funding
This study was funded by Novartis Pharma Schweiz AG. Funding of the University of Basel authors was through a research grant to the employment institution, i.e. no personal funding.
Author information
Authors and Affiliations
Contributions
AB, MS, CSS, MG and DD undertook validation and re-parametrisation of the global model. AB, MS, CSS analysed the version re-parameterised for Switzerland. All authors contributed to writing of the manuscript and interpretation of findings. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
CJ is an employee of Novartis Pharma Schweiz AG. JB is an employee of Novartis Pharma AG. MS receives grants, via employment institution, from Novartis. CSS has received funding via contracted consulting services from Vifor Pharma, Mitsubishi Tanabe Pharma and F. Hoffman La-Roche. Rest of the authors have no Conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Appendix table 1. Description of VA/VF-based health states. Appendix table 2. Multistate model parameters based on different distributions, used to calculate transition probabilities between health states (HSs) for the long-term phase.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bhadhuri, A., Dröschel, D., Guldimann, M. et al. Cost-effectiveness of voretigene neparvovec in the treatment of patients with inherited retinal disease with RPE65 mutation in Switzerland. BMC Health Serv Res 22, 837 (2022). https://doi.org/10.1186/s12913-022-08211-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-022-08211-y