Abstract
Background
The lack of medication standards is a serious problem in paediatrics mainly because of age-related differences in organ development and physiological functions in children. Consequently, dosage measurement becomes inaccurate. For this reason, methods for evaluating and monitoring rational paediatric medications should be developed. Drug use indicators, such as those similar to the drug utilisation index (DUI) based on the Anatomical Therapeutic Chemical/Defined Daily Dose (DDD) and widely used for the assessment of appropriate dosage in adults, should be explored in terms of their applicability to children.
Methods
A total of 5,538 prescriptions of antibiotics selected from a general teaching hospital were included. Drug, dose, frequency and treatment duration were obtained from each prescription. The prescription daily dose (PDD) of each antibiotic drug was calculated as the average of the daily doses. Underdose and overdose were determined in terms of the PDD/DDD ratio for each prescription. Children’s DUI (cDUI) was explored in terms of the appropriate dosage for children as follows: the meaning of children’s DDD (cDDD) and the evaluation of paediatric drug dosage.
Results
The top five antibiotics and their utilisation rates were as follows: cefmetazole sodium injection (18.47 %), erythromycin lactobionate injection (15.07 %), amoxicillin/clavulanate potassium injection (10.72 %), ceftriaxone sodium injection (9.50 %) and azithromycin dry suspension (8.02 %). The ratio of cDUI and PDD/cDDD was mostly not close to 1.
Conclusions
The establishment of a cDUI system is an effective means of paediatric dosage evaluation. In addition to DDDs, cDUI and PDD/cDDD should be used to analyse the utilisation of antibiotics in children.
Similar content being viewed by others
Background
The irrational use of antibiotics is a public health problem and has an impact on people’s health and economic development [1,2,3]. In the 1990 s, many countries, such as the United States, launched antimicrobial stewardship programmes [4, 5] to promote the rational use of antibiotics.
Having a low immune function, children are prone to infectious diseases. As such, they have emerged as one of the groups that widely use antibiotics, and their medication safety has remarkably concerned the society. Paediatric dosage cannot be directly normalised on the basis of adult dosage [6] because the exposure of antibiotics largely differs between paediatric patients and adults [7].
In contrast to adults who are mature in all aspects, in a healthy paediatric population, age directly affects the development status and physiological functions of organs; consequently, paediatric drug use cannot be accurately measured, off-label drug use behaviour [8, 9] is inevitable, and a series of drug safety problems in serious cases occurs [10]. The medication error rate without injury amongst paediatric patients is three times higher than that amongst adults [11], and the medication error rates of causing injury and death in the former are 31 and 13 % higher than those in the latter [12]. Therefore, evaluating the rational use of antibiotics in paediatrics is a difficult problem. The index of drug intensity based on a limited daily dose is recommended by the World Health Organisation (WHO) and the health administration department of China. However, the limited daily dose has restrictions in the children’s population. For this reason, studies should explore how to scientifically and accurately understand the current situation of the use of antibiotics in children and reasonably evaluate the clinical use of antibiotics.
This study analysed the utilisation of antibiotics in paediatric inpatients in the comprehensive teaching hospital of Anhui Medical University as an example and the indicators of paediatric drug utilisation index (DUI) and prescription daily dose (PDD) in the clinical comprehensive evaluation of paediatric antibiotics to protect children’s health rights and interests, avoid the risk of drug use and improve the rationality and safety of antibiotic use in paediatrics. This study also aimed to establish a methodology for the evaluation of antimicrobial agents in children and provide a reference for rational clinical drug use.
Data and methods
Sources
In this study, the comprehensive teaching hospital of Anhui Medical University was selected as the sample point by convenience sampling to collect all the prescriptions of antibiotic treatment for children hospitalised from 1 to 2019 to 31 May 2020 via the hospital information system. The inclusion criteria were the prescriptions for patients aged 0–14 years [13,14,15] and the exclusion criteria were the prescriptions with missing information or obviously wrong data. Inconsistencies with the complete prescription definition in the Prescription Management Measures and inaccuracies in the writing of the data on review by the pharmacist are considered to be data errors or omissions [16].
