Abstract
Background
Urinary retention is common in elderly patients undergoing acute hip fracture surgery. Avoiding overfilling the urinary bladder is important to avoid detrusor muscle damage and associated motility problems. The aim of this study was to analyse associations between the co-creation of a nurse-driven urinary catheterisation protocol and the incidence of bladder distension in patients undergoing hip fracture surgery.
Methods
This is a single-centre implementation intervention with a retrospective longitudinal observation design, using five measures points, spanning from June 2015 to March 2020. The intervention was theory driven and the participants, together with the facilitators and researcher, co-created a nurse-driven urinary catheterisation protocol. Data were retrieved from the hip fracture register. Uni- and multivariable logistic regressions were used for analyses of changes in bladder distension and urinary volume of ≥500 ml over the years.
Results
A total of 3078 patients were included over a five-year period. The implementation intervention was associated with a reduction in the proportion of patients with bladder distension of 31.5% (95% confidence interval 26.0–37.0), from year 1 to year 5. The multivariable analysis indicated a 39% yearly reduction in bladder distension, OR 0.61 (95% confidence interval 0.57–0.64, p < 0001). There was a reduction in the proportion of patients with a bladder volume of ≥500 ml of 42.8% (95% confidence interval 36.2–49.4), from year 1 to year 5. The multivariable analysis found a 41% yearly reduction in patients with a bladder volume of ≥500 ml, OR 0.59 (95% confidence interval 0.55–0.64, p < 0.0001). The intervention was associated with improved documentation of both catheter indications and removal plans.
Conclusion
The use of predefined catheter indications and a tighter bladder scanning schedule were associated with a reduction in the incidence of both bladder distension and urine volume ≥ 500 ml in hip fracture patients. Registered nurses can play an active role in the facilitation of timely and appropriate catheter treatment in patients with hip fractures.
Trial registration
Clinical Trial Registry ISRCTN 17022695 registered retrospectively on 23 December 2021, in the end of the study, after data collection.
Similar content being viewed by others
Background
Since To Err is Human report [1], healthcare has a good knowledge of hospital-related adverse events, their associated preventive strategies, but also how challenging it can be to implement and routinise evidence-based best practice in clinical daily work [2, 3]. In high-income countries, one in ten patients is estimated to suffer from adverse events [3]. In Sweden, adverse events occurs in almost 98,000 patients every year, of which bladder distension was reported in approximately 10% of the adverse events [4]. Further, bladder distension is a largely preventable adverse event if evidence-based best practice is adhered to [4, 5]. Bladder distension occurs when the bladder is overfilled with urine. Even though the bladder threshold varies [6], and reduces with age [7], bladder capacity is commonly reported to range between 400 and 600 ml and, in some patients, a volume of between 500 and 1000 ml might be unharmful, if treated within one to 2 hours [8, 9], However, if undetected, the tension of the bladder wall when it is overfilled can damage the detrusor muscle [8, 10, 11]. Iatrogenic bladder damage has been shown to affect patients’ daily life substantially, due to chronic catheter treatment or straight in-out self-catheterisation, recurrent urinary tract infections (UTIs) and/or urosepsis and a mistrust to the healthcare system [12]. Moreover, insufficient routines, lack of knowledge and poor communication between healthcare workers (HCW) and patients have been identified as factors contributing to the development of bladder damage [12].
Orthopaedic patients are especially prone to develop bladder distension compared to other specialities [5, 13]. Specifically, most patients undergoing hip fracture surgery, have several intrinsic and extrinsic factors that increase the risk of urinary retention (UR) [7, 8, 14, 15]. The reported incidence of pre- and post-operative UR varies between 4 and 82% [16,17,18,19,20,21,22,23] and has proven to be a persistent problem in rehabilitation units [24]. Variations in UR rates may explain some of these findings.
