Abstract
Background
Authors in previous studies demonstrated that centralising acute stroke care is associated with an increased chance of timely Intra-Venous Thrombolysis (IVT) and lower costs compared to care at community hospitals. In this study we estimated the lower bound of the causal impact of centralising IVT on health and cost outcomes within clinical practice in the Northern Netherlands.
Methods
We used observational data from 267 and 780 patients in a centralised and decentralised system, respectively. The original dataset was linked to the hospital information systems. Literature on healthcare costs and Quality of Life (QoL) values up to 3 months post-stroke was searched to complete the input. We used Synthetic Control Methods (SCM) to counter selection bias. Differences in SCM outcomes included 95% Confidence Intervals (CI). To deal with unobserved heterogeneity we focused on recently developed methods to obtain the lower bounds of the causal impact.
Results
Using SCM to assess centralising acute stroke 3 months post-stroke revealed healthcare savings of $US 1735 (CI, 505 to 2966) while gaining 0.03 (CI, − 0.01 to 0.73) QoL per patient. The corresponding lower bounds of the causal impact are $US 1581 and 0.01. The dominant effect remained stable in the deterministic sensitivity analyses with $US 1360 (CI, 476 to 2244) as the most conservative estimate.
Conclusions
In this study we showed that a centralised system for acute stroke care appeared both cost-saving and yielded better health outcomes. The results are highly relevant for policy makers, as this is the first study to address the issues of selection and unobserved heterogeneity in the evaluation of centralising acute stroke care, hence presenting causal estimates for budget decisions.
Similar content being viewed by others
Background
The care that patients receive following hospital discharge largely determined the high costs of stroke [1, 2]. Treatment with Intra-Venous Thrombolysis (IVT) is cost-effective as the health benefits outweighed the initial costs [3, 4]. Although IVT treatment rates have risen in the last decade [5], there is still substantial undertreatment given the fact that currently between 8 and 10% of patients were treated [6, 7], whereas treatment rates up to 30% have been achieved in optimised and dedicated settings [8]. There are various reasons for the current undertreatment of patients with IVT. These can largely be grouped in themes such as patient delay, performance of the stroke pathway and the organisational system in place for providing acute care [8].
Centralising care in designated stroke centres resulted in more patients arriving in time for treatment, improved outcomes and lowered mortality rates compared to care provided in community hospitals [9,10,11,12,13]. Potential factors influencing more timely hospital arrival of patients within centralised settings were a lower threshold for using ambulance services and preferential routing of patients with suspected stroke candidate for acute treatment [14]. Also a greater awareness and readiness for IVT may exist among healthcare professionals in a centralised organisational system [9]. This can be the result of a combination of experience and exposure to IVT, continued medical training and new trainees entering the workforce [15, 16]. Improvement in outcome is based on a larger proportion of patients arriving on time for treatment at the hospital and a shorter time to treatment (door-to-needle time) within the hospital [9, 17]. In the Northern Netherlands a centralised organisational system for acute stroke care was developed in which patients with suspected stroke are transported to a single tertiary university hospital for acute treatment [9]. We have learned from previous research that a centralised system can be associated with a 50% increased chance of treatment compared to a decentralised system in which treatment is offered in community hospitals.
Using a probabilistic simulation modelling, a recent study showed that centralising IVT would substantially lower mean annual costs per patient compared to improving care at community hospitals separately [17]. However, the causal impact of centralising acute stroke care within clinical practice remained unclear. There is previously demonstrated that centralising stroke care systems was cost-effective, improved outcomes and reduced mortality and costs [3, 18, 19]. Yet, these studies did not adequately counter the endogeneity in the comparison, which limited a causal interpretation of the delivered estimates. Specifically, both selection into centralised stroke care systems and the inference on assessed outcomes are potentially driven by other factors. Hence, not taking these (un) observables into account may have yielded biased estimates, possibly resulting in suboptimal policy decisions. In this study we specifically link this omitted variable bias to the coefficient stability, enabling identification of the lower bound of impact on cost and health outcomes 3 months post-stroke.
