Abstract
Background
The study objective was to identify and describe the process, resources and expertise required for the revision of accreditation standards, and report outcomes arising from such activities.
Methods
Secondary document analysis of materials from an accreditation standards development agency. The Royal Australian College of General Practitioners’ (RACGP) documents, minutes and reports related to the revision of the accreditation standards were examined.
Results
The RACGP revision of the accreditation standards was conducted over a 12 month period and comprised six phases with multiple tasks, including: review methodology planning; review of the evidence base and each standard; new material development; constructing field trial methodology; drafting, trialling and refining new standards; and production of new standards. Over 100 individuals participated, with an additional 30 providing periodic input and feedback. Participants were drawn from healthcare professional associations, primary healthcare services, accreditation agencies, government agencies and public health organisations. Their expertise spanned: project management; standards development and writing; primary healthcare practice; quality and safety improvement methodologies; accreditation implementation and surveying; and research. The review and development process was shaped by five issues: project expectations; resource and time requirements; a collaborative approach; stakeholder engagement; and the product produced. The RACGP evaluation was that participants were positive about their experience, the standards produced and considered them relevant for the sector.
Conclusions
The revision of accreditation standards requires considerable resources and expertise, drawn from a broad range of stakeholders. Collaborative, inclusive processes that engage key stakeholders helps promote greater industry acceptance of the standards.
Similar content being viewed by others
Background
Government, quality improvement and accreditation agencies frequently engage in the development or revision of clinical and organisational standards. These are significant tasks that utilise considerable human and financial resources [1]. Different organisations produce standards according to their own processes and requirements, and it is believed that inclusive processes result in greater acceptance of the standards produced [1]. However, we do not know what might be evidence-informed practice in the development or revision of accreditation standards [2]. To date, no empirical study has been published that sought to identify the process, resources and expertise required for either of these endeavours [2]. This is a significant gap in the evidence base for the healthcare accreditation field [2]-[4], as accreditation programs have increasingly become an important strategy by which governments seek to regulate healthcare quality and safety [5],[6]. There are now more than 44 health service accreditation programs which have been implemented in over 70 countries [3],[7].
The aim of this study was to identify and describe the process, resources and expertise required for, and to report evaluation outcomes from, a revision of a set of healthcare accreditation standards. The standards and associated revision activities under examination concern the Standards for General Practice (4th Edition) developed and revised by The Royal Australian College of General Practitioners (RACGP), which are used for accrediting general practice nationally. Through implementing an instrumental case study [8],[9] of the RACGP accreditation standards we sought to highlight the process, resource and expertise issues relevant for other accreditation standard setting bodies. The RACGP is representative of other bodies that similarly have responsibility for the development and revision of standards, but do not themselves apply or assess services using the standards [10]. Previous studies have revealed the common issues and challenges facing standard setting and accreditation bodies [7],[11].
Methods
Study context
The Accreditation Collaborative for the Conduct of Research, Evaluation and Designated Investigations through Teamwork (ACCREDIT) was funded across 2011-15 to investigate health service accreditation in Australia [12]. The collaboration comprises university researchers, accreditation agency personnel and staff from leading quality improvement bodies in Australia (Table 1). The collaboration was awarded an Australian Research Council Linkage Project grant (LP100200586) in 2010. Ethics approval for the study was given by the University of New South Wales (UNSW) Human Research Ethics Committee (approval number HREC 10274). The ACCREDIT study protocols are publically available [12]-[15] and are informed by previous accreditation research conducted by UNSW and The Australian Council on Healthcare Standards (ACHS) [10]-[12],[16]-[24], including reviews of the healthcare accreditation literature [2]-[4].
Setting
Australia has over 7,100 general practices in which more than 23,500 doctors work. There were 125 million consultation services provided during 2010-11, costed at A$5.3 billion through the Medicare Benefits Scheme [25] (this excludes out of pocket expenses of patients). In 2011-12 there were over 4000 general practices accredited against the RACGP Standards for General Practice [26]. The standards cover five areas: practice services (7 standards and 19 criteria); rights and needs of patients (1 standard and 3 criteria); safety, quality improvement and education (2 standards and 7 criteria); practice management (2 standards and 4 criteria); and physical factors (3 standards and 8 criteria). (See: http://www.racgp.org.au/your-practice/standards/standards4thedition/).