In 2015, we initiated the Safe Hands project [25, 26], where new preventive bundle routines were co-created with HCW. One routine aiming to improve hand hygiene, the use of aseptic insertion techniques and indwelling urinary catheter (IDC) care was associated with a reduction in urinary catheter (UC)-associated UTI from 18 to 4%, after introduction in the care pathway of hip fracture patients, which was the first step in our bladder bundle [27]. As a co-finding, we observed a high incidence of bladder distension, a lack of appropriate use of IDC indications removal plan and related documentation, as well as a timely bladder scan. As a result, increased awareness and the use of preventive strategies were needed [28,29,30,31,32,33]. Given this, a joint decision was taken by managers, leaders, quality co-ordinators and researchers to also address these problems by including a co-created nurse-driven UC protocol and timely bladder scanning schedule as a second step in our bladder bundle intervention. The overall aim of this study was to analyse association between the co-creation of a nurse-driven UC protocol and the incidence of bladder distension in patients undergoing hip fracture surgery.
Methods
Design
This is a single-centre implementation intervention with a retrospective longitudinal observation design, using five measures points, spanning from June 2015 to March 2020. For patient outcomes, data from the local hip fracture quality register were retrieved and analysed. The primary outcome: Changes in the incidence of bladder distension before and after the intervention. Bladder distension were defined as; a) urine volume ≥ 500 ml twice or ≥ 1000 ml once, according to the Swedish national trigger tool [34], b) a physician-diagnosed bladder distension with a urine volume of < 1000 ml or no volume documented and with an IDC present at discharge. Not defined as bladder distension; patients with a documented urine volume of < 1000 ml once or no volume documented, treated with an IDC due to indications other than UR or residual urine.
Secondary outcomes: Changes in the incidence before and after the intervention of, i) a bladder volume of ≥500 ml, ii) the largest urine volume documented during hospital stay and iii) changes in documented catheter indication(s) and removal plan over the years. Patients with no urine volume documented and an IDC indication other than UR or residual urine were considered as not having a urine volume of ≥500 ml. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) criteria for reporting observational studies were followed [35].
Setting and participants
The study setting was an orthopaedic department at a university hospital performing 800–900 acute hip fracture surgeries annually. The participating wards were selected as they were involved in the care of acute hip fracture patients, ≥ 65 years of age: the emergency department (ED), three ortho-geriatric wards, the operating room (OR) and the post-anaesthesia care unit (PACU)/intensive care units (ICU). The HCW participating in the intervention program were registered nurses (RNs) approximately 400, of which some were specialist within critical care, anestesia care nurses, OR-nurses and within surgical care and nurse assistants. Appointed local facilitators, called expert nurses, participated in the learning lab meetings describe below. The register nurses in the involved units assessed patients with hip fracture according to the protocol, described below. These patients did not participate in the intervention program.
Prior to the intervention, the hospital’s routine for preventing UTI was to use straight in-out catheterisation if the UR was ≥400 ml, with a six- to eight-hour bladder scanning timespan to measure UR or residual urine. An IDC was used, if prescribed by a physician, and routinely removed on day one post-surgery, unless a need to continue was identified, using an IDC marker on the patient board alerting that an IDC was in situ.
Theoretical foundation and implementation strategies
This study was based on integrated knowledge translation (iKT) to facilitate knowledge transfer, i.e. the researcher and main facilitators work in partnership and collaborate with the local expert nurses during the implementation period [36]. The iKT processes used were informed by theories of dialogue and organisational learning [37, 38]. Facilitation was used as a means of overcoming barriers and supporting the participants [39, 40]. The implementation process had an emergent and flexible approach. The main facilitators were:
-
i.
a senior researcher, RN and expert in infection prevention and implementation
-
ii.
an RN specialising in critical care and anaesthesia nursing
-
iii.
a senior researcher, consultant specialist in anaesthesiology and expert on hip fracture patients
-
iv.
a consultant specialist in gerontology
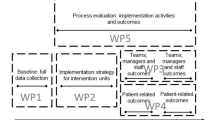
As part of the iKT process, the main facilitators and research together with the local expert nurses, appointed physicians, first-line leaders and quality co-ordinators set out goals and plans for the intervention. The local expert nurses were appointed by the first-line leaders, either one to two RNs and/or nurse assistants, who functioned as internal facilitators. Moreover, in the pre-planning of the intervention, potential barriers and enablers were considered, as well as specific contextual features [41, 42]. The time frame of the implementation intervention, strategies and components are presented in Fig. 1.