Drug use research
To compare drug use at a general level, the WHO has adopted Anatomical Therapeutic Chemical (ATC) and defined daily dose (DDD) system [17] referred to as the ATC/DDD system. DDD is defined as the average daily dose of adults used for therapeutic purposes [18]. The main indicators commonly used in drug utilisation research and rational drug use evaluation includes the following based on the DDD method: DDDs, DUI and PDD [18,19,20]. DUI represents the trend of drug use and can be used as a criterion to judge whether clinical drug use is reasonable. PDD is the actual daily dose of the drug at the time of prescription. The combination of DUI and PDD was helpful to explain the specific details of drug utilization.
The WHO and related studies have concluded that prescription drug use is basically reasonable when DUI is close to 1. Considering the influence of other factors during the course of medication, this study regarded DUI as close to 1 when it was between 0.9 and 1.1 [21,22,23]. DUI > 1.1 indicates that the actual daily dose of a given drug exceeds DDD and implies drug abuse. Conversely, DUI < 0.9 denotes that the actual daily dose of this drug is lower than DDD and may affect the therapeutic effect because of the excessively low dose. Of course, DUI is mainly used in clinical practice to monitor drug utilization trends, and a more accurate dose needs to be determined by clinicians and pharmacists after diagnosis and discussion.
Paediatric patients were the main object of this study. Indicators such as children’s defined daily dose (cDDD) and children’s DUI (cDUI) were used to distinguish adult dose and calculated using the same formula described above. The dosage of paediatric drugs is directly related to age, so the children were divided into five age groups in accordance with the New Edition of Pharmacology (17th edition) and the child growth standards of the WHO [14, 22]. Each age group had the corresponding cDDD combined with the actual prescription situation of hospitals: newborns (1–28 days): cDDD = 1/10 − 1/8DDD; infants (28 days-1 year): cDDD = 1/8 − 1/4 DDD; toddlers (1–3 years): CDDD = 1/4 − 1/3DDD; preschool (4–5 years): cDDD = 1/3 − 1/2DDD; school age (6–14 years): cDDD = 1/2–2/3DDD [14].
In New Edition of Pharmacology, a body surface area method is introduced to calculate dose, but the exact dose for clinical use needs to be determined by clinician’s diagnosis and clinical pharmacist’s evaluation. The calculation of body weight and gestational age are also introduced in this book, but these are factors that have been comprehensively considered by clinical pharmacists in the process of prescription review and this study is not authorized to further review, so we temporarily do not calculate these two factors.
Rationality evaluation
In accordance with Chinese Pharmacopoeia (2015 Edition), Guidelines for Clinical Application of Antimicrobial Agents (2015 Edition), China National Formulary [24, 25] and package inserts, the rationality evaluation standard of paediatric antibacterial drug prescription was established, whether the use of antibacterial drugs met the relevant specifications was evaluated, and the medication characteristics of irrational prescriptions were analysed. A prescription is considered reasonable when it meets all the criteria. The rationality evaluation standard of paediatric antibacterial drug prescription was as follows: 1.there should be obvious symptoms; 2.the appropriate drug should be selected according to according to the symptoms; 3.the dosage should be adjusted according to different ages; 4.the dosage form and route should be selected according to the drug and patient compliance; 5.there should be no incompatibility or adverse reactions(Due to the route of administration, dosage form and dosage and other reasons).
Statistical analysis
The prescriptions were sorted out in Excel 2010 software. Factors related to patient age, drug class, infection type, dosage form, specification, price and time of administration were included, and the baseline data table of patients was drawn for the corresponding calculation. Statistical analysis was conducted in SPSS 23.0 software. Differences were considered clinically significant when P < 0.05.
Results
Basic information
After the data were screened, 5,538 prescriptions that met the inclusion and exclusion criteria were collected. After classification, the baseline data of the patients were obtained. The average age of the patients was 2.72 ± 3.35 years, the average hospitalisation time was 8.96 ± 9.60 days, and the average medication time was 2.97 ± 2.95 days (Table 1).