Methods
Stroke system characteristics
In the Northern Netherlands, a centralised and decentralised stroke care system for acute stroke care co-exist [9]. Within the centralised system acute stroke treatment is performed in the University Medical Centre Groningen (UMCG), a tertiary university hospital. Within the catchment area of four hospitals, arrangements were made with hospitals, General Practitioners (GPs) and Emergency Medical Services (EMS) to bypass the local three community hospitals, and transfer potential stroke victims directly to the UMCG for acute stroke treatment. Approximately 580.000 inhabitants are served by the centralised system, with a population density of 250 inhabitants per square kilometer. The decentralised system consists of nine community hospitals all offering IVT to patients with suspected acute stroke in their catchment area. Both stroke care systems conform to the national guidelines. All hospitals practice identical protocols for identification of patients with suspected stroke, triage and 911 systems, ambulance transport and finally IVT treatment.. For the patients within the centralised system this meant possibly bypassing a community hospital and being taken to a comprehensive stroke center directly. A total of 1.14 million inhabitants are served by the decentralised system, with an average population density of 189 inhabitants per square kilometer. For the whole of the Northern Netherlands, geography is quite similar with low levels of traffic congestion, the absence of mountains and a temperate maritime climate.
Data sources
We used patient-level data from 1047 stroke patients who were part of a large observational study carried out in the Northern Netherlands in 2010 over the course of 6 months [9]. Of these patients, 780 patients were admitted to community hospitals all part of a decentralised stroke care system, and 267 patients were admitted to a centralised stroke care system. The descriptive statistics of the patients are presented in Table 1. Within the centralised system ischemic stroke patients from all four hospitals were considered. The original dataset was linked to the hospital information systems to acquire additional information for the calculation of hospital costs, such as length of stay. A description of the number of stroke presentations at each included hospital is provided in Table 6 in Appendix.
Approach
We used patient-level data from a previously published study on a central and decentral stroke care system in the Northern Netherlands [9]. Costs from onset to treatment had been collected in prior work [17] and extended by linking the original dataset [9] to the hospital information system to include intra-hospital costs. The Costs after hospital discharge up to 3 months were based on the literature [20]. Functional disability and independence at 3 months was assessed with the modified Rankin Scale (mRS). mRS scores were subsequently mapped into Quality of Life (QoL) values using a validated algorithm [21, 22].
Health measures
Short National Institutes of Health stroke scale (sNIHSS)
The sNIHSS is a commonly used scale to measure stroke severity in the pre-hospital phase, but has also been used in hospital settings [23]. We used the 5-item sNIHSS, covering gaze, visual fields, motor function in both legs and language. The sNIHSS scores were recorded in the original dataset and used as a measure for patients’ health upon hospital arrival.
Quality of life (QoL) values
The mRS score is a commonly used scale to measure disability and independence in stroke victims [24]. The scale consists of seven grades, from 0 to 6, with 0 corresponding to no symptoms, 5 corresponding to severe disability and 6 to indicate mortality. The mRS scores at 3 months were recorded in the original dataset and mapped into QoL values between 0 and 1 using a validated algorithm [21], implemented with the corresponding STATA package mrs2eq [22].
The EQ. 5D questionnaire is a standardized instrument developed by the EuroQol Group as a measure of QoL that can be used in a wide range of health conditions and treatments [25]. The QoL values were used as a one-time measure for patients’ health at 3 months post stroke. Pre-stroke QoL values were missing, making the calculation of Qualtiy-Adjusted Life Years (QALYs) not straightforward as information on time spent within the first 3 months is missing.
Cost calculation
The health care use of both systems was ascertained and valued. Unit costs were obtained from the Dutch Manual of Costing [26]. The costs associated with healthcare use are presented in Table 2. The original dataset [9] was linked to the hospital information systems to collect the intra-hospital costs. Data linkage with the hospital information system, PoliPlus, was requested by the researchers and performed by hospital’s neurology department. All patients in the original dataset [9] were linked with the system. Costs in the post-hospital phase were based on cost estimates previously published in a Dutch setting [21] combined with the observed destination and functional independence at hospital discharge. Costs were determined from a healthcare provider perspective. Productivity losses due to functional impairments were not considered, since the average age of the sample is above retirement age and relevant measures for the sample below retirement age were not available in the dataset.
