Abstract
Purpose
In this study, exercise interventions were evaluated for their effects on cancer-related fatigue (CRF) and quality of life (QoL) among cancer patients.
Design
A meta-analysis was performed.
Methods
We systematically searched the PubMed/Medline, Web of Science, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), PsycINFO, and CINAHL databases, and gray literature sources including the Virginia Henderson International Nursing Library and Google Scholar. This study only included randomized controlled trials (RCTs) examining how exercise interventions affect CRF and QoL among cancer patients. Based on the Cochrane Risk-of-Bias Assessment Tool, version 2 (RoB 2) and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, the methodological quality of the included studies was evaluated. In addition, standardized mean differences (SMDs) and 95% confidence intervals (CIs) were applied to assess the intervention effect with respect to CRF and QoL. Data analysis was performed using Review Manager (version 5.4).
Results
There were a total of 1573 participants in the 28 included articles. According to the meta-analysis, CRF (SMD = -0.35, 95% CI: -0.63 to -0.07, p = 0.01) and QoL (SMD = 0.36, 95% CI: 0.20 to 0.53, p < 0.01) were positively affected by exercise interventions. Subgroup analyses revealed considerable improvements in CRF (SMD = -0.54, 95% CI: -1.00 to -0.09, p = 0.02) and QoL (SMD = 0.38, 95% CI: 0.16 to 0.59, p < 0.01) from aerobic exercise. An intervention duration less than 12 weeks had a better effect on CRF (SMD = -0.80, 95% CI: -1.43 to -0.17, p = 0.01) and QoL (SMD = 0.53, 95% CI: 0.21 to 0.85, p < 0.01), and three times per week was the most effective frequency in improving QoL (SMD = 0.69, 95% CI: 0.28 to 1.11, p < 0.01). Exercise intervention was more successful in improving CRF (SMD = -0.66, 95% CI: -1.10 to -0.21, p < 0.01) and QoL (SMD=-0.50, 95% CI: 0.23 to 0.78, p < 0.01) in female cancer patients. Sensitivity analyses showed that the pooled outcomes were reliable and stable.
Conclusion
Exercise interventions are a workable approach to improve CRF and QoL among cancer patients. An aerobic exercise intervention of less than 12 weeks might be most effective in improving CRF and QoL, and three times per week might be the most appropriate frequency. Exercise might have a more positive effect on improving CRF and QoL in female cancer patients. Additionally, a larger number of high-quality RCTs should be conducted to further confirm the efficacy of exercise interventions on CRF and QoL among cancer patients.
Registration number
CRD42022351137.
Similar content being viewed by others
Introduction
The World Health Organization states that the incidence and mortality rates of cancer are increasing rapidly worldwide, making it the world’s second leading cause of death [1]. The International Agency for Research on Cancer predicts that 30.2 million cases of cancer will be diagnosed worldwide by 2040, a 36.1% increase from 2020 (19.3 million) [2]. With improvements in cancer diagnosis and treatment approaches, the survival years of cancer patients have greatly increased [3]. However, their QoL has not significantly improved [3]. Specifically, cancer patients’ QoL is markedly affected for up to 2 to 26 years after cancer diagnosis with the influence of a variety of problems [4, 5], among which CRF is one of the most common causes [6, 7].
CRF is a multidimensional, persistent, and painful feeling experienced in relation to cancer and its treatment, which is described as a prolonged debilitating condition and interferes with cancer patients’ body functions and their daily activities [8,9,10]. CRF is one of the most common symptoms observed among cancer patients, and its incidence during and after positive cancer treatment is 40% ~ 100% and 14% ~ 40%, respectively [11]. Notably, CRF is stubborn in cancer patients and can last up to five years or more during their survival phases and lead to substantial impairments in various aspects [12,13,14]. For example, CRF increases the physical and mental burden of cancer patients, resulting in physical dysfunctions such as pain and insomnia and psychological problems including anxiety and depression [15, 16], which can seriously impair their QoL [7, 17]. QoL for cancer patients refers to a dynamic and subjective feeling that involves all aspects of their lives and needs [18]. Poor QoL is associated with a series of adverse events, such as aggravating physical dysfunction and delaying cancer rehabilitation, leading to an increase in symptom burden, including persistent CRF, and a decrease in survival rate [19,20,21]. Thus, there is an urgency to implement effective and sustainable interventions to address CRF and QoL among cancer patients. Many studies have reported that non-pharmacological interventions could improve CRF and QoL [22, 23]. These non-pharmacological interventions do not lead to any serious adverse side-effects and are free from drug interactions, which makes them easier to accept by cancer patients than drug treatments [24, 25].
Exercise interventions are one of the most effective non-pharmacological interventions to address the side effects of treatment, with the aims of improving physical fitness and helping symptom management in a systematic and scientific manner [26,27,28]. In recent years, exercise interventions have been widely conducted among patients diagnosed with various chronic diseases, including chronic obstructive pulmonary disease, high blood pressure, and diabetes, and have been considered effective for protecting the heart, strengthening body immunity, as well as improving fatigue and QoL [29,30,31]. Some studies have implemented exercise interventions in cancer care and have revealed benefits for cancer patients from both physical (e.g., improving sleep) and mental (e.g., reducing psychological distress) aspects [32, 33].
Nevertheless, studies on exercise interventions for cancer patients remain limited, and the majority of these studies have targeted patients with breast and lung cancer [34, 35]. Current studies have revealed the effectiveness of exercise interventions for cancer patients’ physical health, including pain and insomnia, and mental health, including anxiety and depression [36, 37]. However, the impact on CRF and QoL is controversial. Results from previous randomized controlled trials have shown that exercise fails to improve CRF and QoL [38, 39], whereas some studies have reported that exercise is effective for CRF and QoL [40, 41]. Hence, further studies are needed. Moreover, to the best of our knowledge, few studies have conducted systematic and quantitative assessments of CRF and QoL among cancer patients with respect to the type, duration, and frequency of exercise interventions, as well as the gender of cancer patients. Thus, there is a need to perform further quantitative synthesis. The purposes of this meta-analysis were (1) to determine the overall effect of exercise interventions on CRF and QoL among patients with cancer and (2) to identify the effects of different exercise types, durations, and frequencies, as well as cancer patient gender on CRF and QoL.
Methods
This meta-analysis was registered in the International Prospective Register of Systematic Reviews (CRD42022351137), and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [42] were implemented for reporting. Since the data of this review were obtained from formerly published studies, there was no requirement for informed consent or ethical permission.
Data sources and search strategies
We searched the PubMed/Medline, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), PsycINFO, CINAHL, and Embase electronic databases to find relevant articles up to 31 October 2022. In addition, searching of the gray literature was conducted, including the Virginia Henderson International Nursing Library and Google Scholar. A combination of MeSH terms and free-text terms was used. In the Supplementary Materials, we provided a detailed description of the search strategy (see Supplementary file Table 1s), which focuses solely on the study of humans and adults. The list of references to relevant articles was examined to find additional articles.