Types of infection
We double-checked the admission and discharge diagnoses of patients to confirm the type and number of infections in each patient, and counted them by person. Amongst the 5,538 prescriptions, 10 kinds of infections were involved, and some patients had multiple infections, with a total of 9,253 infections. Upper respiratory tract, lower respiratory tract and blood infections were the common types, accounting for 72.30 % (Table 1).
Rationality evaluation of initial prescription antibiotics
According to the Guidelines for Clinical Application of Antimicrobial Agents (2015 Edition) and New Edition of Pharmacology (17th edition), the rationality of the sample prescriptions was preliminarily evaluated by more than two pharmacists in charge. According to the Prescription Management Measures issued by the Health Commission, PRC, a three-level quality control mode for prescription comment was established. Level 1 quality control is completed by the dispensing pharmacist, who evaluates the legality and normative suitability of the prescription. Secondary quality control was completed by multiple clinical pharmacists. The prescriptions were evaluated by referring to prescription evaluation standards, and the results of primary quality control were verified. Clinical pharmacists are independent of each other and do not interfere with each other. Finally, the results are summarized. Third-level quality control is performed by the pharmacy department and the pharmaceutical board, mainly to review the prescriptions that have differences in the second-level quality control. Of the 5,538 prescriptions, 4,604 (83.13 %) were rational prescriptions, and 934 (16.87 %) were unreasonable prescriptions. The specific manifestations and composition of unreasonable prescriptions are shown in Table 1. Amongst the irrational prescriptions, the most common drug use without obvious indications accounted for 72.16 %(Table 1), which means that the use of antimicrobial drugs in the absence of clinical indications of infection or the scope of treatment of antimicrobial drugs inconsistent with clinical diagnosis.
Research on drug utilisation trend
Amongst the 5,538 prescriptions, 41 kinds of antibiotics, namely, 18 kinds of nonrestricted antibiotics, 13 kinds of restricted antibiotics and 10 kinds of special antibiotics, were involved. Some prescriptions were used in combination. A total of 15,119 patients were treated with antimicrobial agents. The top 20 antibacterial drug varieties in the prescription frequency of use and the corresponding DDD [26] and PDD are presented in Table 2.
Rationality evaluation of initial prescription antibiotics
Four factors, namely, patients’ gender, age, department and length of stay, were statistically analysed to determine the relationship between the patients’ basic information and the rationality of prescriptions (Table 3).
The chi-square test revealed that gender, age, department and length of stay significantly differed (P < 0.05). The rationality of prescription might be related to gender, age, department and length of stay. However, the correlation coefficient between gender and length of stay was less than 0.1, suggesting that they were not the main influencing factors. Therefore, this study focused on the use of antibiotics in children with different ages.
Drug utilisation analysis
In this study, the top five drugs were taken as an example to analyse the drug utilisation of children in different age groups. The main analysis indices were cDUI and PDD/cDDD [28] (PDD and cDDD are taken as the mean value in calculation). Similar to the criteria of cDUI, PDD/cDDD close to 1 indicated that the prescription dosage is basically appropriate, and the range from 0.9 to 1.1 could be regarded as close to 1. PDD/cDDD > 1.1 implied the possibility of excessive drug use. PDD/cDDD < 0.9 suggested an insufficient drug dosage.
Cefmetazole sodium for injection
Drug utilisation amongst children treated with cefmetazole sodium injection was analysed on the basis of different age groups (Table 4). In the 5 age groups, cDUI and PDD/cDDD were basically less than 0.9, which indicated that cefmetazole sodium injection was underdosed in different age groups of paediatric patients.
Erythromycin lactobionate for injection
Drug utilisation amongst children treated with erythromycin cefose injection according to different age groups (Table 4). Among the five age groups, the cDUI (taking the mean) and PDD/ CDDD of newborns were less than 1 and close to 1 in the other age groups. This result showed that the dosage of erythromycin cefaloctate injection was too low in newborns, but it was basically appropriate in other age groups.