Interventional components and activities
The facilitators held several educational meetings at the involved units and with the expert nurses. The meetings with the expert nurses took place in learning labs, which aimed to create a safe place for learning together through dialogue [37, 38]. See Table 1 for the educational components and implementation process, Additional file 1 for a patient case used for educational purposes and Additional file 2 for a brief version of the protocol.
The implementation process of the nurse-driven urinary catheterisation protocol
For 10 months, RNs consecutively assessed patients with hip fractures, ≥ 65 years of age, on admission, according to the UC protocol. The protocol followed the patients until discharge. The RNs were encouraged to consult a colleague, the expert nurses, or a physician if in doubt about indication(s) or catheter removal plan. A weekly evaluation of adherence to the protocol was performed by two of the main facilitators. Direct feedback was given to the RN, expert nurse and participating physicians, if any incorrect assessments were identified.
Data collection
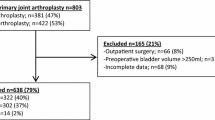
Outcome data were extracted from the hospital-based quality register of orthogeriatric hip fractures. Patients were included in the register by the discharge nurse. Thereafter the data were validated against the electronic medical records by a research nurse, a senior nurse anaesthetist specialist in infection control. We used the same exclusion criteria from the first step of our bladder bundle [27]. Patients with a hospital stay of ≤2 days, distal fracture, resection arthroplasty or previously included due to contralateral hip fracture, no catheterisation/chronic catheter/suprapubic/urostomy/dialysis, or straight in-out catheterisation/self-catheterisation were excluded.
The extracted variables were age, gender, ASA-classification score I-IV [55], hospital length of stay (LOS), diabetes mellitus type I and II, type of catheterisation treatment (indwelling, straight in-out or both), catheter days (including re-catheterisation days), documented catheter indication and removal plan, number of straight in-out catheterisations, re-catheterisation catheter present at discharge, largest bladder volume documented during hospital stay, urine volume ≥ 500 ml (yes/no) and bladder distension (yes/no). The hospital procedure was to measure the volume after catheterisation either by reading the marker on the urine bag or by pouring it into a litre measuring cup.
Assessment of the protocol nurse-driven urinary catheterisation protocol
The data from the nurse-driven protocol was descriptively described using numbers and percentages. We used both RN documentation in the UC protocol and the electronical medical records for assessing adherence to the protocol. If correct IDC indication(s) and/or removal plan were identified in the electronical medical records but not in the protocol, this was counted as a successful identification and vice versa. If they were correct but differed, they were assessed as more than one indication. If one or more were not an appropriate indication this was assessed as incorrect indication(s). If the RN had documented remove after surgery as removal plan, we assessed it as correct even though it was not pre-defined removal plan, and removal plan uncertain was assessed as incorrect. Timely insertions were assessed by setting for first IDC insertion and changes in the number of patients treated with both IDC and straight in-out catheterisation.
Statistical analysis
The categorical variables were presented as numbers and percentages and continuous variables as means, standard deviation or median, quartile 1 and quartile 3. For ordered group comparisons, the Mantel-Haenszel chi-square test was used for ordered categorical variables and dichotomous variables and Jonckheere-Terpstra test for continuous variables. For comparisons between two groups, Fisher’s exact test was used for dichotomous variables and Fisher’s non-parametric permutation test was used for continuous variables. Mean changes between year 1 and year 5 with 95% CI are given for bladder distension, a urine volume ≥ 500 ml and the largest observed urine volume. The confidence interval for dichotomous variables was the unconditional exact confidence limits and, if no exact limits could be computed, the asymptotic Wald confidence limits with continuity correction were calculated instead. The confidence interval for the mean difference between groups was based on Fisher’s non-parametric permutation test. Univariable logistic regression analysis was performed. Multivariable logistic regression was used to analyse the effectiveness of the intervention over years on bladder distension and a urine volume ≥ 500 ml, with adjustment for ASA-classification score, age, gender, LOS and diabetes. The results are given as odds ratio (OR) with 95% confidence interval (CI). To describe the goodness of fit of the model, we calculated the area under the ROC curve (AUC) [56]. All significance tests were two-sided and conducted at the 5% significance level. SAS version 9.4 was used for all these analyses [57].