Inclusion and exclusion criteria
The studies were independently screened and selected by two investigators based on the following inclusion criteria: (1) the study participants were patients aged over 18 years and diagnosed with any type of cancer, regardless of sex and cancer stage; (2) the intervention group used exercise interventions only; (3) the comparators of the studies were routine care, usual care, wait list control, standard treatment, or conventional care interventions; (4) the outcomes of the studies were CRF and QoL; and (5) the studies were in English or Chinese. The exclusion criteria were as follows: (1) the studies were research protocols, conference abstracts, reviews, systematic evaluations, meta-analyses, pilot research, or duplicate reports; and (2) the studies failed to record available data. There were no restrictions in terms of publication dates.
Study selection and data extraction
Data management was enabled by the reference management program Endnote X9. After removal of duplicates, two authors individually filtered all titles and abstracts that met the eligibility criteria. Then, the full texts of any citations deemed possibly related by either author were retrieved and assessed. We resolved disagreements through discussion or through consultation with third authors as necessary.
The relevant data were extracted from the included studies by means of a predesigned data gathering form. Extracted data covered the study’s first author, date of publication, country, sample size, characteristics of participants (mean age, cancer type and stage), intervention and control type, and intervention duration.
Quality assessment
Included studies were independently evaluated for methodological quality by two authors applying the Cochrane Risk-of-Bias Assessment Tool, version 2 (RoB2) [43], which included five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reporting results. Within each field, the studies were evaluated as having a low risk of bias, some concerns, or high risk of bias. If disagreement occurred, the reviewers reached a consensus, with a third reviewer resolving disagreements or discussing them within the team, if needed.
The evidence quality was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [44] according to the following domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The total classification of the evidence was assessed as “very low”, “low”, “moderate” or “high”. Methodologically, the evidence quality for RCTs was originally classified as high, with a reduction to moderate, low or very low if limitations were detected in any of the above domains. Nonetheless, the evidence could be escalated through a dose‒response gradient and a large effect. Two researchers independently scored evidence for quality in accordance with the GRADE manual. Disagreements were settled with discussion or with a third author’s assistance.
Data synthesis and analysis
Data synthesis and analysis in this study were carried out using Review Manager 5.4 software. Means and standard deviations (SDs) at post-intervention were extracted for meta-analysis. Due to the different measurements used in these enrolled studies, standardized mean differences (SMDs) and 95% confidence intervals (CIs) were used to evaluate the intervention effect with respect to CRF and QoL. A two-tailed p value < 0.05 was applied to denote statistical significance. The I2 statistic and p value were used to assess heterogeneity. If I2 ≤ 50% and p > 0.1, the heterogeneity was considered statistically significant and was aggregated by a fixed effects model. If I2 > 50% and p < 0.1, a random effects model was used. Sensitivity analyses were performed to examine the stability of the pooled outcomes, and meta-regression analyses were conducted for exploring potential sources of heterogeneity. Subgroup analyses based on intervention type, duration, and frequency, as well as gender of cancer patient were also conducted in a predesigned manner. Funnel plots were examined to assess potential publication bias for CRF and QoL. Additionally, if the included study reported more than two arms, the method of splitting shared groups was used [45].
Results
Study selection
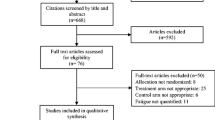
The selection process of our study is illustrated in Fig. 1. In brief, 26,187 references were retrieved from the databases; these were reduced to 352 after excluding references due to duplicate papers (11,161 studies), meta-analyses (1073 studies), reviews (1306 studies), animal testing studies (471 studies), subject nonconformity (6329 studies), initial checking by title/abstract (5478 studies), conference abstracts (15 studies), and dissertations (2 studies). A full-text review and quality assessment of the remaining 352 studies was performed. After full-text review, 324 studies were excluded for the reasons outlined in Fig. 1. Finally, a total of 28 RCTs [38,39,40,41, 46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] were subjected to data extraction.
Characteristics of the included studies
Study characteristics
Across studies, sample sizes ranged from 20 to 133, totalling 1573 samples. In the intervention group, the mean age (SD) ranged from 42.70 (9.60) to 71.40 (5.40) years, while in the control group, it ranged from 43.50 (8.60) to 72.50 (4.20) years. Among the 28 studies, 13 included only breast cancer survivors, four included prostate cancer patients, three included lung cancer patients, and two included colorectal cancer patients. And 24 studies reported the cancer stages of the participants. One study was a 3-arm RCT. The studies were published from 2003 to 2022, and their characteristics are shown in Table 1.
Characteristics of exercise interventions
Most exercise interventions were supervised or home-based. The types of exercise interventions varied across studies and included walking, cycling, Tai Chi, yoga, and jogging. Specifically, 14 studies [41, 46, 48, 50, 51, 53, 55,56,57,58, 60, 65,66,67] evaluated aerobic exercise, four studies [47, 49, 50, 63] evaluated resistance exercise including recumbent or upright cycle ergometers, leg extensions, and leg curls, three studies [40, 59, 61] evaluated aerobic and resistance exercise, and eight studies [38, 39, 52, 54, 62, 64, 68, 69] evaluated mixed exercise. And 12 studies reported an intervention duration < 12 weeks, and 20 studies reported an intervention duration ≥ 12 weeks. In addition, exercise interventions ranged in frequency from two to five times per week (n = 24), four to five days per week (n = 1), 150 min per week (n = 2), endurance training five days per week and strength training every other day (n = 1), and 18 metabolic equivalent task (MET) hours per week for the first six weeks and 27 MET hours per week after six weeks (n = 1).
Characteristics of comparators
Within the control groups, 23 studies used usual care/conventional care/standard treatment or standard care, two studies used wait list controls, three studies provided participants with simple stretching and/or relaxation exercises.
Outcome measurements
Among these included studies, the outcome assessment tools differed. In terms of CRF, three studies applied the European Organization for Research and Treatment of Cancer QoL Questionnaire Core 30 (EORTC-QLQ-C30), five studies applied the Functional Assessment of Cancer Therapy-Fatigue (FACT-F), and three studies applied the Functional Assessment of Cancer Therapy-Anemia (FACT-An) scales. Regarding QoL, seven studies applied the Functional Assessment of Cancer Therapy scale (FACT), and three of the seven applied the Functional Assessment of Cancer Therapy-General (FACT-G) scale.
Risk of bias
For each study, RoB 2 was applied to evaluate the risk of bias, and the outcomes are displayed in Fig. 2. Specifically, although all included studies were reported as randomized, selective bias remained, as 15 studies did not mention allocation concealment. All studies were evaluated as having some concerns regarding deviations from intended intervention because no blind method on participants was reported. Additionally, one study reported incomplete data, resulting in a high risk of attrition bias. Regarding bias in outcome measures, ten studies were blinded to the outcome assessor, 16 studies were concerned, and the other two studies were at high risk. In addition, three studies presented inadequate information to make judgments, which may result in effects being overvalued and a selection bias in reported results being introduced. Overall, one study was assessed as “low-risk bias”, twenty-three studies were classified as “some concern” and four studies were evaluated as “high risk of bias”. The funnel plots were distributed roughly symmetrically, with no apparent differences (see Figure S1a and Figure S1b of Supplementary file).
Quality of evidence
The evidence certainty for CRF and QoL was rated as low and high, respectively. The total sample size for all two study indicators was greater than 300. The results of the evaluation and further detailed information are displayed in Table 2.