Amoxicillin/clavulanate potassium for injection
The use of amoxicillin/potassium clavulanate injection was analysed on the basis of different age groups (Table 4). In the 5 age groups, the cDUI and PDD/cDDD of newborns and infants were less than 0.9, the cDUI and PDD/cDDD of infants were close to 1, and the cDUI and PDD/cDDD of preschool and school-aged children were more than 1.1. These results indicated that amoxicillin/potassium clavulanic injection was underused in newborns and infants, appropriately used in infants and overdosed in preschool and school-aged children.
Ceftriaxone Sodium for Injection
Drug utilization analysis was performed on children treated with ceftriaxone sodium for injection according to different age groups(Table 4). The cDUI and PDD/cDDD were all greater than 1.1, indicating the overdose of ceftriaxone sodium for injection in pediatric patients.
Azithromycin dry suspension
Drug utilisation amongst children treated with azithromycin dry suspension was analysed on the basis of different age groups (Table 4). Amongst the 5 age groups, cDUI and PDD/cDDD of newborns were close to 1, whereas the cDUI and PDD/cDDD of the 4 other groups were greater than 1.1. This result showed that azithromycin dry suspension was used appropriately in newborns. In other age groups, this drug was overdosed.
Clinical feasibility analysis
The dosages of different drugs used in the five age groups (based on the mean PDD) were compared via ANOVA(Table 5). The results showed that the doses significantly differed between the age group and the drug group (F1 = 10.419, P1 < 0.01; F2 = 9.319 and P2 < 0.01).
In Fig. 1, the drug utilisation trend of the five drugs in different age groups of paediatric patients was basically the same regardless of the analysis index used. Therefore, cDUI and PDD/cDDD could be preliminarily considered and used for the comprehensive evaluation of the clinical application of antibiotics in paediatrics. These indices also had clinical significance and feasibility for the analysis of paediatric drug utilisation.
Discussion
According to the WHO, DDD is the average daily dose for adult indications, so it cannot be directly used as the basis for prescribing children’s medication. In the absence of specific medication standards, some hospitals directly reduce the adult dose in half to obtain the children’s medication standard. However, this method has no scientific clinical basis, and it leads to the deviation of medication results. In the present study, cDDD, cDUI, PDD/cDDD and other indicators were introduced to analyse the drug use status amongst children by dividing them into different age groups and preliminarily establish a rational evaluation method of antimicrobial drug use in children.
Use of antibiotics
In this study, the irrational prescriptions of antibiotics for children in the sample hospitals accounted for 16.87 %, which indicated that irrational drug use in medical institutions is relatively common. This phenomenon is attributed to two possible reasons. From the perspective of prescribing physicians, physicians may not have a comprehensive understanding of antibacterial drug use, so they tend to ignore the principles of the clinical application of antibacterial drugs, or physicians use antibiotics randomly and do not choose broad-spectrum antibacterial drugs in strict accordance with indications. Doctors’ attitude and knowledge level of medication are important factors affecting prescription behaviour [29,30,31]. Therefore, medical institutions should actively implement training on the antimicrobial knowledge of paediatricians, improve the level of drug use and require prescription physicians and reviewers to supervise one another to enhance the quality of paediatric prescriptions. From the perspective of patients receiving drugs, children are a special group, whose body functions develop and change with age, and doctors experience difficulty in mastering the metabolism and reactions of drugs in children. Coupled with the lack of standards for direct use in paediatric patients, deriving paediatric medications from adult doses is difficult and risky. Paediatric medicine is full of uncertainty in terms of individual treatments and lack of experience and standards to refer to. Furthermore, pharmacokinetic studies on paediatric patients should be strengthened by combining with the physiological characteristics of patients of different ages, formulating administration plans and strictly controlling drug indications and dosages.