Pre-hospital costs
Pre-hospital costs were based on mode of referral (GP, 911, self-referral, or intra-hospital), ambulance transportation and distance covered by EMS [17]. The indicators were multiplied with the unit prices as presented in Table 2.
Intra-hospital costs
Intra-hospital costs were based on whether the patient was treated with IVT, length-of-stay in the acute stroke unit and length-of-stay in the neurology ward. For this, the original dataset was linked to the hospital information system which contains detailed medical information on length of stay at the neurology department. Differences in staffing costs between university medical centres and community hospitals were taken into account [26].
Costs after hospital discharge
Costs after hospital discharge up to 3 months were not directly observed. We adopted the strategy of Dirks et al. [20] and related mRS scores at 3 months to average healthcare use after discharge. Patients in the mRS 0–1 category were presumed to be discharged home with no extra costs. Patients in the mRS 2–3 category were presumed to be discharged home with additional home care (1 h/day) and remedial therapy costs (3 sessions/week). Patients in the mRS 4 category were discharged (depending on age) to a rehabilitation centre (if younger than 65 years) or a nursing home (if aged 65 years or older). Patients in the mRS 5 category were discharged to a nursing home. mRS 6 category means deceased with no extra costs.
Adjustment for timing and currency
The index year is 2019. Therefore, costs are corrected with an average annual inflation rate of 1.015% [29]. Furthermore, since costs were collected from a healthcare provider perspective, cost prices are converted using the current Purchasing Power Parity (PPP) of 1.2642$US per 1 Euro [30].
Statistical analysis
Mean differences in the patients’ characteristics, costs and health outcomes were determined with independent samples t-tests (normal distribution) or Mann-Whitney U tests (skewed distribution). Mean differences tests on the cost and health outcomes indicated that mean regressions could be used for the estimation.
The regression formulation of the evaluation in this study is given by
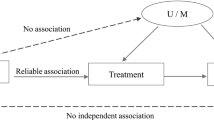
where Yi is the outcome of interest (cost, health) for individual i, c0 the intercept, CSi is a binary variable for the stroke care system with the centralised stroke system as reference category with β as the corresponding coefficient, Xi are the control variables gender, age, IVT received, mode of referral, stroke severity on arrival, and transported by EMS with γ as the vector of corresponding coefficients and ei denotes the error term. Distance to hospital was excluded as control variable due to collinearity with the system indicator variable CSi. As mentioned above, Ordinary Least Squares (OLS) regression of eq. (1) yields a biased estimate of β, as both selection into centralised stroke care systems and the inference on assessed outcomes are potentially driven by other factors, i.e. E[Yi| ei] ≠ 0.
To counter selection bias we use Synthetic Control Methods (SCM) and estimate eq. (1) in two stages. In the first stage we estimate the individual propensity scores of selection in a centralised stroke care system conditional on the control variables Xi with a logit model denoted by
where we followed Rosenbaum and Rubin (1985) and used a preset caliper size of a quarter of a standard deviation of the logit of the propensity score [31, 32]. Mean differences of the raw and matched data and balance plots were used to assess the balancing assumption in the first stage. Subsequently, in the second stage we use the predicted values pi of eq. (2) to obtain the Average Treatment Effect (ATE),
The SCM does not control for unobserved heterogeneity, i.e. factors related to the inference on β that were not observed in the dataset (e.g., socioeconomic status). Therefore, to assess to what extent the inference on coefficient β in eq. (1) is affected by (un) observables we link the omitted variable bias to the coefficient stability using the Altonji ratio [33, 34]. Subsequently, we implement a recently published estimator [35] to obtain the lower bound of the causal effect of centralising acute stroke care denoted by
where RF (RR) and \( {\hat{\beta}}_F \) (\( {\hat{\beta}}_R \)) are the R-squared and obtained estimate of OLS regression on the full (restricted) model of equation (1), respectively, and RMAX is the maximum R-squared. The calculation of RMAX is pre-determined. For example, Bellows & Miquel (2009) suggest RMAX equals RF + (RF − RR) [36]. For that case, Angelini & Mierau (2018) show that \( {\hat{\beta}}_{\ast } \) then reduces to \( 2{\hat{\beta}}_F-{\hat{\beta}}_R \), which is a straightforward way to assess \( {\hat{\beta}}_{\ast } \) without further knowledge of the underlying R-squared [37]. Alternatively, Oster (2017) suggest RMAX equals 1.3 × RF [35], determined from published randomized controlled trials in leading economic journals between 2008 and 2013. We adopted the latter option, as it incorporates both the coefficient movement and the model’s fit.