Meta-analysis results
Effect of exercise interventions on CRF
Twenty-two studies [38,39,40,41, 47, 49,50,51,52,53,54,55, 59,60,61,62,63, 65,66,67] estimated the effects of exercise interventions on CRF among cancer patients. Exercise interventions significantly decreased CRF among cancer patients (SMD = -0.35, 95% CI: -0.63 to -0.07, p = 0.01; I2 = 80%, p < 0.01) (Fig. 3a). A sensitivity analysis was conducted with a leave-one-out approach, and the results were between − 0.28 (95% CI: -0.54 to -0.02) and − 0.40 (95% CI: -0.67 to -0.12).
Subgroup analyses based on exercise intervention type were conducted, including aerobic (SMD = -0.54, 95% CI: -1.00 to -0.09, p = 0.02), resistance (SMD = -0.52, 95% CI: -1.11 to 0.08, p = 0.09), aerobic and resistance (SMD = -0.49, 95% CI: -1.61 to 0.63, p = 0.39), and mixed exercise interventions (SMD = 0.15, 95% CI: -0.16 to 0.47, p = 0.35). Our results showed high heterogeneity in all subgroups (Figure S2a).
In the subgroup analyses, the duration of exercise interventions was evaluated, including less than 12 weeks (SMD = -0.80, 95% CI: -1.43 to -0.17, p = 0.01) and greater than or equal to 12 weeks (SMD = -0.18, 95% CI: -0.46 to 0.10, p = 0.20). High heterogeneity remained in these subgroups (Figure S3a).
Subgroup analyses were also conducted in accordance with the frequency of the exercise interventions. To ensure the accuracy of the results, some articles that did not report the frequency of exercise were excluded from this subgroup analysis [46, 51, 54]. Subgroup analyses included 2 times per week (SMD = -0.15, 95% CI: -1.03 to 0.73, p = 0.75), 3 times per week (SMD = -0.23, 95% CI: -0.60 to 0.15, p = 0.24), and more than 3 times per week (SMD = 0.39, 95% CI: -0.99 to 0.21, p = 0.20). Our results suggested high heterogeneity in all subgroups (Figure S4a).
For the gender of cancer patients, subgroup analyses excluded studies that did not report gender [38, 40, 41, 47, 51, 54, 55, 67] to secure the credibility of this study. Subgroup analysis included female (SMD = -0.66, 95% CI: -1.10 to -0.21, p < 0.01) and male (SMD = 0.40, 95% CI: 0.07 to 0.72, p = 0.02). Our outcomes showed that there was low heterogeneity in the male subgroup (I2 = 0%, P = 0.61) (Figure S5a).
Effect of exercise interventions on QoL
Twelve studies [39,40,41, 46, 48, 52, 54, 56,57,58, 64, 68] estimated the effects of exercise interventions on QoL among cancer patients. Exercise considerably improved the QoL of cancer patients (SMD = 0.36, 95% CI: 0.20 to 0.53, p < 0.01; I2 = 46%, p < 0.05) (Fig. 3b). A sensitivity analysis was carried out with a leave-one-out method, with results ranging from 0.27 (95% CI: 0.10 to 0.45) to 0.42 (95% CI: 0.25 to 0.59).
Subgroup analyses were conducted by the type of exercise intervention, including aerobic (SMD = 0.38, 95% CI: 0.16 to 0.59, p < 0.01) and mixed exercise (SMD = 0.34, 95% CI: 0.08 to 0.59, p = 0.01). Our results indicated that there was low heterogeneity for the subgroup of other interventions (I2 = 0%, p = 0.76) (Figure S2b).
Subgroup analyses on the duration of the exercise interventions were performed and included less than 12 weeks (SMD = 0.53, 95% CI: 0.21 to 0.85, p < 0.01) and greater than or equal to 12 weeks (SMD = 0.30, 95% CI: 0.10 to 0.49, p < 0.01). There was high heterogeneity in subgroups based on interventions less than 12 weeks (I2 = 81%, p < 0.01) (Figure S3b).
Regarding the frequency of exercise interventions, subgroup analyses removed studies [32, 46] that did not specify the frequency of exercise to ensure the credibility of this study. The subgroup analysis included 2 times per week (SMD = 0.15, 95% CI: -0.42 to 0.73, p = 0.60), 3 times per week (SMD = 0.69, 95% CI: 0.28 to 1.11, p < 0.01), and more than 3 times per week (SMD = 0.24, 95% CI: -0.15 to 0.63, p = 0.23). Our results suggested low subgroup heterogeneity for exercise interventions performed 3 times per week (I2 = 45%, p = 0.14) and more than 3 times per week (I2 = 0%, p = 0.59) (Figure S4b).
As for cancer patients’ gender, subgroup analyses eliminated studies that did not report gender [40, 41, 48, 52, 54, 64] to assure the credibility of this study. The subgroup analysis included female (SMD=-0.50, 95% CI: 0.23 to 0.78, p < 0.01) and male (SMD = 0.29, 95% CI: -0.05 to 0.63, p = 0.09). Our outcomes indicated a high heterogeneity in the female subgroup (I2 = 79%, p < 0.01) (Figure S5b).
Results of meta-regression analyses
Results of meta-regression analysis of CRF
The results of the meta-regression included exercise types (R2 = 2.44%, p = 0.28), exercise duration (R2 = 0%, p = 0.43), exercise frequency (R2 = 0%, p = 0.52), gender (R2 = 0%, p = 0.62), and cancer types (R2 = 15.66%, p = 0.14). As a result, exercise type, duration, frequency, gender, and cancer type were not potential sources of heterogeneity (see Supplementary file Table 2s).
Results of meta-regression analysis of QoL
The results of the meta-regression included exercise types (R2 = 0%, p = 0.43), exercise duration (R2 = 0%, p = 0.33), exercise frequency (R2 = 0%, p = 0.75), gender (R2 = 0%, p = 0.40), and cancer types (R2 = 0%, p = 0.43). As a result, exercise type, duration, frequency, gender, and cancer type were not potential sources of heterogeneity (see Supplementary file Table 2s).
Discussion
Summary of main results
Our subgroup results showed that exercise duration of less than 12 weeks was effective in improving CRF, while greater than or equal to 12 weeks was invalid; regarding QoL, the effect of exercise duration less than 12 weeks was significantly superior to that of greater than or equal to 12 weeks. Exercise three times a week was effective for QoL, but not for CRF. Moreover, exercise intervention was effective in improving CRF and QoL in female cancer patients. The quality of evidence ranged from low to high. We explored possible sources of heterogeneity in this review in terms of the characteristics of the intervention (type of exercise, duration of exercise, and frequency of exercise) and participants (gender and different types of cancer), although none was statistically significant.
Effectiveness of exercise interventions on CRF
The pooled results indicated that exercise interventions were effective for improving CRF among patients with cancer, which was in accordance with the results of previous studies [70, 71]. Exercise is generally considered an effective approach to improve dysfunction by increasing daytime activities, which can directly improve CRF [72, 73]. Owing to cancer diagnosis and treatment, cancer patients experience grievous CRF stemming from frequent cancer pain [74]. Regular physical exercise provides long-term benefits in reducing the intensity of cancer pain and its disruption of daily life [75], thereby assisting cancer patients in easing CRF. Furthermore, current evidence identified that CRF is secondary to immune dysregulation and inflammatory problems [16, 22]. One probable mechanism is that exercise interventions help cancer patients to strengthen the immune system, regulate body balance, and control inflammation, thereby alleviating fatigue [32, 76].