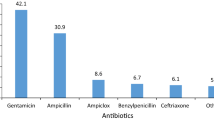
According to prescription analysis, the top five antibiotics commonly used in paediatrics are cefmetazole sodium injection, erythromycin lactate injection, amoxicillin/potassium clavulanate injection, ceftriaxone sodium injection and azithromycin dry suspension, accounting for 61.78 % of the total antibacterial drugs. This result confirmed that injection remains the most commonly used route of administration in paediatrics because of its rapid and reliable action and its advantages of systemic or local localisation. However, the compliance of injections is poor, and direct intravenous injection has a high risk, so the principles of injection administration must be strictly observed. The five commonly used drugs are used to treat the most common upper and lower respiratory tract infections and blood infections in patients, but they are also prone to adverse reactions. With the complex physiological conditions of children, paediatric clinicians should cautiously prescribe such medications.
Drug utilisation analysis
The indices of cefmetazole sodium injection and erythromycin lactobionate injection were less than or close to 1. The index of amoxicillin/clavulanate potassium injection was less than 1 in newborns and infants, close to 1 in infants and greater than 1 in preschool and school age. The index of ceftriaxone sodium injection and azithromycin dry suspension were basically greater than 1. This finding showed that doctors cautiously prescribed cefmetazole sodium injection at a small dosage. The use of erythromycin lactobionate injection was appropriate, and ceftriaxone sodium injection and azithromycin dry suspension were overdosed. Furthermore, the use of amoxicillin/clavulanate potassium injection fluctuated greatly, that is, it was increasingly administered with the age of patients increase the dosage. As for different ages, the utilisation index of 3 drugs in newborns was less than 1, and 2 drug use indices of less than 1 were observed in infants. The utilisation index of 2 drugs in toddlers was close to 1, the index of 3 drugs in preschool and school age was greater than 1. This finding indicated that physicians’ medication changed with patients’ age. In newborns and infants, medications were carefully used, and dosage was reduced. In toddlers, medications fluctuated reasonably. In preschool and school age of over 4 years, the overdose phenomenon appears. This observation might be due to the importance that doctors attach to children of different ages. In comparison with older children, children under 4 years of age have poor drug absorption and metabolism and have a higher probability of adverse reactions [32]. Therefore, physicians cautiously administer drugs.
Limitations
This study has some limitations. Firstly, the study only selected a tertiary hospital with a single sample point. Secondly, the study focused on the analysis of drug use amongst children in different age groups but did not consider the effects of weight and combined medications, which might influence the results. Lastly, cDUI and PDD/cDDD are not commonly used to evaluate the appropriately of paediatric drug use, and the evaluation system needs further improvement and should be jointly discussed by more scholars.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- WHO:
-
World Health Organization
- DUI:
-
Drug Utilisation Index
- DDD:
-
Defined Daily Dose
- DDDs:
-
Defined Daily Doses
- DDDs:
-
Defined Daily Doses
- cDDD:
-
Children Defined Daily Dose
- cDUI:
-
Children Drug Utilisation Index
- ATC:
-
Anatomical Therapeutic Chemical
- ASP:
-
Antimicrobial Stewardship Program
References
Larsson S, Prioux M, Fasth T, Ternhag A, Struwe J, Dohnhammar U, et al. A microsimulation model projecting the health care costs for resistance to antibacterial drugs in Sweden. Eur J Public Health. 2019;29(3):392–6. https://doi.org/10.1093/eurpub/cky209 [PubMed:30304449].
Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183–98. https://doi.org/10.1016/S0140-6736(18)32218-9. [PubMed:30558872].
Trinh NTH, Chahwakilian P, Bruckner TA, Sclison S, Levy C, Chalumeau M, et al. Discrepancies in national time trends of outpatient antibiotic utilization using different measures: a population-based study in France. J Antimicrob Chemother. 2018;73(5):1395–401. https://doi.org/10.1093/jac/dky018. [PubMed:29438535].
Dellit TH, Owens RC, McGowan JE Jr., Gerding DN, Weinstein RA, Burke JP, et al. Clin Infect Dis. 2007;44(2):159–77. https://doi.org/10.1086/510393 [PubMed:17173212].