Study methodology
An expert group was formed by UNSW researchers and RACGP staff. During 2012, they collaboratively conducted a study with three stages. First, informed by the accreditation and evaluation literatures, the expert group purpose-designed an analysis framework with seven categories including: phase, task, objective, time frame, components, people involved and National Expert Committee - Standards for General Practice (NEC-SGP) involvement. The role of the NEC-SGP includes developing and maintaining standards for general practices, and ensuring that the standards reflect quality practice and are independent of government policies and initiatives. The NEC-SGP comprises experts in standards development with professional backgrounds including general practitioners, practice nurses and managers, and a consumer representative. Since 2011 the NEC-SGP is known as the National Standing Committee - Standards for General Practice. Second, using the framework, thematic analysis [8] of RACGP documents, minutes and evaluation reports related to the revision of the accreditation standards was conducted. More than 50 documents were accessed from the RACGP information system. Third, the group reviewed the findings to clarify the process, resources and expertise utilised, and reported evaluation outcomes. Over several months the expert group discussed the findings in meetings and electronic forums to work through the material, with differences resolved by negotiation [8].
Results
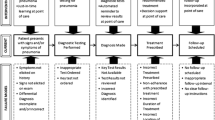
The analysis framework facilitated the identification of a standards review process comprising six sequential and overlapping phases with multiple components (Table 2). The six phases occurred over a 12 month period across 2009-10. Phase 1 comprised the `review methodology planning’ phase, which occurred over two months. This phase involved two tasks: developing the review feedback methodology and tools; and reviewing the evidence base for information on methods of standards development. The review of the evidence base and current standards were the tasks that formed Phase 2, which was completed over a five month period. Following Phase 1, and overlapping with Phase 2, Phase 3 was a five month activity requiring the development of new material for the new standards. The construction of field trial methodology, the sole task in Phase 4, occurred in parallel and was completed in two months. The completion of the initial four phases led to Phase 5 and the combined task of drafting, trailing and refining the new standards, which occupied five months. To complete the project, the final task was the formatting and production of the new standards. This was Phase 6 and occurred over a two month period.
Over 100 individuals substantively participated in the review process, with an additional 30 providing periodic input and feedback. Participants were drawn from general practice stakeholders, including: healthcare professional associations; primary healthcare services; accreditation agencies; government agencies; and public health organisations. Their expertise spanned the fields of: project management; standards development and writing; primary healthcare practice; quality and safety improvement methodologies; accreditation implementation and surveying; and research methodologies.
The review and development process was shaped by five factors. First, identifying and delivering upon the requirements of the RACGP, and stakeholders associated with the standards, was reported as necessary for the credibility of the product. Second, identifying and communicating resource and time restrictions to participants, and observers within the sector, was required to enable the review to be completed as expected. Managing expectations and employing an effective communication strategy reinforced a collaborative approach and facilitated broad stakeholder engagement with the review; these being the third and fourth issues identified as essential for a positive development process leading to the acceptance of revised standards. Finally, the review project had to deliver a well structured, clearly written, evidence based, high quality document that was consistent with previous editions. One significant improvement suggestion emerged from the evaluation: a majority of participants agreed that consideration could be given to altering the standards revision process to conduct periodic reviews and progressive updates.
Discussion
This study provides the first case-study evidence about processes invoked for the development and revision of accreditation standards [2], and lays the foundation for further work in this area [3],[4]. The research reveals that the revision of accreditation standards is a major undertaking requiring considerable resources and expertise, drawn from a broad range of stakeholders. Industry acceptance of the standards produced was found to be related to a collaborative, inclusive process, grounded by clinical evidence and process reviews, which promoted stakeholder participation. These findings support previously reported, non-empirical assessments, of how to approach the task [1]. For other accrediting bodies the study provides three things: insight into a difficult and challenging process; encouragement to investigate and make public their own experiences; and, a template and structure to follow to undertake such forensic examinations.