Effectiveness of exercise interventions on QoL
Regarding QoL, the pooled results showed that exercise interventions were also beneficial in improving QoL among patients with cancer, which was in line with the results described in a prior meta-analysis [77]. One possible explanation is that exercise helps alleviate the symptom distress of cancer patients, which is a critical factor affecting their overall QoL [78]. For example, the release of dopamine from exercise can make patients feel happy, and thus, they can manage negative emotions effectively [79,80,81], and the reinforcement of the immune system can also improve their overall physical fitness [82]. In these ways, cancer patients’ QoL could be improved. Furthermore, unhealthy lifestyles (e.g., a sedentary lifestyle) following a cancer diagnosis could also impact cancer patients’ QoL [83]. Exercise has been demonstrated to be a cost-effective healthy lifestyle habit [84], and maintaining physical activity among cancer patients can benefit the self-management of their cancer-related symptoms. For instance, boosting their motivation to adopt more health-promoting actions and preventing the adverse effects associated with cancer treatment, such as pain and loss of muscle strength, provides them with ongoing benefits [85,86,87].
Subgroup analysis
The results of our subgroup analysis of exercise types suggested that aerobic exercise is most effective for improving CRF and QoL among cancer patients, and the results were in agreement with the findings of previous studies [88, 89]. Cancer patients may tend to avoid exercise due to the burden of symptoms (such as CRF and pain) caused by cancer treatment [90]. Aerobic exercise seems more acceptable by cancer patients than other exercise types because it has lower intensity [91] and fewer potential adverse events, such as falls and muscle soreness [92]. Surprisingly, our results indicated that exercise interventions with durations shorter than 12 weeks showed better effectiveness for cancer patients than those with durations longer than 12 weeks, which was also confirmed in previous studies [93]. This might be because exercise interventions over long periods are likely to result in poor exercise program completion, as cancer patients may lack the motivation to persist [94]. Thus, developing the exercise habits of cancer patients may be a focus of future studies, and longer follow-ups are warranted to verify their exercise compliance and the long-term effects of exercise for patients with cancer.
Regarding the frequency of exercise, the results of our subgroup analysis revealed that exercising three times a week was most effective in enhancing QoL, which is compatible with the 2018 American College of Sports Medicine (ACSM) roundtable [95] stating that engaging in exercise three times a week improves QoL among cancer patients with additional benefits. One possible reason is that an exercise frequency of 3 times a week is easier for cancer patients to accommodate and follow and may also be more appropriate for their exercise tolerance [86], thus contributing to substantial improvements in their health-related well-being and QoL. However, only four studies in this meta-analysis reported that exercising three times a week significantly improved QoL; therefore, further studies with larger sample sizes are warranted. On the other hand, our results indicated that exercising three times per week did not alleviate CRF, which is not in line with the 2018 ACSM roundtable [95] results. CRF is among the most common adverse events during cancer treatment, and cancer patients may suffer from stubborn CRF, even with exercise interventions [96]. Frequent exercise interventions may cause severe postexercise discomfort in cancer patients, which may exacerbate CRF, especially during active cancer treatment [91, 97]. Therefore, further studies are needed to evaluate the viability and rationality of exercise interventions based on the specificity of CRF and to explore appropriate exercise frequencies that effectively improve CRF.
In addition, our subgroup results indicated that exercise greatly improved CRF and QoL in female cancer patients, while the effects were not significant in male. Previous studies have reported higher levels of CRF and worse QoL in females with cancer [98, 99], with the possibility that this may lead to a more significant effect of exercise interventions, from a statistical aspect. On the other hand, females with cancer have more acceptability and greater use of positive adaptive coping strategies, such as healthy behaviors like exercise, to face the disease compared to male cancer patients who tend to adopt an attitude of avoidance and denial [100,101,102]. Hence, female cancer patients may have higher adherence to exercise interventions, leading to better outcomes. Due to the small sample size, the results need to be treated with caution. Therefore, future studies could focus more on the effect of exercise on CRF and QoL in different gender cancer patients to validate our results.
Strengths and limitations
This meta-analysis had several strengths: (a) to the best of our knowledge, this study is the first to examine how different types, durations and frequencies of exercise affect cancer patients’ CRF and QoL; (b) with only the RCTs included in this meta-analysis, there was an enhancement in the methodological quality of the study; (c) the searches were conducted in six major electronic databases by using a combination of MeSH terms and keywords covering cancer and exercise to minimize potential publication bias; and (d) our findings were reliable based on the sensitivity analysis. However, the present study also had some limitations: (a) the majority of studies suffered from a lack of blinding of participants and assessors; (b) due to the small number of relevant included studies, we were unable to perform subgroup analyses of cancer stage, thus failing to identify the heterogeneity of patient groups and the potential association with outcomes; and (c) despite a comprehensive search strategy, we were unable to uncover the full texts of some studies. Thus, our conclusions need to be interpreted with caution.
Implications for nursing practice and further research
Based on the results of our study that we recommend aerobic exercise 3 times a week for cancer patients. Notably, our results suggested that short-term exercise interventions are more effective for cancer patients compared to long-term exercise. Thus, future studies should consider tailoring stage-specific exercise programs to cancer patients in different stages. Moreover, previous studies have shown the effectiveness of online exercise interventions [103], whereas the studies included in our meta-analysis were based entirely on offline activities. Hence, it is imperative to transfer exercise interventions from the real world to online against the background of the COVID-19 epidemic as a serious worldwide public health problem [104]. For example, an artificial intelligence exercise app for cancer patients led by medical staff and a social platform similar to WeChat can be developed to give cancer patients good health education and guidance to improve cancer-related symptoms and QoL. In addition, future studies with larger sample sizes are needed to focus on the impact of gender in the exercise of cancer patients.
Conclusions
In brief, exercise interventions are effective in relieving CRF and improving QoL among patients with cancer. In this study, the most effective improvement in CRF and QoL was an aerobic exercise intervention of less than 12 weeks, and regarding the frequency of the intervention, three times a week was the most beneficial. Exercise intervention was more significant in improving CRF and QoL in female cancer patients. Moreover, stage-specific exercise interventions should be developed for patients with different cancer stages, and online factors should receive further attention and exploration and be introduced into future exercise intervention studies.
Data Availability
All data generated or analyzed during this study is included in this published article.
Abbreviations
- CRF:
-
Cancer-related fatigue
- QoL:
-
Quality of life
References
World Health Organization., https://www.who.int/health-topics/cancer/.
International Agency for Research on Cancer., https://gco.iarc.fr/.
Li J, Liu Y, Jiang J, Peng X, Hu X. Effect of telehealth interventions on quality of life in cancer survivors: a systematic review and meta-analysis of randomized controlled trials. Int J Nurs Stud. 2021;122:103970. https://doi.org/10.1016/j.ijnurstu.2021.103970.