Deterministic sensitivity analyses were undertaken to test the stability of the observed estimates. First, we focused on the mapping method of the QoL values. In the sensitivity analysis we used the second validated algorithm of Rivero-Arias et al. (2010) [21] and replicated the OLS regression option using Monte Carlo simulation with 10,000 iterations, again implemented with the STATA package mrs2eq [22]. Second, we focused on the uncertainty underlying the cost derivation of costs after hospital discharge, as this part is largely determined from previously published cost estimates for the Dutch setting [20]. Specifically, we modified the assumptions in the main analysis and presumed that patients in the mRS 4 category either go home during the weekends or receive informal care half a week.
Differences in outcomes include 95% Confidence Intervals (CI). All of above statistical analyses were performed with STATA/SE 15.0 (STATA; https://www.stata.com/).
Results
Comparing stroke care systems
A summary on patient recruitment, baseline patient characteristics, access to healthcare services and health outcomes of both stroke care systems is provided in Table 1. Mean differences were determined with independent samples t-tests (normal distribution) or Mann-Whitney U tests (skewed distribution). We observed that while stroke severity on arrival does not differ between the two systems (P = 0.132), at 3 months after hospital discharge the level of disability and dependence is greater in the decentralised system than in the centralised system (P = 0.012).
In Table 3 the cost composition of both systems is provided. We observed that while the mean pre-hospital costs were greater for the centralised system (P = 0.000), the total costs up to 3 months were less than for the decentralised system (P = 0.009).
Estimation results
Synthetic control methods
As mentioned above, we followed Rosenbaum and Rubin (1985) and used a preset caliper size of a quarter of a standard deviation of the logit of the propensity score [31, 32]. No observations were excluded. The systems were balanced in the first stage on the included covariates, as demonstrated with mean differences of the raw and matched data in Table 7 in Appendix and illustrated in the balance plot in Figure 1 in Appendix. The balancing assumption enables to estimate the ATE in the second stage. Using SCM we obtain a \( \hat{\beta} \) for healthcare savings and QoL gain of $US 1735 (CI, 505 to 2966) (P = 0.006) and 0.03 (CI, − 0.01 to 0.73) (P = 0.093), respectively.
Causal approach
In Tables 4 and 5 we present the restricted and full coefficients for β in equation (1) for incremental healthcare costs and QoL values, respectively. Using \( {\hat{\beta}}_R \) and \( {\hat{\beta}}_F \) in the first row in combination with RR and RF in the last row enables to determine the lower bounds of the causal effect according to equation (4) [35]. Hence, centralising acute stroke leads to a lower bound causal effect on healthcare savings and QoL gain of $US 1581 and 0.01 respectively.
Sensitivity analyses
Deterministic sensitivity analyses were undertaken to test the stability of the observed dominant causal effect of centralising acute stroke care. First, implementing the second validated algorithm to mapp QoL values from the observed mRS scores revealed no change in results (P = 0.124). Second, adopting the alternative assumptions underlying the derivation of cost after hospital discharge in the SCM yields healthcare savings of $US 1561 (CI, 524 to 2597) (P = 0.003) and $US 1360 (CI, 476 to 2244) (P = 0.003), respectively.
Discussion
In this study we evaluated the causal impact of a centralised stroke care system on healthcare costs and QoL values up to 3 months after hospital discharge, compared to a decentralised stroke care system. To this end we linked the original dataset [9] to the hospital information system comprising patient-level data and used previously published cost estimates [20] and algorithms [21, 22]. We show that centralising IVT lowers costs and increases patients’ health – proving dominance over the decentralised system. On average, the lower bound of the causal impact on healthcare savings was $US 1581, while similarly health outcomes in terms of QoL gain were 0.014 higher. Indeed, studies that did not adequately account for omitted variables bias may have overestimated the effects of centralising IVT, potentially leading to suboptimal budget allocation if adopted by policy makers.