Results
Primary outcome
Data from 3078 patients were assessed for yearly incidence of bladder distension over 5 years, see Fig. 2 for the excluded 625 patients. Patient demographics did not differ over the years (Table 2). We observed a reduction in hospital LOS of 5 days from year one to year five (Table 2).
Bladder distension was reduced over the years, from 40.6% in year 1 to 9.1% (p < 0.0001) in year 5 (Table 3). The mean difference over the years in bladder distension were 31.5 (95% CI 26.0; 37.0).
The odds of contracting bladder distension were reduced by 40% per year and did not differ substantially after adjustment for age, gender, hospital LOS and ASA-classification score (Table 4). The univariable logistic regression analysis showed that the odds of bladder distension were higher in men and when having a longer hospital LOS. The multivariable regression analysis shows that the years of the intervention, gender and hospital LOS were independent risk factors (Table 4).
Secondary outcomes
Changes in a urine volume ≥ 500 ml and largest observed urine volume is presented in Table 3. The mean difference over the years in urine volume ≥ 500 ml were 42.8 (95% CI 36.2;49.4) and largest volume 147.3 (95% CI, 122.7; 171.4). The odds of a urine volume of ≥500 ml were reduced by 42% yearly, OR 0.58, (95% CI 0.54–0.62, p < 0.0001) and did not differ substantially after adjustment for age, gender, hospital LOS and ASA-classification score (Additional file 3). The multivariable regression analysis shows that years 1–5, LOS and ASA-classification score were all independent risk factors to urine volume of ≥500 ml (Additional file 3).
Significant improvements were found over the 5 years, in documentation related to catheter indication, the present of a removal plan and urine volume when inserting the IDC (Table 3). We found more patients with a first IDC insertion earlier in the patient pathway (Additional file 4). Significant reductions were found in patients treated with both an IDC and straight in-out catheterisation during hospital stay, as well as the number of straight in-out catheterisations. We found no significant reductions in IDC days, re-catheterisation and catheter presented at discharge over the years.
Findings related to the nurse-driven urinary catheterisation protocol
The RN assessed 586 patients for 10 months. Of these, 544 patients had documented IDC indication(s) of which most were correctly assessed. The most common indications were related to UR/residual urine, morbidity or to ensure haemodynamic stability (Additional file 5). Patient involvement and removal plan is presented in Additional file 6.
Discussion
We found that the intervention was associated with a reduced incidence of bladder distension over 5 years, from 4 to 1 in 10 patients, and the mean yearly incidence of patients with a bladder volume of ≥500 ml was almost halved. The incidence of bladder distension was high the first 2 years. The findings from other studies using the same trigger in orthopaedic patients found bladder distension in a small percentage of patients [5, 16, 58]. However, comparisons with our study are problematic, due to the different study design and case mix. Further, the incidence of a urine volume of ≥500 ml is in the higher range in year 1, while those in years 4 and 5 are in the lower ranges, when compared with other studies [20, 22, 23]. Still, comparison with other studies is difficult as the definition of UR differ. Moreover, contrary to Adunsky et al. [22], we found that diabetes did not predict bladder distension or a urine volume of ≥500 ml in our cohort.