The project was completed through the combined efforts, or distributed leadership [19], of more than 100 people over a 12 month period. Key influences on the review process were: project requirements and stakeholder expectations; resource and time restrictions; collaborative team approach; stakeholder engagement; and the product required. The revision process necessitated the delicate balancing of these issues to maintain cohesion and continued participation between diverse and distributed stakeholders over an extended time period. Methodological rigour, as recognised by the six phases, was applied by the RACGP in the development, piloting and revision of materials. The commitment and effort of agency staff and committee members, who efficiently used resources with strict time constraints, enabled the efficient completion of the project. RACGP evaluation showed that stakeholder acceptance of the revision process and the revised standards produced was based on their perception of a transparent, inclusive and rigorous process implemented by the College [1].
Conclusion
The revision of accreditation standards requires collaboration from a diverse range of professionals, with considerable resources and expertise. The collaborative, inclusive process employed engaged stakeholders and promoted the acceptance of the revised standards by the sector.
Authors’ contributions
DG, RH, JW and JB conceived of the study, and participated in its design and coordination and helped to draft the manuscript. AD participated in the data collection and analysis, and along with AH and MC assisted in the revising of the manuscript. All authors read and approved the final manuscript.
References
McGlynn E, Shekelle P, Hussey P: Developing, Disseminating and Assessing Standards for the National Health Service: An Assessment of Current Status and Opportunities for Improvement. RAND Health; 2008.
Greenfield D, Pawsey M, Hinchcliff R, Moldovan M, Braithwaite J: The standard of healthcare accreditation standards: a review of empirical research underpinning their development and impact. BMC Health Serv Res 2012, 12:329..
Greenfield D, Braithwaite J: Health sector accreditation research: a systematic review. Int J Qual Health Care. 2008, 20 (3): 172-183. 10.1093/intqhc/mzn005.
Hinchcliff R, Greenfield D, Moldovan M, Pawsey M, Mumford V, Westbrook J, Braithwaite J: Narrative synthesis of health service accreditation literature. BMJ Qual Saf. 2012, 21: 979-991. 10.1136/bmjqs-2012-000852.
National Safety and Quality Health Service Standards. 2011, Commonwealth of Australia, Sydney, Australia
Access to Standards. [], [www.accreditation.ca/review-our-standards]
Braithwaite J, Shaw C, Moldovan M, Greenfield D, Hinchcliff R, Mumford V, Kristensen M, Westbrook J, Nicklin W, Fortune T, Whittaker S: Comparison of health service accreditation in low- and middle-income countries with those in higher income countries: a cross sectional study. Int J Qual Health Care. 2012, 24 (6): 568-577. 10.1093/intqhc/mzs064.
Liamputtong P: Qualitative Research Methods. 2009, Oxford University Press, Melbourne, 3
Creswell J: Qualitative Inquiry and Research Design: Choosing Among Five Approaches. 2007, Sage Publications, London
Greenfield D, Pawsey M, Braithwaite J: Accreditation: A Global Regulatory Mechanism to Promote Quality and Safety. Continuous Quality Improvement in Health Care. Edited by: Sollecito W, Johnson J. 2013, Jones and Barlett Learning, New York, 513-531. 4
Greenfield D, Pawsey M, Braithwaite J: The role and impact of accreditation on the healthcare revolution. O papel e o impacto da acreditação na revolução da atenção à saúde. Acreditação. 2012, 1 (2): 1-14.
Braithwaite J, Westbrook J, Johnston B, Clark S, Brandon M, Banks M, Hughes C, Greenfield D, Pawsey M, Corbett A, Georgiou A, Callen J, Øvretveit J, Pope C, Suñol R, Shaw C, Debono D, Westbrook M, Hinchcliff R, Moldovan M: Strengthening organizational performance through accreditation research: the ACCREDIT project. BMC Res Notes 2011, 4:390..
Greenfield D, Hinchcliff R, Moldovan M, Mumford V, Pawsey M, Westbrook JJB, Braithwaite J: A multi-method research investigation of consumer involvement in Australian health service accreditation programs: the ACCREDIT-SCI study protocol. BMJ Open 2012, 2:329..
