Abstract
Background
Back pain is an extensive burden to our healthcare system, yet few studies have explored modifiable prognostic factors associated with high costs related to healthcare utilization, especially among older back pain patients. The aims of this study were to identify modifiable prognostic factors for high costs related to healthcare utilization among older people seeking primary care with a new episode of back pain; and to replicate the identified associations in a similar cohort, in a different country.
Methods
Data from two cohort studies within the BACE consortium were used, including 452 and 675 people aged ≥55 years seeking primary care with a new episode of back pain. High costs were defined as costs in the top 25th percentile. Healthcare utilization was self-reported, aggregated for one-year of follow-up and included: primary care consultations, medications, examinations, hospitalization, rehabilitation stay and operations. Costs were estimated based on unit costs collected from national pricelists. Nine potential modifiable prognostic factors were selected based on previous literature. Univariable and multivariable binary logistic regression models were used to identify and replicate associations (crude and adjusted for selected covariates) between each modifiable prognostic factor and high costs related to healthcare utilization.
Results
Four modifiable prognostic factors associated with high costs related to healthcare utilization were identified and replicated: a higher degree of pain severity, disability, depression, and a lower degree of physical health-related quality of life. Kinesiophobia and recovery expectations showed no prognostic value. There were inconsistent results across the two cohorts with regards to comorbidity, radiating pain below the knee and mental health-related quality of life.
Conclusion
The factors identified in this study may be future targets for intervention with the potential to reduce high costs related to healthcare utilization among older back pain patients.
Trial registration
ClinicalTrials.gov NCT04261309, 07 February 2020. Retrospectively registered.
Similar content being viewed by others
Background
The burden of back pain has been growing along with an increasing and ageing population [1,2,3,4,5]. Back pain is the number one cause of disability globally [4] and an extensive burden to our healthcare systems [2, 6,7,8]. According to a recent systematic review, the prevalence rate of healthcare utilization for back pain ranges from 28 to 92% [9]. Back pain is one of the most prevalent complaints encountered in primary care [4, 5, 8, 10].
To improve use of scarce healthcare resources and reduce the burden on our healthcare systems, where possible and appropriate, researchers have highlighted the importance of monitoring and understanding healthcare utilization and related costs [3, 11]. It is well known that most of healthcare utilization and related costs stems from a relatively small group of back pain patients [12], and more importantly, that many of these patients receive unnecessary and ineffective treatment [3, 7]. This suggests that care for this high-cost subgroup requires quality improvement and cost reduction. An important next step towards this would be to identify modifiable prognostic factors associated with high costs related to healthcare utilization, and to replicate initial findings to evaluate the consistency of prognostic value across datasets and settings [13]. Information about such factors could inform development of effective strategies and/or interventions, or new applications of existing interventions.
Only a few prospective studies have explored modifiable prognostic factors associated with high costs related to healthcare utilization among patients with back pain [14,15,16,17], and no such study has been conducted among a sample of exclusively older people with back pain. Patients with high costs related to healthcare utilization are a diverse population [18, 19], and generalization of results cannot be done automatically from younger to older people with back pain [20]. With an ageing population, and the expected rise in older people requiring back care in the years to come [21], it is important to study modifiable prognostic factors of high costs related to healthcare utilization among older people with back pain.
Therefore, the aims of this study were 1) to identify modifiable prognostic factors for high costs related to healthcare utilization among older people seeking primary care with a new episode of back pain and 2) to replicate the identified associations in a similar cohort, in a different country.
Method
This study was designed and performed in accordance with the PROGnosis RESearch Strategy (PROGRESS) framework [22], with aims consistent with prognostic factor research: identification of prognostic factors, including external replication. In line with recommendations from the PROGRESS framework [13] a study protocol (ClinicalTrials.gov NCT04261309, 07 February 2020) including a statistical analysis plan has been published [23], and the REMARK reporting guidelines were followed [24].
Design and setting
This study was carried out in two steps. First, modifiable prognostic factors were identified in the Back Complaints in the Elderly - Norway study (BACE-N), a prospective observational cohort study within Norwegian primary care [25]. Next, a replication analysis was conducted in the Back Complaints in the Elders study (BACE-D), a prospective observational cohort study within Dutch primary care [26]. BACE-N has been classified as a quality assessment study by the Norwegian Regional Committee for medical Research Ethics (ref no. 2014/1634/REK vest) and approved by the Norwegian Social Science Data Service (ref no. 42149). Likewise, the BACE-D study protocol (NL24829.078.08) has been approved by the Medical Ethics Committee of the Erasmus Medical Center, the Netherlands. BACE-N and BACE-D are part of the international BACE consortium [26].