The intervention
We have not found any theory-driven intervention similar to ours to reduce bladder distension or UR. Most nurse-driven protocols have been shown to reduce both the length and use of IDC, associated UTIs and catheter trauma, by using appropriate indications, removal plans and timely bladder scanning [33, 59,60,61,62]. We found that more patients received a catheter over the intervention years and in parallel a decrease he incidence of UC-UTI [27]. This despite no significant reductions in IDC days and re-catheterization rates. It is possible that implementing aseptic insertion techniques and antiseptic prewash to some extent counter the development of bacteriuria. A review by, Zhang et al.’s [63] supports the use of a short-term IDC, removed within 24–48 hours, in preventing post-operative UR compared with straight in-out catheterisation, without increasing the risk of UC-UTI. Moreover, our study confirms the importance of using timely measurement of residual urine, starting in the ED to reduce the risk of overfilling the bladder [13, 15, 30]. Considering the decreased number of straight in-out catheterisation and that the catheter was inserted earlier in the care pathway, the intervention might have contributed to timelier insertion of IDC and thereby avoiding unnecessary catheterisations. Further, the intervention significantly improved RNs catheter related documentation and confirmed the lack in documentation among both RNs and physicians [64, 65].
To facilitate for RNs to rethink and relearn “new” catheter best practices through “embracing” doubts as well as allowing participants to examine the problem from different perspectives [37, 38], seems to be a way forward in preventing bladder distension in hip fracture patients. Our findings support the belief that bladder distension is a nurse-sensitive adverse event [58] and, by allowing nurses to initiate catheter treatment, through pre-defined clinical decision tool, a timelier catheter insertion can be facilitated. Moreover, we agree with Rutberg et al. [5] that avoiding an IDC to prevent a UTI might increase the risk of bladder distension and that preventive strategies ought to address both types of adverse events. Further, either routine insertion of an IDC or the strict use of straight in-out catheterisation may be recommended in this patient group [17, 66, 67]. Instead, an individual assessment of each patient is important [44, 46, 49,50,51,52].
Strength and limitations
The strength of our study is the large cohort size and the longitudinal observation period with continuous validation of the data. However, it is a single-centre study using outcome data from a specific patient cohort and we have not controlled for all potential confounding factors that could have affected our outcomes. For example, UR or residual urine on admission, or comorbidities such as Parkinson’s and stroke which increases the risk of lower urinary tract problems as these data were not available in the register. The lack in follow up after discharge is also a limitation. Moreover, LOS is difficult to interpret, as changes related to LOS may have several other explanations, such as changes in discharge routines or other adverse events. Further, urinary retention has been shown to increase hospital LOS in orthopaedic patients [68, 69] but not in hip fracture patients [17, 22]. We did not include UC-UTI as a covariate as it is difficult to single out the dependency between UTI and UR.
The initial lower completeness of data can be regarded as a limitation. During the first year, the register suffered from organisational issues and the completeness was approximately 50–60% if we anticipated a yearly incidence of 800–900 hip fracture patients. However, the yearly incidence also includes those admitted to the orthopaedic wards and thereby not reported to the register.
Conclusion
This study provides new insights in how an intervention which includes the co-creation of a nurse-driven UC-protocol and timely bladder scanning schedule can reduce bladder distension and urine volume ≥ 500 ml in patients with hip fracture. The findings suggest that RNs, in line with their core competencies, can use supporting tools to ensure timely and appropriate catheter insertion. Still more studies are needed to investigate if this approach is applicable to other settings.
Availability of data and materials
The summary data are available in the main document. We have no ethical approval to share the datasets generated and analysed during the current study. If anyone wishes to request the datasets from this study, the corresponding author can be contacted.
Abbreviations
- CI:
-
Confidence interval
- CRM:
-
Crew Resource Management
- ED:
-
Emergency Department
- ICU:
-
Intensive care unit
- IDC:
-
Indwelling urinary catheter
- LOS:
-
Length of stay
- OD:
-
Operating room
- OR:
-
Odds ratio
- PACU:
-
Post-anaesthesia care units
- SD:
-
Standard Deviation
- UC:
-
Urinary catheter
- UR:
-
Urinary retention
References
Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press; 2000.