Firkins J, Hansen L, Driessnack M, Dieckmann N. Quality of life in “chronic” cancer survivors: a meta-analysis. J Cancer Surviv. 2020;14(4):504–17. https://doi.org/10.1007/s11764-020-00869-9.
Zhang Y, Li J, Hu X. The effectiveness of dignity therapy on hope, quality of life, anxiety, and depression in cancer patients: a meta-analysis of randomized controlled trials. Int J Nurs Stud. 2022;132:104273. https://doi.org/10.1016/j.ijnurstu.2022.104273.
Huizinga F, Westerink NL, Berendsen AJ, Walenkamp AME, MHG DEG, Oude Nijeweeme JK, GH DEB, Berger MY, Brandenbarg D. Home-based physical activity to alleviate fatigue in Cancer Survivors: a systematic review and Meta-analysis. Med Sci Sports Exerc. 2021;53(12):2661–74. https://doi.org/10.1249/MSS.0000000000002735.
Zetzl T, Renner A, Pittig A, Jentschke E, Roch C, van Oorschot B. Yoga effectively reduces fatigue and symptoms of depression in patients with different types of cancer. Support Care Cancer. 2021;29(6):2973–82. https://doi.org/10.1007/s00520-020-05794-2.
Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713–23. https://doi.org/10.1016/j.annonc.2020.02.016.
Chen YJ, Li XX, Ma HK, Zhang X, Wang BW, Guo TT, et al. Exercise training for improving patient-reported outcomes in patients with Advanced-Stage Cancer: a systematic review and Meta-analysis. J Pain Symptom Manage. 2020;59(3):734–749e710. https://doi.org/10.1016/j.jpainsymman.2019.09.010.
Matias M, Baciarello G, Neji M, Di Meglio A, Michiels S, Partridge AH, et al. Fatigue and physical activity in cancer survivors: a cross-sectional population-based study. Cancer Med. 2019;8(5):2535–44. https://doi.org/10.1002/cam4.2060.
Lobefaro R, Rota S, Porcu L, Brunelli C, Alfieri S, Zito E, et al. Cancer-related fatigue and depression: a monocentric, prospective, cross-sectional study in advanced solid tumors. ESMO Open. 2022;7(2):100457. https://doi.org/10.1016/j.esmoop.2022.100457.
Tian L, Lu HJ, Lin L, Hu Y. Effects of aerobic exercise on cancer-related fatigue: a meta-analysis of randomized controlled trials. Support Care Cancer. 2016;24(2):969–83. https://doi.org/10.1007/s00520-015-2953-9.
Akbas M, Surucu SG, Akca E, Koroglu CO. Determination of the relationship between the fatigue and social support levels of cancer patients: a cross-sectional study. Korean J Intern Med. 2021;36(Suppl 1):207–S216. https://doi.org/10.3904/kjim.2019.010.
Hilfiker R, Meichtry A, Eicher M, Nilsson Balfe L, Knols RH, Verra ML, Taeymans J. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. 2018;52(10):651–8. https://doi.org/10.1136/bjsports-2016-096422.
Ma Y, He B, Jiang M, Yang Y, Wang C, Huang C, Han L. Prevalence and risk factors of cancer-related fatigue: a systematic review and meta-analysis. Int J Nurs Stud. 2020;111:103707. https://doi.org/10.1016/j.ijnurstu.2020.103707.
Weber D, O’Brien K. Cancer and Cancer-Related fatigue and the Interrelationships with Depression, stress, and inflammation. J Evid Based Complementary Altern Med. 2017;22(3):502–12. https://doi.org/10.1177/2156587216676122.
Gong X, Rong G, Wang Z, Zhang A, Li X, Wang L. Baduanjin exercise for patients with breast cancer: a systematic review and meta-analysis. Complement Ther Med. 2022;71:102886. https://doi.org/10.1016/j.ctim.2022.102886.
Lewandowska A, Rudzki G, Lewandowski T, Prochnicki M, Rudzki S, Laskowska B, Brudniak J. Quality of life of Cancer Patients treated with chemotherapy. Int J Environ Res Public Health. 2020;17(19):6938. https://doi.org/10.3390/ijerph17196938.
van Nieuwenhuizen AJ, Buffart LM, Langendijk JA, Vergeer MR, Voortman J, Leemans CR, Verdonck-de Leeuw IM. Health-related quality of life and overall survival: a prospective study in patients with head and neck cancer treated with radiotherapy. Qual Life Res. 2021;30(4):1145–53. https://doi.org/10.1007/s11136-020-02716-x.
Haraldstad K, Wahl A, Andenaes R, Andersen JR, Andersen MH, Beisland E, et al. A systematic review of quality of life research in medicine and health sciences. Qual Life Res. 2019;28(10):2641–50. https://doi.org/10.1007/s11136-019-02214-9.
Ozdemir K, Keser I, Sen I, Ozgur Tan M. Investigating the relationships between quality of life, fatigue and leisure time physical activity in prostate cancer patients. J Back Musculoskelet Rehabil. 2019;32(3):497–503. https://doi.org/10.3233/BMR-181220.
Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. https://doi.org/10.1038/nrclinonc.2014.127.
Duncan M, Moschopoulou E, Herrington E, Deane J, Roylance R, Jones L, et al. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open. 2017;7(11):e015860. https://doi.org/10.1136/bmjopen-2017-015860.
Zachariae R, Lyby MS, Ritterband LM, O’Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia – a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. https://doi.org/10.1016/j.smrv.2015.10.004.
He Z, Song A, Zhang Z, Zhang Y, Liu X, Zhao L, Lv X, Ren G, Li Y. Comparative efficacy of non-pharmacological adjuvant therapies for quality of life in the patients with breast cancer receiving chemo- or radio-therapy: a protocol for systematic review and bayesian network meta-analysis. Med (Baltim). 2018;97(35):e12096. https://doi.org/10.1097/MD.0000000000012096.
Thong MSY, van Noorden CJF, Steindorf K, Arndt V. Cancer-Related fatigue: causes and current treatment options. Curr Treat Options Oncol. 2020;21(2):17. https://doi.org/10.1007/s11864-020-0707-5.
Khosravi N, Stoner L, Farajivafa V, Hanson ED. Exercise training, circulating cytokine levels and immune function in cancer survivors: a meta-analysis. Brain Behav Immun. 2019;81:92–104. https://doi.org/10.1016/j.bbi.2019.08.187.
Luan X, Tian X, Zhang H, Huang R, Li N, Chen P, Wang R. Exercise as a prescription for patients with various diseases. J Sport Health Sci. 2019;8(5):422–41. https://doi.org/10.1016/j.jshs.2019.04.002.
Montalvo RN, Hardee JP, VanderVeen BN, Carson JA. Resistance Exercise’s ability to Reverse Cancer-Induced Anabolic Resistance. Exerc Sport Sci Rev. 2018;46(4):247–53. https://doi.org/10.1249/JES.0000000000000159.
Paneroni M, Simonelli C, Vitacca M, Ambrosino N. Aerobic Exercise training in very severe chronic obstructive Pulmonary Disease: a systematic review and Meta-analysis. Am J Phys Med Rehabil 2017, 96(8):541–8. https://doi.org/10.1097/PHM.0000000000000667.