Participants and recruitment procedure
Eligible participants within BACE-N were people ≥55 years of age seeking primary care (physiotherapist, chiropractor, or General Practitioner (GP)) with a new episode of back pain (preceded by 6 months without visiting primary care for similar complaints). Eligible participants within BACE-D were people > 55 years of age seeking primary care (GP) with a new episode of back pain (preceded by 6 months without visiting a GP for similar complaints). Patients were excluded from both studies if they had difficulties completing the questionnaires due to language barriers, or if they had difficulties completing the physical examination (e.g. are wheelchair bound). Patients within BACE-N were recruited by 110 physiotherapists, chiropractors and GPs in urban and rural parts of Norway between April 2015 and February 2020. Patients within BACE-D were recruited by 49 GPs in and around Rotterdam between March 2009 and September 2011. All included patients signed an informed consent form before study enrolment.
Data collection, outcome, modifiable prognostic factors, and covariates
At baseline all patients responded to a comprehensive questionnaire and went through a standardized physical examination. Follow-up questionnaires were sent at 3, 6, and 12 months after inclusion within BACE-N and at 3, 6, 9 and 12 months after inclusion within BACE-D. All questionnaires were preferably completed electronically, but paper versions were available for patients not familiar with electronic data collection. Within this study, only data from questionnaires were used.
Outcome
The outcome of this study was costs related to healthcare utilization aggregated over one-year of follow up and dichotomized as high and low. Having high costs related to healthcare utilization was defined as patients with costs in the top 25th percentile [15, 16].
Healthcare utilization within BACE-N and BACE-D were self-reported and included: consultation to healthcare professionals (type and frequency), use of back medication (prescription and over-the-counter, type and frequency), number of diagnostic examinations (type and frequency), number of days of hospitalization and/or rehabilitation stay (within BACE-N) and back operations. Within BACE-N, consultations to healthcare professionals and use of back medication were reported with a 3-months recall period at each timepoint of follow-up. Number of diagnostic examinations and days of hospitalization and/or rehabilitation stay were reported with a 3-months recall period at 3- and 6-months follow-up, and a 6-months recall period at 12-months follow-up. Back operations were reported with a 12-months recall period at 12-months follow-up. Within BACE-D, all variables, except back operations, were reported with a 3-months recall period at each timepoint of follow-up. Back operations were reported with a 12-months recall period at 12-months follow-up. Total costs of healthcare utilization per patient were estimated by multiplying frequency of use by unit costs collected from national pricelists (see Table 1).
Modifiable prognostic factors
Potential modifiable prognostic factors were factors expected to have the potential to be modified or improved by appropriate care or treatment, and therefore classified as modifiable. Potential modifiable prognostic factors of high costs related to healthcare utilization were based on previous scientific literature on (primarily) middle-aged back pain patients as well as patients with musculoskeletal disorders, and included the following self-reported variables measured at baseline:
-
Pain severity [14,15,16, 28,29,30] measured by a Numeric Rating Scale (NRS) (range 0-10, higher score indicating higher pain severity) [31].
-
Disability [14,15,16, 28, 29, 32] measured by the Roland-Morris Disability questionnaire (RMDQ) (range 0-24, higher score indicating higher degree of back-related disability) [33].
-
Health-related quality of life [14, 30] measured by the Short-Form Health Survey 36-item (SF36) physical and mental summary score (range 0-100, higher score indicating better health-related quality of life) [34].
-
Emotional well-being [15, 16, 19, 32, 35] measured by the Center for Epidemiological Studies-Depression questionnaire (CES-D) (range 0-60, higher score indicating more signs of depression) [36].
-
Kinesiophobia [15, 35] measured by the Fear Avoidance Beliefs Questionnaire - Physical Activity subscale (FABQ-PA) (range 0-24, higher score indicating higher levels of kinesiophobia) [37].
-
Comorbidity [17, 30] measured by the Self-Administered Comorbidity Questionnaire (SCQ) (range 0-15, thirteen pre-defined comorbidities and two optional comorbidities. Item no. 12 (back pain) was replaced with a third optional comorbidity) [38].
-
Radiating pain below the knee [15] measured by the question “did your back pain radiate to your legs last week? If yes, how far down did the pain radiate?” and categorized into yes/no.
-
Expectations of recovery from back pain within the next 3 months measured with a five-point scale and categorized into “recovered”, “much better” or “no change or worse”.