Hinchcliff R, Greenfield D, Moldovan M, Pawsey M, Mumford V, Westbrook JJB, Braithwaite J: Evaluation of current Australian health services accreditation processes (ACCREDIT-CAP): a protocol for a mixed method research project. BMJ Open 2012, 0:e001726..
Mumford V, Greenfield D, Hinchcliff R, Moldovan M, Forde K, Westbrook J, Braithwaite J: Economic evaluation of Australian acute care accreditation (ACCREDIT-CBA [acute]): study protocol for a mixed-method research project. BMJ Open 2013, 3:e002381..
Braithwaite J, Greenfield D, Westbrook J, Pawsey M, Westbrook M, Gibberd R, Naylor J, Nathan S, Robinson M, Runciman B, Jackson M, Travaglia J, Johnston B, Yen D, McDonald H, Low L, Redman S, Johnson B, Corbett A, Hennessy D, Clark J, Lancaster J: Health service accreditation as a predictor of clinical and organisational performance: a blinded, random, stratified study. Qual Saf Health Care. 2010, 19 (1): 14-21. 10.1136/qshc.2009.033928.
Greenfield D, Braithwaite J: Developing the evidence base for accreditation of healthcare organizations: a call for transparency and innovation. Qual Saf Health Care. 2009, 18: 162-163. 10.1136/qshc.2009.032359.
Greenfield D, Braithwaite J, Pawsey M: Health care accreditation surveyor styles typology. Int J Health Care Qual Assur. 2008, 21 (5): 435-443. 10.1108/09526860810890422.
Greenfield D, Braithwaite J, Pawsey M, Johnson B, Robinson M: Distributed leadership to mobilise capacity for accreditation research. J Health Organ Manage. 2009, 23 (2): 255-267. 10.1108/14777260910960975.
Greenfield D, Hinchcliff R, Westbrook M, Jones D, Low L, Johnston B, Banks M, Pawsey M, Moldovan M, Westbrook J, Braithwaite J: An empirical test of accreditation patient journey surveys: randomised trial. Int J Qual Health Care. 2012, 24 (5): 495-500. 10.1093/intqhc/mzs035.
Greenfield D, Moldovan M, Westbrook M, Jones D, Low L, Johnston B, Clark S, Banks M, Pawsey M, Hinchcliff R, Westbrook J, Braithwaite J: An empirical test of short notice surveys in two accreditation programs. Int J Qual Health Care. 2012, 24 (1): 65-71. 10.1093/intqhc/mzr074.
Greenfield D, Pawsey M, Braithwaite J: What motivates health professionals to engage in the accreditation of healthcare organizations?. Int J Qual Health Care. 2011, 23 (1): 8-14. 10.1093/intqhc/mzq069.
Greenfield D, Pawsey M, Naylor J, Braithwaite J: Are accreditation surveys reliable?. Int J Health Care Qual Assur. 2009, 22 (2): 105-116. 10.1108/09526860910944601.
Greenfield D, Pawsey M, Naylor J, Braithwaite J: Researching the reliability of accreditation survey teams: lessons learnt when things went awry. Health Inform Manage. 2013, 42 (1): 4-10.
Australia’s Health 2012. 2012, Australian Institute of Health and Welfare, Canberra
Australian General Practice Accreditation Limited and Quality in Practice: Australian General Practice Accreditation Limited and Quality in Practice Pty Ltd Annual Report. In . Brisbane: 2012.
Acknowledgements
This research was supported under Australian Research Council’s Linkage Projects funding scheme (project number LP100200586). The RACGP staff and members of the National Standing Committee - Standards for General Practice are thanked for access to documentation and contribution to this study. We also recognise our national and international collaborators for their contributions to the ACCREDIT project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests regarding the publication of this article.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Greenfield, D., Civil, M., Donnison, A. et al. A mechanism for revising accreditation standards: a study of the process, resources required and evaluation outcomes. BMC Health Serv Res 14, 571 (2014). https://doi.org/10.1186/s12913-014-0571-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-014-0571-8