Wu G, Zhang X, Gao F. The epigenetic landscape of exercise in cardiac health and disease. J Sport Health Sci. 2021;10(6):648–59. https://doi.org/10.1016/j.jshs.2020.12.003.
Schmidt T, Jonat W, Wesch D, Oberg HH, Adam-Klages S, Keller L, Rocken C, Mundhenke C. Influence of physical activity on the immune system in breast cancer patients during chemotherapy. J Cancer Res Clin Oncol. 2018;144(3):579–86. https://doi.org/10.1007/s00432-017-2573-5.
Galvao DA, Newton RU, Chambers SK, Spry N, Joseph D, Gardiner RA, Fairman CM, Taaffe DR. Psychological distress in men with prostate cancer undertaking androgen deprivation therapy: modifying effects of exercise from a year-long randomized controlled trial. Prostate Cancer Prostatic Dis. 2021;24(3):758–66. https://doi.org/10.1038/s41391-021-00327-2.
O’Neill M, Samaroo D, Lopez C, Tomlinson G, Santa Mina D, Sabiston C, Culos-Reed N, Alibhai SMH. The effect of yoga interventions on Cancer-Related fatigue and quality of life for women with breast Cancer: a systematic review and Meta-analysis of Randomized controlled trials. Integr Cancer Ther. 2020;19:1534735420959882. https://doi.org/10.1177/1534735420959882.
Scott JM, Thomas SM, Herndon JE 2nd, Douglas PS, Yu AF, Rusch V, et al. Effects and tolerability of exercise therapy modality on cardiorespiratory fitness in lung cancer: a randomized controlled trial. J Cachexia Sarcopenia Muscle. 2021;12(6):1456–65. https://doi.org/10.1002/jcsm.12828.
Nakano J, Hashizume K, Fukushima T, Ueno K, Matsuura E, Ikio Y, et al. Effects of Aerobic and Resistance exercises on physical symptoms in Cancer Patients: a Meta-analysis. Integr Cancer Ther. 2018;17(4):1048–58. https://doi.org/10.1177/1534735418807555.
Pearce M, Garcia L, Abbas A, Strain T, Schuch FB, Golubic R, et al. Association between Physical Activity and Risk of Depression: a systematic review and Meta-analysis. JAMA Psychiatry. 2022;79(6):550–9. https://doi.org/10.1001/jamapsychiatry.2022.0609.
McNeely ML, Parliament MB, Seikaly H, Jha N, Magee DJ, Haykowsky MJ, Courneya KS. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer. 2008;113(1):214–22. https://doi.org/10.1002/cncr.23536.
Villumsen BR, Jorgensen MG, Frystyk J, Hordam B, Borre M. Home-based ‘exergaming’ was safe and significantly improved 6-min walking distance in patients with prostate cancer: a single-blinded randomised controlled trial. BJU Int. 2019;124(4):600–8. https://doi.org/10.1111/bju.14782.
Kim JY, Lee MK, Lee DH, Kang DW, Min JH, Lee JW, et al. Effects of a 12-week home-based exercise program on quality of life, psychological health, and the level of physical activity in colorectal cancer survivors: a randomized controlled trial. Support Care Cancer. 2019;27(8):2933–40. https://doi.org/10.1007/s00520-018-4588-0.
Broderick JM, Guinan E, Kennedy MJ, Hollywood D, Courneya KS, Culos-Reed SN, Bennett K, DM OD, Hussey J. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7(4):551–62. https://doi.org/10.1007/s11764-013-0294-6.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Higgins JP, Thomas J, Chandler J et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3, 2022 (updated March 2023). Cochrane, 2023. Retrieved from: https://training.cochrane.org/handbook/)
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
Rucker G, Cates CJ, Schwarzer G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res Synth Methods. 2017;8(4):392–403. https://doi.org/10.1002/jrsm.1259.
Bourke L, Stevenson R, Turner R, Hooper R, Sasieni P, Greasley R, et al. Exercise training as a novel primary treatment for localised prostate cancer: a multi-site randomised controlled phase II study. Sci Rep. 2018;8(1):8374. https://doi.org/10.1038/s41598-018-26682-0.
Cataldi S, Latino F, Greco G, Fischetti F. Multilateral training improves physical fitness and fatigue perception in cancer patients. In: Journal of Human Sport and Exercise – 2019 - Spring Conferences of Sports Science 2019, 14: S916-S926. https://doi.org/10.14198/jhse.2019.14.Proc4.54.
Christensen JF, Sundberg A, Osterkamp J, Thorsen-Streit S, Nielsen AB, Olsen CK, et al. Interval walking improves Glycemic Control and Body Composition after Cancer Treatment: a Randomized Controlled Trial. J Clin Endocrinol Metab. 2019;104(9):3701–12. https://doi.org/10.1210/jc.2019-00590.
Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–8. https://doi.org/10.1200/JCO.2003.04.093.
Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, et al. Six-month follow-up of patient-rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2572–8. https://doi.org/10.1158/1055-9965.EPI-07-0413.
Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27(27):4605–12. https://doi.org/10.1200/JCO.2008.20.0634.
Donnelly CM, Blaney JM, Lowe-Strong A, Rankin JP, Campbell A, McCrum-Gardner E, Gracey JH. A randomised controlled trial testing the feasibility and efficacy of a physical activity behavioural change intervention in managing fatigue with gynaecological cancer survivors. Gynecol Oncol. 2011;122(3):618–24. https://doi.org/10.1016/j.ygyno.2011.05.029.
Gokal K, Wallis D, Ahmed S, Boiangiu I, Kancherla K, Munir F. Effects of a self-managed home-based walking intervention on psychosocial health outcomes for breast cancer patients receiving chemotherapy: a randomised controlled trial. Support Care Cancer. 2016;24(3):1139–66. https://doi.org/10.1007/s00520-015-2884-5.
Henke CC, Cabri J, Fricke L, Pankow W, Kandilakis G, Feyer PC, de Wit M. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer. 2014;22(1):95–101. https://doi.org/10.1007/s00520-013-1925-1.
Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer. 2012;20(12):3169–77. https://doi.org/10.1007/s00520-012-1452-5.
Kang DW, Fairey AS, Boule NG, Field CJ, Wharton SA, Courneya KS. A Randomized Trial of the Effects of Exercise on anxiety, fear of Cancer Progression and Quality of life in prostate Cancer patients on active surveillance. J Urol. 2022;207(4):814–22. https://doi.org/10.1097/JU.0000000000002334.
Mijwel S, Jervaeus A, Bolam KA, Norrbom J, Bergh J, Rundqvist H, Wengstrom Y. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J Cancer Surviv. 2019;13(2):244–56. https://doi.org/10.1007/s11764-019-00747-z.
Murtezani A, Ibraimi Z, Bakalli A, Krasniqi S, Disha ED, Kurtishi I. The effect of aerobic exercise on quality of life among breast cancer survivors: a randomized controlled trial. J Cancer Res Ther. 2014;10(3):658–64. https://doi.org/10.4103/0973-1482.137985.
Ndjavera W, Orange ST, O’Doherty AF, Leicht AS, Rochester M, Mills R, Saxton JM. Exercise-induced attenuation of treatment side-effects in patients with newly diagnosed prostate cancer beginning androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2020;125(1):28–37. https://doi.org/10.1111/bju.14922.