Covariates
Prognostic factor research may vary depending on context (time, place, healthcare setting) and characteristics of the study population. We therefore adjusted for potential covariates when evaluating the modifiable prognostic factors. Potential covariates were based on previous scientific literature on (primarily) middle-aged back pain patients as well as patients with musculoskeletal disorders, and included the following self-reported variables measured at baseline:
-
Education level [32, 41] measured as the highest education completed and categorised into low (elementary and high school level) or high (university level).
-
Employment status measured by the question “do you have a paying job?” and categorized into yes/no.
-
Pain duration [16] measured by the question “how many days have you had your current back pain?” and categorized into < 6 weeks, 6 weeks to 3 months or > 3 months.
-
Pain history [29] measured by the question “have you had back pain before?” and categorized into yes/no.
-
First healthcare provider [42] (physiotherapist, chiropractor, or GP).
-
Total costs related to healthcare utilization during a period of 6 (BACE-N) or 12 (BACE-D) weeks prior to inclusion. Healthcare utilization prior to inclusion was self-reported and included: primary care consultations, use of back medication and number of diagnostic examinations. Total cost of healthcare utilization was estimated by multiplying frequency of use by unit costs collected from national pricelists (see Table 1).
Analyses
All analyses are outlined in the statistical analysis plan published a priori [25] and preformed using the IBM SPSS version 26 (IBM Corporation, Armonk, NY, USA). We considered our study as explanatory. Thus, no correction for multiple testing was performed and p-values < 0.05 were considered statistically significant. All statistical tests were two-sided.
Study flow
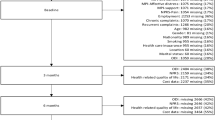
The flow of patients through the studies were reported with a flow chart according to the REMARK guidelines [24]. Reasons for dropout were provided where known. Dropouts at 12-months follow-up were removed from the analyses. Differences in baseline characteristics between responders and non-responders at 12-months follow-up were evaluated.
Missing data
Whitin BACE-N, missing value pattern was visually explored, and missingness at random was assumed. Also, we found evidence against the hypothesis that values were not missing completely at random (Little’s test, p > 0.05). Missing baseline data was handled by multiple imputation. Five multiple imputation datasets with 10 iterations were created using regression estimation. We did not impute missing outcome values, as the imputation model had poor predictive performance and caused a clear trend of values being overestimated. Instead, missing values on variables used to estimate the outcome score were filled in with; 1) each patient’s individual average of observed values for the variables: consultations to healthcare professionals and medication use, 2) a value of zero costs for the variables: diagnostic examinations, hospitalization, rehabilitation stay and back operations. Within BACE-D, missing value pattern was visually explored, and missingness at random was assumed. Missing values on variables used to estimate the outcome score were filled in with; 1) each patient’s individual average of observed values for the variables: consultations to healthcare professionals and medication use, 2) a value of zero costs for the variables: diagnostic examinations and back operations.
Healthcare utilization and cost estimation
Type and frequency of use of different healthcare resources were calculated for each of the follow-up periods. Costs of healthcare utilization per patient were estimated by multiplying frequency of use by unit prices collected from national pricelists (see Table 1). Costs related to back medication were estimated based on medication type and frequency of use (data on dosage were not available). All costs were presented in Euros (€) for 2020 and estimated for the entire follow-up period with both mean and median values with 95% CI, using bias-corrected and accelerated (BCa) bootstrapping. The BCa was conducted with a bootstrap sample size of 1000. Cost data are commonly skewed thus both mean and median values were presented to inform interpretation. Norwegian prices were recalculated to Euros using the exchange rate from the National Bank of Norway from February 2020 (1€ = NOK 10).
Identification analysis
Univariable and multivariable binary logistic regression models were used to investigate associations (crude and adjusted for selected covariates) between each predefined modifiable prognostic factor and costs related to healthcare utilization (within BACE-N). The cost score was entered into the model as a dependent dichotomous variable (high costs defined as patients with cost in the top 25th percentile, yes/no). Linearity of continuous independent variables were examined using Box-Tidwell transformations [43]. Independent variables that demonstrated a non-linear relationship with the dependent variable where categorized. The results were presented as crude and adjusted odds ratios (OR) with 95% CI.
Replication analysis
Univariable and multivariable binary logistic regression models were used, as described above, to replicate findings from the identification analysis within BACE-D. The results were presented as crude and adjusted OR with 95% CI. The decision on whether findings were replicated were based on the direction and magnitude of the association, and the size of the CI for each of the predefined modifiable prognostic factors [44].