Drew RH. Antimicrobial stewardship programs: how to start and steer a successful program. J Manag Care Pharm. 2009;15(2 Suppl):S18-23. https://doi.org/10.18553/jmcp.2009.15.s2.18. [PubMed:19236137].
Anderson BJ, Holford NH. Understanding dosing: children are small adults, neonates are immature children.Arch Dis Child. 2013;98(9):737–44. https://doi.org/10.1136/archdischild-2013-303720. [PubMed:23832061].
Le J, Bradley JS. Optimizing Antibiotic Drug Therapy in Pediatrics: Current State and Future Needs. J Clin Pharmacol. 2018;58 Suppl 10:S108-S22. https://doi.org/10.1002/jcph.1128. [PubMed:30248202].
Allen HC, Garbe MC, Lees J, Aziz N, Chaaban H, Miller JL, et al. Off-Label Medication use in Children, More Common than We Think: A Systematic Review of the Literature. J Okla State Med Assoc. 2018;111(8):776–83. [PubMed:31379392].
Chauthankar SA, Marathe PA, Potey AV, Nanavati RN. Drug Utilization in Neonatal Intensive Care Unit of a Tertiary-care Hospital in Mumbai, India. Indian Pediatr. 2017;54(11):931–4. https://doi.org/10.1007/s13312-017-1184-1. [PubMed:28849769].
Abrams E, Netchiporouk E, Miedzybrodzki B, Ben-Shoshan M. Antibiotic Allergy in Children: More than Just a Label. Int Arch Allergy Immunol. 2019;180(2):103–12. https://doi.org/10.1159/000501518. [PubMed:31394524].
Benjamin L, Frush K, Shaw K, Shook JE, Snow SK, Medicine AAOPCoPE, et al. Pediatric Medication Safety in the Emergency Department. Pediatrics. 2018;141(3). https://doi.org/10.1542/peds.2017-4066. [PubMed:30352389].
Kennedy AR, Massey LR. Pediatric medication safety considerations for pharmacists in an adult hospital setting. Am J Health Syst Pharm. 2019;76(19):1481–91. https://doi.org/10.1093/ajhp/zxz168. [PubMed:31532506].
Dictionary Office, Institute of Linguistics, Chinese Academy of Social Sciences. Modern Chinese Dictionary. 7th edition [M]. The Commercial Press, 2016.
Chen Xq, Jin Yy, Tang G. New edition of Pharmacology. 17nd ed. Beijing: People’s Medical Publishing House, 2011: 34–959.
Ministry of Health, People's Republic of China. Measures for prescription management [S]. Decree 53 of Ministry of Health.
Anon. Prescription management method. J Law Med. 2007.
Dragojevic-Simic V, Rancic N, Stamenkovic D, Simic R. Utilization of Parenteral Morphine by Application of ATC/DDD Methodology: Retrospective Study in the Referral Teaching Hospital. Front Public Health. 2017;5:232. https://doi.org/10.3389/fpubh.2017.00232. [PubMed:28913333].
Leucht S, Samara M, Heres S, Davis JM. Dose Equivalents for Antipsychotic Drugs: The DDD Method. Schizophr Bull. 2016;42 Suppl 1:S90-4. https://doi.org/10.1093/schbul/sbv167. [PubMed:27460622].
Bin Z, Maobai L, Na L. The control indexes of antimicrobial agents in different departments were established by the method of limited daily dose[J]. Chin J Clin Pharmacol. 2015;31(08):661–663.
Stanic Benic M, Milanic R, Monnier AA, Gyssens IC, Adriaenssens N, Versporten A, et al. Metrics for quantifying antibiotic use in the hospital setting: results from a systematic review and international multidisciplinary consensus procedure. J Antimicrob Chemother. 2018;73(suppl_6):vi50-vi8. https://doi.org/10.1093/jac/dky118. [PubMed:29878222].