The results are mainly determined by the differences in patient health, as measured with mRS scores, in both stroke care systems. This corroborates our expectation that patients’ health is influenced by the organisation of the healthcare system. Although pre-hospital costs were greater in a centralised system, on average a larger portion of patients in the centralised system become functionally independent again at 3 months (mRS scores 0–1), thereby saving significant healthcare costs by avoiding care in either a nursing home or rehabilitation centre. This is could suggest that higher pre-hospital costs for the centralised system are offset by a decreased length-of-stay in the hospital and avoiding institutional care after hospital discharge due to improved patients’ health. These results suggest that centralising services could contribute to further improving healthcare, as short-term stroke severity is an important predictor of QoL years after the stroke [38]. From a societal perspective it would be interesting to see whether centralisation of acute stroke care would lead to a shift in costs associated with productivity, informal care and additional transport for caregivers. Better outcomes as obtained in the centralised system would have led to higher productivity, and thus added to a more favourable cost difference. Indeed, dominance would have only increased. Furthermore, also the long-term costs incurred for informal care would have been lower in the centralised system, simply as fewer stroke victims would need less of it. Further research is needed to prove these arguments, as data on productivity, informal care and additional transport costs for caregivers are missing in this study.
It is increasingly recognized that stroke care systems centralised at highly specialised tertiary hospital may generate better patient outcomes at lower costs, compared to care offered at community hospitals [12, 39]. Nationally acute stroke care treatment consists of admission to a stroke unit and treatment with IVT, which is currently administered to approximately 15% of the Dutch incident stroke population [9]. Due to an ageing population the number of patients receiving acute treatment is expected to increase substantially in the near future. Expanding services to other hospitals and regions therefore appears to provide great potential for economic as well as patient value. Importantly, costs per patient will likely decrease with large patient volumes due to economies of scale associated with lower training costs of medical specialists and overhead costs for materials and equipment. Additionally, more costly because of economies of scale certainly will apply also in the Netherlands, yet rurality of the Netherlands may be a relative issue. The nearest comprehensive stroke center will hardly ever be further out than say 50 kms. Indeed in a Scandinavian, US or Canadian settings this may be a different issue. In such settings travel time will become a real issue up to a point where certain services simply may no longer be accessible. In acute stroke telemedicine, not taken into account in this study, may become a viable option.
We acknowledge that our study design has some limitations. For example, patient-level data could not be retrieved for actual costs made by patients after hospital discharge. Therefore, we relied on previously published cost estimates in a Dutch setting [20]. We acknowledge this affects the size of the estimate for incremental healthcare costs, but we argue it would not have altered out conclusions, as it has been shown in the literature that healthcare costs increase with functional disability and dependence [3,4,5, 16,17,18,19,20]. Furthermore, after manipulating the assumptions underlying healthcare use in the deterministic sensitivity analyses, we found that the coefficient only changed moderately. Hence, the dominant effect remained stable. To further understand the effect of centralised stroke care systems on societal costs within clinical practice, future studies may consider following cohorts prospectively from onset to 3 months post-stroke. Furthermore, stroke severity may have been slightly underestimated by using the 5-item short version of the NIHSS. The sNIHSS has been validated for the pre-hospital setting, however the subset of impairments scored is still lower compared to the full version of the NIHSS potentially leading to loss of information on stroke severity. However, this will marginally affect our results, as the sNIHSS is only included as control variable.
Since the results suggest centralising IVT is both cost saving and yields better health outcomes, we dare conclude dominance in terms of cost-effectiveness. We acknowledge that a full cost-utility analysis requires to adopt the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [40]. This is not feasible as important components are missing in the dataset. For example, extrapolating the results over patients’ lifetime would introduce too much uncertainty, as we would have to rely on transition rates from the literature since follow-up data within applicable cycle-lengths is missing. The latter, however, would not alter the outcome of dominance as after initial treatment failure or success the long-term prognosis is more or less determined, i.e., a higher initial success rate implies both lower long-term costs as well as health benefits [38].
Conclusions
From this study we conclude that a centralised system for acute stroke care lowers healthcare costs and improves health outcomes within clinical practice. The results are highly relevant for policy makers, as this is the first study to address the issues of selection and unobserved heterogeneity in the evaluation of centralising acute stroke care, hence presenting causal estimates for budget decisions.