Sensitivity analysis
To assess credibility of the identification analysis and possible bias introduced by the imputation procedure, the univariable and multivariable logistic regression analyses were performed on complete case data (within BACE-N).
Sample size
This study contains secondary analyses embedded in the BACE-N and BACE-D. Details of the sample size calculation related to the original aims of the cohorts are provided in the BACE-N and BACE-D protocols [25, 26]. To determine statistical power for this particular study, we used number of events per variable (EPV) [45,46,47,48,49] and the rule-of-thumb of “10 events per 1 analysed variable” [50,51,52,53]. With a sample size of 450 participants within BACE-N, we anticipated 112 participants to be in the top 25th percentile of costs and categorized as having high costs (yes/no) (events). An EPV of 10 would allow a maximum of 11 prognostic variables to be included in the final multivariable prediction model. With a sample size of 675 participants in BACE-D, we anticipated 168 participants to be in the top 25th percentile of costs and defined as having high costs (yes/no) (events). An EPV of 10 would allow a maximum of 16 prognostic variables to be included in the final multiple prediction model.
Results
A total of 452 (BACE-N) and 675 (BACE-D) patients were included in the identification and the replication sample, respectively. Table 2 shows patient characteristics and clinical status at baseline, along with the proportion of missing data per variable. Flow of patients through the studies are shown in Fig. 1. Fourteen patients (3%) in BACE-N and 22 patients (3%) in BACE-D were dropouts at 12-months follow-up. We removed these cases from the analyses. Within BACE-N, there was a larger proportion of females (55 vs. 42%) among the responders as compared to non-responders. Within BACE-D, there was a larger proportion of people not in paid work (26 vs. 38%) and people with short pain duration < 6 weeks (56 vs. 39%) among the responders as compared to non-responders. Otherwise, there were no differences between responders and non-responders in the two cohorts. The BACE-N and BACE-D samples were also largely comparable, although there were some differences that might have impacted healthcare utilization. BACE-N had a larger proportion of people with high education level (44 vs. 17%), people in paid work (47 vs. 27%), and people with short pain duration < 6 weeks (67 vs. 54%).
Within BACE-N, missing data ranged from 0.0 to 16.8% for included baseline variables and 18.4 to 26.0% for included follow-up variables. Total missingness was 4.9 and 23.3% for all baseline and follow-up values, respectively. Variables on medication use at 12-months follow-up had most missing values. Within BACE-D, missing data ranged from 0.0 to 11.8% for included baseline variables and 7.3 to 18.1% for included follow-up variables. Total missingness was 2.2 and 9.6% for all baseline and follow-up values, respectively. Variables on examination at 12-months follow-up had most missing values.
Healthcare utilization and cost estimation
Table A1 and A2 in the Additional file 1 and Additional file 2 shows healthcare utilization throughout one-year of follow-up for the BACE-N and BACE-D sample, respectively. Costs related to healthcare utilization aggregated for the one-year of follow-up are shown in Table 3. Within BACE-N, 87% of all patients used healthcare during the one-year of follow-up, and a total of 110 patients (25%) were defined as having high costs (≥ € 789). Within BACE-D, 78% of all patients used healthcare during the one-year of follow-up, and a total of 163 patients (25%) were defined as having high costs (≥ € 664).
Identification analysis
All continuous independent variables, aside from the two SF-36 variables, demonstrated a linear relationship with the dependent variable. Table 4 shows crude and adjusted OR with 95% CI for the association between each of the modifiable prognostic factor and being in the high costs group. All analyses showed a statistical significant crude association between the factors and the outcome. After adjustment for covariates, only the following factors remained significantly associated with the outcome: pain severity, disability, depression, comorbidity, radiating pain below the knee, and physical and mental health-related quality-of-life.
The sensitivity analysis (Table A3 in the Additional file 3) showed no substantial change in point estimates when comparing complete case analysis to the main analysis. There were some minor changes in p-values for the two SF-36 variables: In the complete case analysis of crude associations, the SF-36 mental second percentile group were not significantly associated with the outcome, and in the complete case analysis of adjusted associations, the SF-36 physical and mental second percentile groups were not significantly associated with the outcome.
Replication analysis
Table 4 also shows results of the replication analysis. Except for the SF-36 mental second percentile group, findings were replicated with respect to the direction of the association between each of the factors and the outcome. Though, the magnitude of the association varied > 20% for the following factors: comorbidity, radiating pain below the knee, and physical and mental health-related quality-of-life.