Bates DW, Singh H. Two decades since to err is human: an assessment of Progress and emerging priorities in patient safety. Health Aff (Millwood). 2018;37(11):1736–43. https://doi.org/10.1377/hlthaff.2018.0738.
Global patient safety action plan 2021–2030: towards eliminating avoidable harm in health care [https://www.who.int/publications/i/item/9789240032705].
Markörbaserad journalgranskning, Skador i somatisk vård januari 2013 december 2019, nationell nivå [https://webbutik.skr.se/bilder/artiklar/pdf/7585-836-4.pdf].
Rutberg H, Borgstedt-Risberg M, Gustafson P, Unbeck M. Adverse events in orthopedic care identified via the global trigger tool in Sweden–implications on preventable prolonged hospitalizations. Patient Saf Surg. 2016;10(1):1–9. https://doi.org/10.1186/s13037-016-0112-y.
Brouwer TA, Rosier PF, Moons KG, Zuithoff NP, van Roon EN, Kalkman CJ. Postoperative bladder catheterization based on individual bladder capacity: a randomized trial. Anesthesiology. 2015;122(1):46–54. https://doi.org/10.1097/ALN.0000000000000507.
Siroky MB. The aging bladder. Rev Urol. 2004;6 Suppl 1(Suppl 1):S3–7.
Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention anesthetic and perioperative considerations. Anesthesiology. 2009;110(5):1139–57. https://doi.org/10.1097/ALN.0b013e31819f7aea.
Bjerregaard LS, Hornum U, Troldborg C, Bogoe S, Bagi P, Kehlet H. Postoperative urinary catheterization thresholds of 500 versus 800 ml after fast-track total hip and knee arthroplasty: a randomized, open-label, controlled trial. Anesthesiology. 2016;124(6):1256–64. https://doi.org/10.1097/ALN.0000000000001112.
Madersbacher H, Cardozo L, Chapple C, Abrams P, Toozs-Hobson P, Young JS, et al. What are the causes and consequences of bladder overdistension? Neurourol Urodyn. 2012;31(3):317–21.
Joelsson-Alm E. Bladder distension: aspects of a healthcare-related injury, dissertation. Inst för klinisk forskning och utbildning, Södersjukhuset/Dept of Clinical Science and Education, Södersjukhuset; 2012.
Joelsson-Alm E, Nyman CR, Svensén C, Ulfvarson J. Micturition problems after bladder distension during hospitalization in Sweden: “I’m not ill, just damaged for the rest of my life”. Nurs Res. 2014;63(6):418–25. https://doi.org/10.1097/nnr.0000000000000057.
Joelsson-Alm E, Nyman CR, Lindholm C, Ulfvarson J, Svensén C. Perioperative bladder distension: a prospective study. Scand J Urol Nephrol. 2009;43(1):58–62. https://doi.org/10.1080/00365590802299122.
Ringdal M, Borg B, Hellström A. A survey on incidence and factors that may influence first postoperative urination. Urol Nurs. 2003;23(5):341–54.
Brouwer TA, van Roon EN, Rosier PFWM, Kalkman CJ, Veeger N. Postoperative urinary retention: risk factors, bladder filling rate and time to catheterization: an observational study as part of a randomized controlled trial. Perioper Med. 2021;10(1):2. https://doi.org/10.1186/s13741-020-00167-z.
Pettersson PK, Sköldenberg O, Samuelsson B, Stark A, Muren O, Unbeck M. The identification of adverse events in hip fracture patients using the global trigger tool: a prospective observational cohort study. Int J Orthop Trauma Nurs. 2020;38:100779. https://doi.org/10.1016/j.ijotn.2020.100779.
Thomas S, Harris N, Dobransky J, Grammatopoulos G, Gartke K, Liew A, et al. Urinary catheter use in patients with hip fracture: are current guidelines appropriate?. A retrospective review. Can J Surg. 2021;64(6):E630–5. https://doi.org/10.1503/cjs.014620.