Teng L, Xin HW, Blix HS, Tsutani K. Review of the use of defined daily dose concept in drug utilisation research in China. Pharmacoepidemiol Drug Saf. 2012;21(10):1118–24. https://doi.org/10.1002/pds.3240. [PubMed:22438276].
Zhang L, Li Y, Zeng L, Wang Z. Applying “children defined daily dose” to assess appropriate dose in pediatrics. J Evid Based Med. 2012;5(1):2–5. https://doi.org/10.1111/j.1756-5391.2012.01166.x. [PubMed:23528114].
The WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Available from: https://www.who.int/toolkits/child-growth-standards/standards/.
Xiao Y, Li L. Legislation of clinical antibiotic use in China. Lancet Infect Dis. 2013;13(3):189–91. https://doi.org/10.1016/S1473-3099(13)70011-2. [PubMed:23427881].
State Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science and Technology Press, 2015:382.
World Health Organization Collaborating Centre for Drug Statistics Methodology and Norwegian Institute of Public Health. Guidelines for ATC Classification and DDD Assignment. Oslo: World Health Organization Press; 2018. ISBN 9788280828965.
Guillot J, Lebel D, Roy H, Ovetchkine P, Bussieres JF. Usefulness of defined daily dose and days of therapy in pediatrics and obstetrics-gynecology: a comparative analysis of antifungal drugs (2000–2001, 2005–2006, and 2010–2011). J Pediatr Pharmacol Ther. 2014;19(3):196–201. https://doi.org/10.5863/1551-6776-19.3.196. [PubMed:25309150].
Sanchez-Huesca R, Lerma A, Guzman-Saldana RME, Lerma C. Prevalence of Antibiotics Prescription and Assessment of Prescribed Daily Dose in Outpatients from Mexico City. Antibiotics (Basel). 2020;9(1). https://doi.org/10.3390/antibiotics9010038. [PubMed:31968527].
Teixeira Rodrigues A, Roque F, Falcao A, Figueiras A, Herdeiro MT. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–12. https://doi.org/10.1016/j.ijantimicag.2012.09.003. [PubMed:23127482].
Lopez-Vazquez P, Vazquez-Lago JM, Figueiras A. Misprescription of antibiotics in primary care: a critical systematic review of its determinants. J Eval Clin Pract. 2012;18(2):473–84. https://doi.org/10.1111/j.1365-2753.2010.01610.x. [PubMed:21210896].
Bassetti M, Righi E. Development of novel antibacterial drugs to combat multiple resistant organisms. Langenbecks Arch Surg. 2015;400(2):153–65. https://doi.org/10.1007/s00423-015-1280-4. [PubMed:25667169].
Grant C C, Harnden A, Mant D, Emery D, Coster G. Why do children hospitalised with pneumonia not receive antibiotics in primary care?. Archives of disease in childhood. 2012,97(1):21–7. https://doi.org/10.1007/s00423-015-1280-4.
Acknowledgements
The authors would like to express appreciation for all the research assistants in data collection. Particularly thank for the cooperation and participation of the physicians and other staff at the sample hospitals.
Statement
The authors confirm that all methods used in this study were carried out in accordance with relevant guidelines and regulations, and that all experimental protocols have been approved by the Biomedical Ethics Committee of Anhui Medical University. The detailed documents have been uploaded to the attachment.
Funding
Funding This research was supported by the National Natural Science Foundation of China (grant number 71503006); and the Program for Excellent Talents in University of Anhui Province (grant number gxyq2018006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
XFX designed the study. XH performed the statistical analysis. XTZ, XH and YW contributed to the interpretation of the data. XH and XFX drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Biomedical Ethics Committee of Anhui Medical University (No.2021H002) exempted this study from the requirement to obtain informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, X., Zhang, X., Wang, Y. et al. Cross-sectional study on the drug utilization and evaluation indicator of antibiotics used in pediatric population. BMC Health Serv Res 21, 1091 (2021). https://doi.org/10.1186/s12913-021-06727-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-021-06727-3