In both the identification and replication analysis, after adjustment for selected covariates and with the “low cost group” as the reference, factors associated with increased odds of being in the high costs group were a higher degree of pain severity, disability and depression, and a lower degree of physical health-related quality of life. No association was found between being in the high costs group and the degree of kinesiophobia or expectations of recovery.
Discussion
The present study identified and replicated associations between modifiable prognostic factors and high costs related to healthcare utilization among older people seeking primary care with a new episode of back pain. Four modifiable prognostic factors associated with high costs related to healthcare utilization were identified and replicated in a similar cohort, in a different country, reflecting slightly different sociodemographic characteristics and healthcare setting: pain severity, disability, depression and physical health-related quality of life. Kinesiophobia and expectations of recovery showed no prognostic value. There were inconsistent results across the two cohorts with regards to comorbidity, radiating pain below the knee and mental health-related quality of life.
To the best of our knowledge, no similar study has been conducted among a sample of exclusively older people with back pain. Thus, direct comparability of this study with other studies is limited. Nevertheless, our findings are generally in accordance with previous research on (primarily) middle-aged back pain patients [14,15,16, 28, 29, 32, 35], as well as patients with musculoskeletal disorders [30, 54, 55]. For example, pain severity, disability and depression have been shown to be significantly associated with high costs related to healthcare utilization in studies on patients with back pain [14,15,16, 28, 29, 32, 35] and musculoskeletal disorders [30, 54, 55]. Physical health-related quality of life has also previously been reported to be a prognostic factor of high societal costs among back pain patients [14], and high costs related to healthcare utilization among patients with musculoskeletal disorders [30]. Our findings regarding kinesiophobia and radiating pain below the knee are also in line with a previous study [15], which showed that these factors were of minor importance when predicting future costs related to healthcare utilization among back pain patients. Our finding regarding mental health-related quality of life is, however, contrary to a study on patients with musculoskeletal disorders [30], which found that this factor was associated with persistent high costs related to healthcare utilization. Our finding regarding comorbidity is also contrary to previous research. In a recent systematic review, comorbidity was pointed out as a consistent prognostic factor of high costs related to healthcare utilization in general [18], and similar conclusions have been drawn in single studies among patients with back pain [17] and musculoskeletal disorders [55]. This discrepancy might be explained by the fact that we included costs related to back pain specific healthcare utilization, whereas other studies have included healthcare costs related to all musculoskeletal disorders [17, 55] and healthcare costs in general [18]. To the best of our knowledge, the prognostic value of recovery expectations for high costs related to healthcare utilization has not been reported previously.
The main limitation of this study is missing data on variables used to estimate the outcome score, thus we had to manually replace missing values. It is well-known that healthcare utilization is prone to missing data [56,57,58]. Also, that missing data should be replaced in order to make use of all reported data [56, 57]. Unfortunately, due to poor predictive performance, multiple imputation could not be used on follow-up data in this study. We therefore chose a frequently used, though not optimal, method for replacing missing values [58] and have tried to be transparent in our reporting. A second potential limitation is that we used self-reported data on healthcare utilization. Self-reports tend to underestimate the true value of healthcare utilization due to potential recall bias [59,60,61,62]. Nevertheless, we consider the impact of recall bias to be of only minor importance in this study as the outcome variable was dichotomized into high or low costs. In future studies, the limitations of missing data and recall bias could to some extent be overcome by including registry data on healthcare utilization. A third potential limitation is that costs related to hospitalization and rehabilitation stays were not measured in BACE-D. Thus, the risk of misclassification bias related to whether patients were classified as having high or low costs might be present in BACE-D. However, if costs related to hospitalization and rehabilitation stays were removed from the cost calculations in BACE-N, only 4 patients (< 1%) switched cost group. A fourth potential limitation is that we could not adjust for possibly important covariates of healthcare utilization, such as the patient’s disposition to access and pay for healthcare services, and health insurance status. According to the Behavioral Model of Health Services Use from Andersen [63], healthcare utilization is a function of people’s predisposition to use services, factors which enable or impede use and need for care. Certainly, including these enabling factors is recommended. However, it is likely to assume that these factors are of less importance in countries such as Norway and the Netherlands, where health services are largely available and covered by the public sector. A fifth limitation is the lack of data on eligible participants that declined to participate or for other reasons were not invited. Due to limited resources and practical reasons related to recruitment from a broad network of clinicians, it was not possible to record information on all eligible participants during the BACE-N and BACE-D data collection period. Thus, the risk of selection bias is present. To compensate for this limitation and assess representativeness of the BACE-N sample, key sociodemographic variables have been compared with a large population study on older people; The Norwegian study on life course, ageing and generation (NORLAG) [64, 65]. A subsample of the NORLAG (NORLAG MSK) was used, which is expected to be a representative sample of people aged ≥55 years with musculoskeletal complaints. Characteristics of the two samples were largely comparable, though BACE-N had more men, and more with higher education level [65]. Previous studies have shown that women [14, 28, 29] are more likely to seek care for their back pain as are people with lower education level [28, 32, 41]. Hence, it is likely to assume that the amount of healthcare utilization presented in BACE-N is somewhat underestimated. Furthermore, the BACE-N sample is largely comparable to younger Norwegian back pain cohorts [66, 67] and to the BACE-D sample [26].