Nyrop KA, Callahan LF, Cleveland RJ, Arbeeva LL, Hackney BS, Muss HB. Randomized Controlled Trial of a home-based walking program to reduce moderate to severe aromatase Inhibitor-Associated Arthralgia in breast Cancer survivors. Oncologist. 2017;22(10):1238–49. https://doi.org/10.1634/theoncologist.2017-0174.
Paulo TRS, Rossi FE, Viezel J, Tosello GT, Seidinger SC, Simoes RR, de Freitas R Jr, Freitas IF Jr. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial. Health Qual Life Outcomes. 2019;17(1):17. https://doi.org/10.1186/s12955-019-1090-4.
Reis D, Walsh ME, Young-McCaughan S, Jones T. Effects of Nia exercise in women receiving radiation therapy for breast cancer. Oncol Nurs Forum. 2013;40(5):E374–381. https://doi.org/10.1188/13.ONF.E374-E381.
Santagnello SB, Martins FM, de Oliveira Junior GN, de Freitas Rodrigues J, Nomelini RS, Murta EFC, Orsatti FL. Improvements in muscle strength, power, and size and self-reported fatigue as mediators of the effect of resistance exercise on physical performance breast cancer survivor women: a randomized controlled trial. Support Care Cancer. 2020;28(12):6075–84. https://doi.org/10.1007/s00520-020-05429-6.
Saraboon C, Siriphorn A. Effects of foam pad balance exercises on cancer patients undergoing chemotherapy: a randomized control trial. J Bodyw Mov Ther. 2021;28:164–71. https://doi.org/10.1016/j.jbmt.2021.07.013.
Shobeiri F, Masoumi SZ, Nikravesh A, Heidari Moghadam R, Karami M. The impact of Aerobic Exercise on Quality of Life in women with breast Cancer: a Randomized Controlled Trial. J Res Health Sci. 2016;16(3):127–32.
Taso CJ, Lin HS, Lin WL, Chen SM, Huang WT, Chen SW. The effect of yoga exercise on improving depression, anxiety, and fatigue in women with breast cancer: a randomized controlled trial. J Nurs Res. 2014;22(3):155–64. https://doi.org/10.1097/jnr.0000000000000044.
Zhou W, Wan YH, Chen Q, Qiu YR, Luo XM. Effects of Tai Chi Exercise on Cancer-Related fatigue in patients with nasopharyngeal carcinoma undergoing chemoradiotherapy: a Randomized Controlled Trial. J Pain Symptom Manage. 2018;55(3):737–44. https://doi.org/10.1016/j.jpainsymman.2017.10.021.
Hwang JH, Chang HJ, Shim YH, Park WH, Park W, Huh SJ, Yang JH. Effects of supervised exercise therapy in patients receiving radiotherapy for breast cancer. Yonsei Med J. 2008;49(3):443–50. https://doi.org/10.3349/ymj.2008.49.3.443.
Adams SC, DeLorey DS, Davenport MH, Fairey AS, North S, Courneya KS. Effects of high-intensity interval training on fatigue and quality of life in testicular cancer survivors. Br J Cancer. 2018;118(10):1313–21. https://doi.org/10.1038/s41416-018-0044-7.
Gebruers N, Camberlin M, Theunissen F, Tjalma W, Verbelen H, Van Soom T, van Breda E. The effect of training interventions on physical performance, quality of life, and fatigue in patients receiving breast cancer treatment: a systematic review. Support Care Cancer. 2019;27(1):109–22. https://doi.org/10.1007/s00520-018-4490-9.
Medeiros Torres D, Jorge Koifman R, da Silva Santos S. Impact on fatigue of different types of physical exercise during adjuvant chemotherapy and radiotherapy in breast cancer: systematic review and meta-analysis. Support Care Cancer. 2022;30(6):4651–62. https://doi.org/10.1007/s00520-022-06809-w.
Chen D, Yin Z, Fang B. Measurements and status of sleep quality in patients with cancers. Support Care Cancer. 2018;26(2):405–14. https://doi.org/10.1007/s00520-017-3927-x.
Lin PJ, Kleckner IR, Loh KP, Inglis JE, Peppone LJ, Janelsins MC, et al. Influence of yoga on Cancer-Related fatigue and on Mediational Relationships between changes in Sleep and Cancer-Related fatigue: a Nationwide, Multicenter Randomized Controlled Trial of yoga in Cancer Survivors. Integr Cancer Ther. 2019;18:1534735419855134. https://doi.org/10.1177/1534735419855134.
Ebede CC, Jang Y, Escalante CP. Cancer-Related fatigue in Cancer Survivorship. Med Clin North Am. 2017;101(6):1085–97. https://doi.org/10.1016/j.mcna.2017.06.007.
Reis AD, Pereira P, Diniz RR, de Castro Filha JGL, Dos Santos AM, Ramallo BT, Filho FAA, Navarro F, Garcia JBS. Effect of exercise on pain and functional capacity in breast cancer patients. Health Qual Life Outcomes. 2018;16(1):58. https://doi.org/10.1186/s12955-018-0882-2.
Daniela M, Catalina L, Ilie O, Paula M, Daniel-Andrei I, Ioana B. Effects of Exercise Training on the autonomic nervous system with a focus on anti-inflammatory and Antioxidants Effects. Antioxid (Basel). 2022;11(2). https://doi.org/10.3390/antiox11020350.
Rendeiro JA, Rodrigues C, de Barros Rocha L, Rocha RSB, da Silva ML, da Costa Cunha KC. Physical exercise and quality of life in patients with prostate cancer: systematic review and meta-analysis. Support Care Cancer. 2021;29(9):4911–9. https://doi.org/10.1007/s00520-021-06095-y.
Huang HY, Tsai WC, Chou WY, Hung YC, Liu LC, Huang KF, et al. Quality of life of breast and cervical cancer survivors. BMC Womens Health. 2017;17(1):30. https://doi.org/10.1186/s12905-017-0387-x.
Fischetti F, Greco G, Cataldi S, Minoia C, Loseto G, Guarini A. Effects of Physical Exercise intervention on psychological and physical fitness in Lymphoma Patients. Med (Kaunas). 2019;55(7). https://doi.org/10.3390/medicina55070379.
Greenwood BN. The role of dopamine in overcoming aversion with exercise. Brain Res. 2019;1713:102–8. https://doi.org/10.1016/j.brainres.2018.08.030.
Martinez-Moreno A, Ibanez-Perez RJ, Cavas-Garcia FF, Cano-Noguera F. The influence of physical activity, anxiety, Resilience and Engagement on the optimism of older adults. Int J Environ Res Public Health. 2020;17(21). https://doi.org/10.3390/ijerph17218284.
Gustafson MP, Wheatley-Guy CM, Rosenthal AC, Gastineau DA, Katsanis E, Johnson BD, Simpson RJ. Exercise and the immune system: taking steps to improve responses to cancer immunotherapy. J Immunother Cancer. 2021;9(7). https://doi.org/10.1136/jitc-2020-001872.
Lynch BM, Nguyen NH, Moore MM, Reeves MM, Rosenberg DE, Boyle T, et al. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: the ACTIVATE Trial. Cancer. 2019;125(16):2846–55. https://doi.org/10.1002/cncr.32143.