Availability of data and materials
The data that support the findings of this study are available from the UMCG but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of UMCG.
Abbreviations
- ATE:
-
Average Treatment Effect
- CHEERS:
-
Consolidated Health Economic Evaluation Reporting Standards
- CI:
-
Confidence Intervals
- EMS:
-
Emergency Medical Services
- EVT:
-
Endo-Vascular Treatment
- GP:
-
General Practitioners
- IVT:
-
Intra-Venous Thrombolysis
- mRS:
-
modified Rankin Scale
- OLS:
-
Ordinary Least Squares
- PPP:
-
Purchasing Power Parity
- QALYs:
-
Qualtiy-Adjusted Life Years
- QoL:
-
Quality of Life
- SCM:
-
Synthetic Control Methods
- sNIHSS:
-
short National Institutes of Health Stroke Scale
- UMCG:
-
University Medical Centre Groningen
References
Demaerschalk BM, Hwang HM, Leung G. US cost burden of ischemic stroke: a systematic literature review. Am J Manag Care. 2010;16:525–33.
Dewey HM, Thrift AG, Mihalopoulos C, et al. Cost of stroke in Australia from a societal perspective: results from the north East Melbourne stroke incidence study (NEMESIS). Stroke. 2001;32:2409–16.
Guzauskas GF, Boudreau DM, Villa KF, et al. The cost-effectiveness of primary stroke centers for acute stroke care. Stroke. 2012;43:1617–23.
Tan Tanny SP, Busija L, Liew D, et al. Cost-effectiveness of thrombolysis within 4.5 hours of acute ischemic stroke: experience from Australian stroke center. Stroke. 2013;44:2269–74.
Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the United States. J Hosp Med. 2010;5:406–9.
Bauer A, Limburg M, Visser MC. Variation in clinical practice of intravenous thrombolysis in stroke in the Netherlands. Cerebrovasc Dis Extra. 2013;3:74–7.
Adeoye O, Hornung R, Khatri P, et al. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–5.
Boode B, Welzen V, Franke C, et al. Estimating the number of stroke patients eligible for thrombolytic treatment if delay could be avoided. Cerebrovasc Dis. 2007;23:294–8.
Lahr MM, Luijckx GJ, Vroomen PC, et al. Proportion of patients treated with thrombolysis in a centralised versus a decentralised acute stroke care setting. Stroke. 2012;43:1336–40.
Bekelis K, Marth NJ, Wong K, et al. Primary stroke center hospitalization for elderly patients with stroke: implications for case fatality and travel times. JAMA Intern Med. 2016;176:1361–8.
Morris S, Hunter RM, Ramsay AI, et al. Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. BMJ. 2014;349:g4757.
Rinaldo L, Brinjikji W, Rabinstein AA. Transfer to high-volume centers associated with reduced mortality after endovascular treatment of acute stroke. Stroke. 2017;48:1316–21.
Ramsay AI, Morris S, Hoffman A, et al. Effects of Centralising acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke. 2015;46:2244–51.
Lahr MM, Vroomen PC, Luijckx GJ, et al. Prehospital factors determining regional variation in thrombolytic therapy in acute ischemic stroke. Int J Stroke. 2014;9(Suppl A100):31–5.
Leira EC, Pary JK, Davis PH, et al. Slow progressive acceptance of intravenous thrombolysis for patients with stroke by rural primary care physicians. Arch Neurol. 2007;64:518–21.
Grotta JC, Burgin WS, El-Mitwalli A, et al. Intravenous tissue-type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000. Arch Neurol. 2001;58:2009–13.
Lahr MM, van der Zee DJ, Luijckx GJ, et al. Centralising and optimising decentralised stroke care systems: a simulation study on short-term costs and effects. BMC Med Res Methodol. 2017;17:5.
Hunter RM, Davie C, Rudd A, et al. Impact on clinical and cost outcomes of a centralised approach to acute stroke care in London: a comparative effectiveness before and after model. PLoS One. 2013;8:e70420.
Ganesalingam J, Pizzo E, Morris S, et al. Cost-utility analysis of mechanical Thrombectomy using stent retrievers in acute ischemic stroke. Stroke. 2015;46:2591–8.