The main strength of the present study is that it was conducted in line with the PROGRESS framework including an identification and replication phase [13], pre-planned with a published statistical analysis plan, and reported in line with the REMARK guidelines [24]. Also, that it estimates the prognostic value of modifiable prognostic factors over and beyond a core set of non-modifiable covariates. Prognostic factor studies are an essential step towards quality improvement of clinical practice [13]. Results from such studies have the potential to inform development of effective strategies and/or interventions. Identifying modifiable prognostic factors of high costs related to healthcare utilization among older people is an important step towards addressing the global burden of back pain and decrease waste of valuable healthcare resources [3, 7, 68].
Conclusion
In conclusion, this study identified and replicated four modifiable prognostic factors associated with high costs related to healthcare utilization among older people seeking primary care with a new episode of back pain: pain severity, disability, depression, and physical health-related quality of life. This study contributes to the on-going research into clinical pathways and has the potential to identify future target areas for intervention with the potential to reduce high costs related to healthcare utilization among older back pain patients. Due to differences in healthcare systems between countries, readers are advised to exercise caution with generalizability of the results to other healthcare systems.
Availability of data and materials
Data supporting findings of this study are not public available as participants have consented for their data to be available only to the researcher of this study. However, data are available from the corresponding author upon reasonable request and with permission of the Oslo Metropolitan University and the Erasmus Medical Center (contact through the corresponding author).
Abbreviations
- BCa:
-
Bias-corrected and accelerated
- BACE-D:
-
Back Complaints in the Elders study
- BACE-N:
-
Back Complaints in the Elderly - Norway study
- CES-D:
-
Center for Epidemiological Studies-Depression questionnaire
- CI:
-
Confidence Interval
- EPV:
-
Events per variable
- FABQ-PA:
-
Fear Avoidance Beliefs Questionnaire - Physical Activity subscale
- GP:
-
General Practitioner
- NOK:
-
Norwegian krone
- NORLAG:
-
Norwegian study on life course, ageing and generation
- NRS:
-
Numeric Rating Scale
- OR:
-
Odds ratio
- PROGRESS:
-
PROGnosis RESearch Strategy
- RMDQ:
-
Roland-Morris Disability Questionnaire
- REMARK:
-
REporting recommendations for tumor MARKer prognostic studies
- SCQ:
-
Self-Administered Comorbidity Questionnaire
- SF36:
-
Short-Form Health Survey 36-item
References
Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96.
Hartvigsen J, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–67.
Buchbinder R, et al. Low back pain: a call for action. Lancet. 2018;391(10137):2384–8.
Diseasse GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602.
Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys. Spine (Phila Pa 1976), 2006. 2002;31(23):2724–7.
van Tulder M, Koes B, Bombardier C. Low back pain. Best Pract Res Clin Rheumatol. 2002;16(5):761–75.
Traeger AC, et al. Care for low back pain: can health systems deliver? Bull World Health Organ. 2019;97(6):423–33.
Kinge JM, et al. Musculoskeletal disorders in Norway: prevalence of chronicity and use of primary and specialist health care services. BMC Musculoskelet Disord. 2015;16(1):1–9.
Beyera GK, O'Brien J, Campbell S. Health-care utilisation for low back pain: a systematic review and meta-analysis of population-based observational studies. Rheumatol Int. 2019;39(10):1663–79.
Licciardone JC. The epidemiology and medical management of low back pain during ambulatory medical care visits in the United States. Osteopath Med Prim Care. 2008;2:11.
Foster NE, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018.
Vlaeyen JWS, et al. Low back pain. Nat Rev Dis Primers. 2018;4(1):52.
Riley RD, et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS Med. 2013;10(2):e1001380.