Liu ZY, Wang C, Zhang YJ, Zhu HL. Combined lifestyle, mental health, and mortality in US cancer survivors: a national cohort study. J Transl Med. 2022;20(1):376. https://doi.org/10.1186/s12967-022-03584-4.
Kelley MM, Kue J, Brophy L, Peabody AL, Foraker RE, Yen PY, Tucker S. Mobile Health Applications, Cancer Survivors, and Lifestyle Modification: an integrative review. Comput Inf Nurs. 2021;39(11):755–63. https://doi.org/10.1097/CIN.0000000000000781.
Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psychooncology. 2018;27(3):713–24. https://doi.org/10.1002/pon.4612.
Wang Q, Zhou W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J Sport Health Sci. 2021;10(2):201–10. https://doi.org/10.1016/j.jshs.2020.07.008.
Yang M, Liu L, Gan CE, Qiu LH, Jiang XJ, He XT, Zhang JE. Effects of home-based exercise on exercise capacity, symptoms, and quality of life in patients with lung cancer: a meta-analysis. Eur J Oncol Nurs. 2020;49:101836. https://doi.org/10.1016/j.ejon.2020.101836.
Batalik L, Winnige P, Dosbaba F, Vlazna D, Janikova A. Home-Based Aerobic and Resistance Exercise Interventions in Cancer Patients and Survivors: a systematic review. Cancers (Basel). 2021;13(8):1915. https://doi.org/10.3390/cancers13081915.
Granger CL, Connolly B, Denehy L, Hart N, Antippa P, Lin KY, Parry SM. Understanding factors influencing physical activity and exercise in lung cancer: a systematic review. Support Care Cancer. 2017;25(3):983–99. https://doi.org/10.1007/s00520-016-3484-8.
Kessels E, Husson O, van der Feltz-Cornelis CM. The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2018;14:479–94. https://doi.org/10.2147/NDT.S150464.
Miller KJ, Areerob P, Hennessy D, Goncalves-Bradley DC, Mesagno C, Grace F. Aerobic, resistance, and mind-body exercise are equivalent to mitigate symptoms of depression in older adults: a systematic review and network meta-analysis of randomised controlled trials. F1000Res. 2020;9:1325. https://doi.org/10.12688/f1000research.27123.2.
Li CQ, Wang YC, Shen SQ, Zhang YL, Zhao JQ, Zou WB, Ge RL. Effects of exercise by type and duration on quality of life in patients with digestive system cancers: a systematic review and network meta-analysis. J Sport Health Sci. 2022. https://doi.org/10.1016/j.jshs.2022.12.008.
Kampshoff CS, van Dongen JM, van Mechelen W, Schep G, Vreugdenhil A, Twisk JWR, et al. Long-term effectiveness and cost-effectiveness of high versus low-to-moderate intensity resistance and endurance exercise interventions among cancer survivors. J Cancer Surviv. 2018;12(3):417–29. https://doi.org/10.1007/s11764-018-0681-0.
Campbell kL kM Winters-stone, j. Wiskemann, a. M.may, Schwartz aL, Courneya kS, Zucker dS, Matthews cE, Ligibel jA, Gerber lH, Morris gS, Patel aV, t., Hue F, Perna fM. and k. H. Schmitz.: Exercise Guidelines for Cancer Survivors:Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 2019, 51(11):2375–2390. https://doi.org/10.1249/MSS.0000000000002116.
Li J, Zhu C, Liu C, Su Y, Peng X, Hu X. Effectiveness of eHealth interventions for cancer-related pain, fatigue, and sleep disorders in cancer survivors: a systematic review and meta-analysis of randomized controlled trials. J Nurs Scholarsh. 2022;54(2):184–90. https://doi.org/10.1111/jnu.12729.
Schaffer K, Panneerselvam N, Loh KP, Herrmann R, Kleckner IR, Dunne RF, et al. Systematic review of Randomized controlled trials of Exercise Interventions using Digital Activity Trackers in patients with Cancer. J Natl Compr Canc Netw. 2019;17(1):57–63. https://doi.org/10.6004/jnccn.2018.7082.
Laghousi D, Jafari E, Nikbakht H, Nasiri B, Shamshirgaran M, Aminisani N. Gender differences in health-related quality of life among patients with colorectal cancer. J Gastrointest Oncol. 2019;10(3):453–61. https://doi.org/10.21037/jgo.2019.02.04.
Darezzo Rodrigues Nunes M, Jacob E, Adlard K, Secola R, Nascimento L. Fatigue and sleep experiences at home in children and adolescents with Cancer. Oncol Nurs Forum. 2015;42(5):498–506. https://doi.org/10.1188/15.ONF.498-506.
Park JH, Lee JS, Ko YH, Kim YH. Physical activity of korean cancer survivors is associated with age and sex. Korean J Intern Med. 2021;36(Suppl 1):225–S234. https://doi.org/10.3904/kjim.2019.240.
Jehn P, Linsen SS, Zeller AN, Eckstein FM, Neuhaus MT, Gellrich NC, et al. Gender-specific differences concerning psychosocial aspects and functional impairments that influence quality of life in oral cancer treatment. Support Care Cancer. 2022;30(6):4905–15. https://doi.org/10.1007/s00520-022-06907-9.
Bottaro R, Faraci P. The influence of socio-demographics and clinical characteristics on coping strategies in cancer patients: a systematic review. Support Care Cancer. 2022;30(11):8785–803. https://doi.org/10.1007/s00520-022-07267-0.
Galiano-Castillo N, Cantarero-Villanueva I, Fernandez-Lao C, Ariza-Garcia A, Diaz-Rodriguez L, Del-Moral-Avila R, Arroyo-Morales M. Telehealth system: a randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122(20):3166–74. https://doi.org/10.1002/cncr.30172.
Hu X, Huang W. Protecting the psychological well-being of healthcare workers affected by the COVID-19 outbreak: perspectives from China. Nurs Health Sci. 2020;22(3):837–8. https://doi.org/10.1111/nhs.12727.
Acknowledgements
We would like to thank the Professor Mary for her support and guidance.
Funding
The study is supported by the National Natural Sciences Foundation of China (82172842 and 81803104), Sichuan Science and Technology Program and TianFu Laboratory (2022YFSY0012 and 2021ZYCD011), the National Key Research and Development Program of China (2021YFE0206600), the Post-Doctor Research Project, West China Hospital, Sichuan University (2020HXBH119), West China Nursing Discipline Development Special Fund Project, Sichuan University (HXHL21008), and the China Medical Board (Grant #22–482).
Author information
Authors and Affiliations
Contributions
Study Design and writing: XLC, JJL, XLH, and CCC. Data Collection: SZ, YZ, and LZ.Data Analysis and Interpretation: XLC, JJL and YLZ. Manuscript Writing: XLC, JJL and XLH.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No conflict of interest exists in the submission of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, X., Li, J., Chen, C. et al. Effects of exercise interventions on cancer-related fatigue and quality of life among cancer patients: a meta-analysis. BMC Nurs 22, 200 (2023). https://doi.org/10.1186/s12912-023-01363-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12912-023-01363-0