Dirks M, Baeten SA, Dippel DW, et al. Real-life costs and effects of an implementation program to increase thrombolysis in stroke. Neurology. 2012;79:508–14.
Rivero-Arias O, Ouellet M, Gray A, et al. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Mak. 2010;30:341–54.
Ramos-Gonñi JM, Rivero-Arias O, Dakin H. Response mapping to translate health outcomes into the generic health-related quality-of-life instrument EQ-5D: introducing the mrs2eq and oks2eq commands. Stata J. 2013;13:474–91.
Tirschwell DL, Longstreth WT Jr, Becker KJ, et al. Shortening the NIH stroke scale for use in the prehospital setting. Stroke. 2002;33:2801–6.
Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957;2:200–15.
EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
Hakkaart-van Roijen L, van der Linden N, Bouwmans C, et al. Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Available at: https://www.zorginstituutnederland.nl/binaries/zinl/documenten/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg/Richtlijn+voor+het+uitvoeren+van+economische+evaluaties+in+de+gezondheidszorg+(verdiepingsmodules).pdf. Accessed 5 Oct 2017.
Claes N, Moeremans K, Frank B, et al. Estimating the cost-effectiveness of quality-improving interventions in oral anticoagulation management within general practice. Value Health. 2006;9:369–76.
Zorginstituut Nederland (2019). Medicijnkosten.nl. https://www.medicijnkosten.nl/ Accessed 12 Feb 2019.
OECD. Stat (2019). Consumer price indices (CPIs): annual inflation. https://statsoecdorg/indexaspx?queryid=82174 Accessed 12 Feb 2019.
OECD. Stat (2019). Purchasing Power Parities for GDP and related indicators. https://stats.oecd.org/Index.aspx? DataSetCode=PPPGDP Accessed 12 2019.
Imbens GW, Rubin DB. Causal Inference for Statistics, Social, and Biomedical Sciences: An Introduction. London: Cambridge University Press; 1900. p. 2015.
Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programs. Oxford: Oxford University Press; 2015.
Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–8.
Altonji JG, Elder TE, Taber CR. Selection on observed and unobserved variables: assessing the effectiveness of Catholic schools. J Polit Econ. 2005;113:151–84.
Altonji JG, Elder TE, Taber CR. Using selection on observed variables to assess bias from unobservables when evaluating swan-ganz catheterization. Am Econ Rev. 2008;98:345–50.
Oster E. Unobservable selection and coefficient stability: theory and evidence. J Bus Econ Stat. 2018;37:1–18.
Bellows J, Miguel E. War and local collective action in Sierra Leone. J Public Econ. 2009;93:1144–57.
Angelini V, Mierau J. Late-life health effects of teenage motherhood. Demogr Res. 2018;39:1081–104.
Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Cost Effect Res Alloc. 2013;11:6.
Acknowledgements
N/A
Authors’ disclosures
RDF – Reports no disclosures
JOM – Reports no disclosures
EB – Reports no disclosures
EP – was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care North Thames at Barts Health NHS Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health
GJL – Reports no disclosures
DJZ – Reports no disclosures
MMHL – Reports no disclosures
Funding
N/A
Author information
Authors and Affiliations
Contributions
RDF – study concept and design, statistical analyses, drafting the manuscript. JOM – study concept and design, statistical analyses, critical revision of the manuscript for intellectual content. EB – critical revision of the manuscript for intellectual content. EP – critical revision of the manuscript for intellectual content. GJL – critical revision of the manuscript for intellectual content. DJZ – critical revision of the manuscript for intellectual content. MMHL – study concept and design, study supervision, drafting the manuscript, critical revision of the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We have permission from the Medical Ethics Committee of the University of Groningen to conduct this research (contact: + 31 (0)50 361 4204). This study is related to the original IMPACT study. Individuals in the dataset are aware of the use of hospital information as input for the analysis.
Consent for publication
We have permission on behalf of the individuals (or relatives) from which individual patient data is used to publish the conducted results.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Freriks, R.D., Mierau, J.O., Buskens, E. et al. Centralising acute stroke care within clinical practice in the Netherlands: lower bounds of the causal impact. BMC Health Serv Res 20, 103 (2020). https://doi.org/10.1186/s12913-020-4959-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-020-4959-3