Mutubuki EN, et al. Predictive factors of high societal costs among chronic low back pain patients. Eur J Pain. 2019.
Becker A, et al. Low back pain in primary care: costs of care and prediction of future health care utilization. Spine (Phila Pa 1976). 2010;35(18):1714–20.
Engel CC, von Korff M, Katon WJ. Back pain in primary care: predictors of high health-care costs. Pain. 1996;65(2-3):197–204.
Ritzwoller DP, et al. The association of comorbidities, utilization and costs for patients identified with low back pain. BMC Musculoskelet Disord. 2006;7:72.
Wammes JJG, et al. Systematic review of high-cost patients' characteristics and healthcare utilisation. BMJ Open. 2018;8(9).
Stewart WF, et al. Patterns of health care utilization for low back pain. J Pain Res. 2015;8:523–35.
Paeck T, et al. Are older adults missing from low back pain clinical trials? A systematic review and meta-analysis. Arthritis Care Res. 2014;66(8):1220–6.
Joud A, Petersson IF, Englund M. Low back pain: epidemiology of consultations. Arthritis Care Res. 2012;64(7):1084–8.
Hemingway H, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ. 2013;346:e5595.
Killingmo, R.M., et al., Statistical analysis plan (SAP) for: Modifiable prognostic factors of high cost related to healthcare utilization among older people seeking primary care with a new episode of back pain - an identification and replication study. 2021, ClinicalTrials.gov: https://clinicaltrials.gov/ProvidedDocs/09/NCT04261309/SAP_002.pdf.
McShane LM, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93(4):387–91.
Grotle, M., BACk Pain in Elders in Norway (BACE-N). 2020, ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04261309.
Scheele J, et al. Back complaints in the elders (BACE); design of cohort studies in primary care: an international consortium. BMC Musculoskelet Disord. 2011;12:193.
Kanters TA, et al. Update of the Dutch manual for costing studies in health care. PLoS One. 2017;12(11):e0187477.
Wenig CM, et al. Costs of back pain in Germany. Eur J Pain. 2009;13(3):280–6.
Ferreira M, et al. Factors defining care-seeking in low back pain - A meta-analysis of population based surveys. Eur J Pain. 2010;14(7):747.e1–7.
Lentz TA, et al. Factors associated with persistently high-cost health care utilization for musculoskeletal pain. PLoS One. 2019;14(11):e0225125.
Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine (Phila Pa 1976). 2000;25(24):3140–51.
Lim KL, Jacobs P, Klarenbach S. A population-based analysis of healthcare utilization of persons with back disorders: results from the Canadian Community Health Survey 2000-2001. Spine (Phila Pa 1976). 2006;31(2):212–8.
Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine (Phila Pa 1976). 1983;8(2):141–4.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83.
Keeley P, et al. Psychosocial predictors of health-related quality of life and health service utilisation in people with chronic low back pain. Pain. 2008;135(1-2):142–50.
Radloff LS. The CES-D scale: a Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1(3):385–55.
Waddell G, et al. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–68.
Sangha O, et al. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–63.
Rattay P, et al. Utilization of outpatient and inpatient health services in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2013;56(5-6):832–44.
Chechulin Y, et al. Predicting patients with high risk of becoming high-cost healthcare users in Ontario (Canada). Healthc Policy. 2014;9(3):68–79.
Hoebel J, et al. Socioeconomic Status and Use of Outpatient Medical Care: The Case of Germany. PLoS One. 2016;11(5):e0155982.
Fritz JM, Kim J, Dorius J. Importance of the type of provider seen to begin health care for a new episode low back pain: associations with future utilization and costs. J Eval Clin Pract. 2016;22(2):247–52.
Box GE, Tidwell PW. Transformation of the independent variables. Technometrics. 1962;4:531–50.
Kent P, et al. A conceptual framework for prognostic research. BMC Med Res Methodol. 2020;20(1):172.
Harrell FE Jr, et al. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3(2):143–52.
Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87.
Steyerberg EW, et al. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Mak. 2001;21(1):45–56.
Steyerberg EW, et al. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19(8):1059–79.
Ambler G, Brady AR, Royston P. Simplifying a prognostic model: a simulation study based on clinical data. Stat Med. 2002;21(24):3803–22.
Moons KG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10):e1001744.
Pavlou M, et al. Review and evaluation of penalised regression methods for risk prediction in low-dimensional data with few events. Stat Med. 2016;35(7):1159–77.
Cowley LE, et al. Methodological standards for the development and evaluation of clinical prediction rules: a review of the literature. Diagn Progn Res. 2019;3:16.
Pavlou M, et al. How to develop a more accurate risk prediction model when there are few events. BMJ. 2015;351:h3868.
Budtz CR, Mose S, Christiansen DH. Socio-demographic, clinical and psychological predictors of healthcare utilization among patients with musculoskeletal disorders: a prospective cohort study. BMC Health Serv Res. 2020;20(1):239.
Lentz TA, Beneciuk JM, George SZ. Prediction of healthcare utilization following an episode of physical therapy for musculoskeletal pain. BMC Health Serv Res. 2018;18.
Franklin M, et al. An Educational Review About Using Cost Data for the Purpose of Cost-Effectiveness Analysis. Pharmacoeconomics. 2019;37(5):631–43.
Leurent B, Gomes M, Carpenter JR. Missing data in trial-based cost-effectiveness analysis: An incomplete journey. Health Econ. 2018;27(6):1024–40.
Briggs A, et al. Missing… presumed at random: cost-analysis of incomplete data. Health Econ. 2003;12(5):377–92.
Petrou S, et al. The accuracy of self-reported healthcare resource utilization in health economic studies. Int J Technol Assess Health Care. 2002;18(3):705–10.
Icks A, et al. Agreement found between self-reported and health insurance data on physician visits comparing different recall lengths. J Clin Epidemiol. 2017;82:167–72.
Short ME, et al. How accurate are self-reports? Analysis of self-reported health care utilization and absence when compared with administrative data. J Occup Environ Med. 2009;51(7):786–96.
Hunger M, et al. Official statistics and claims data records indicate non-response and recall bias within survey-based estimates of health care utilization in the older population. BMC Health Serv Res. 2013;13:1.
Andersen RM. National health surveys and the behavioral model of health services use. Med Care. 2008;46(7):647–53.
Slagsvold B, Veenstra M, SO D, Hagestad G, Hansen T, Herlofson K, et al. Life-course, ageing and generations in Norway:the NorLAG study. Norsk Epidemiologi. 2012;22(2).
Vigdal ON, et al. Characteristics of older adults with back pain associated with choice of first primary care provider: a cross-sectional analysis from the BACE-N cohort study. BMJ Open. 2021;11(9):e053229.
Grotle M, et al. Prognostic factors in first-time care seekers due to acute low back pain. Eur J Pain. 2007;11(3):290–8.
Nordstoga AL, et al. Improvement in Work Ability, Psychological Distress and Pain Sites in Relation to Low Back Pain Prognosis A Longitudinal Observational Study in Primary Care. Spine. 2019;44(7):E423–9.
Buchbinder R, et al. The Lancet Series call to action to reduce low value care for low back pain: an update. Pain. 2020;161(Suppl 1):S57–64.
Acknowledgements
We would like to thank all study participants, participating physiotherapists, chiropractors and GPs and their assistants for their contribution to the study. We thank the primary care providers assisting with the clinical examinations and providing baseline questionnaires to study participants. We thank the BACE-N scientific board, and the BACE Consortium.
Funding
This study was supported by the Oslo Metropolitan University, the Norwegian Fund for Post-Graduate Training in Physiotherapy (grant number 90749) and “Et liv i bevegelse” (A life in movement) - Norwegian chiropractors’ research foundation. Funding organisations had no role in the design of the study or collection, analysis, and interpretation of the data or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
RMK originated the idea. RMK, DAW, KS, BWK and MG designed the study. MG and KS contributed to the funding of the study. RMK, ZZK and ØNV collected data for the study. RMK and AC analysed the data. RMK, AC, DAW, KS, SMABZ, MCS, ZZK, BWK and MG contributed to the interpretation of data. RMK drafted the manuscript with all authors contributing in reading, commenting and approving the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The BACE-N study was classified as a quality assessment study by the Norwegian Regional Committee for Medical Research Ethics (ref no. 2014/1634/REK vest) and approved by the Norwegian Social Science Data Service (ref no. 42149). The BACE-D study was approved by the Medical Ethics Committee of the Erasmus Medical Center, the Netherlands (NL24829.078.08). This study was performed in accordance with the PROGRESS framework and the Declaration of Helsinki. All participants provided written informed consent to participate in the studies.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Killingmo, R.M., Chiarotto, A., van der Windt, D.A. et al. Modifiable prognostic factors of high costs related to healthcare utilization among older people seeking primary care due to back pain: an identification and replication study. BMC Health Serv Res 22, 793 (2022). https://doi.org/10.1186/s12913-022-08180-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-022-08180-2