Smith NK, Albazzaz MK. A prospective study of urinary retention and risk of death after proximal femoral fracture. Age Ageing. 1996;25(2):150–4. https://doi.org/10.1093/ageing/25.2.150.
Johansson R, Christensson L. Urinary retention in older patients in connection with hip fracture surgery. J Clin Nurs. 2010;19(15/16):2110–6. https://doi.org/10.1111/j.1365-2702.2010.03261.x.
Teng M, Zerah L, Rouet A, Tomeo C, Verny M, Cohen-Bittan J, et al. Fecal impaction is associated with postoperative urinary retention after hip fracture surgery. Ann Phys Rehabil Med. 2021;64(6):101464. https://doi.org/10.1016/j.rehab.2020.101464.
Kwak D-K, Oh C-Y, Lim J-S, Lee H-M, Yoo J-H. Would early removal of indwelling catheter effectively prevent urinary retention after hip fracture surgery in elderly patients? J Orthop Surg Res. 2019;14(1):315. https://doi.org/10.1186/s13018-019-1360-1.
Adunsky A, Nenaydenko O, Koren-Morag N, Puritz L, Fleissig Y, Arad M. Perioperative urinary retention, short-term functional outcome and mortality rates of elderly hip fracture patients. Geriatr Gerontol Int. 2015;15(1):65–71. https://doi.org/10.1111/ggi.12229.
Skelly J, Guyatt G, Kalbfleisch R, Singer J, Winter L. Management of urinary retention after surgical repair of hip fracture. CMAJ. 1992;146(7):1185.
Cialic R, Shvedov V, Lerman Y. Risk factors for urinary retention following surgical repair of hip fracture in female patients. Geriatr Orthop Surg Rehabil. 2017;8(1):39–43. https://doi.org/10.1177/2F2151458516683507.
Erichsen Andersson A, Frödin M, Dellenborg L, Wallin L, Hök J, Gillespie BM, et al. Iterative co-creation for improved hand hygiene and aseptic techniques in the operating room: experiences from the safe hands study. BMC Health Serv Res. 2018;18(1):2. https://doi.org/10.1186/s12913-017-2783-1.
Wikström E, Dellenborg L, Wallin L, Gillespie BM, Erichsen Andersson A. The safe hands study: implementing aseptic techniques in the operating room: facilitating mechanisms for contextual negotiation and collective action. Am J Infect Control. 2019;47(3):251–7. https://doi.org/10.1016/j.ajic.2018.08.024.
Frödin M, Ahlstrom L, Gillespie BM, Rogmark C, Nellgård B, Wikström E, et al. Effectiveness of implementing a preventive urinary catheter care bundle in hip fracture patients. J Infect Prev. 2022;23(2):41–8. https://doi.org/10.1177/17571774211060417.
Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291–3. https://doi.org/10.1093/cid/cir195.
Lo E, Nicolle LE, Coffin SE, Gould C, Maragakis LL, Meddings J, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(S2):S32–47. https://doi.org/10.1086/675718.
Johansson R-M, Malmvall B-E, Andersson-Gäre B, Larsson B, Erlandsson I, Sund-Levander M, et al. Guidelines for preventing urinary retention and bladder damage during hospital care. J Clin Nurs. 2012;22(3–4):347–55. https://doi.org/10.1111/j.1365-2702.2012.04229.x.
Bladder monitoring during hospital stay-an overview (Swedish, Blåsövervakning vid sjukhusvård-en översikt) [https://www.vardhandboken.se/vard-och-behandling/basal-och-preventiv-omvardnad/blasovervakning-vid-sjukhusvard/oversikt/].
Joelsson-Alm E, Ulfvarson J, Nyman CR, Divander M-B, Svensén C. Preoperative ultrasound monitoring can reduce postoperative bladder distension: a randomized study. Scand J Urol Nephrol. 2012;46(2):84–90.
Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23:277–89. https://doi.org/10.1136/bmjqs-2012-001774.
Marker Based Record Review - Marker with definitions to identify and Measure Harm in Health Care (Markörbaserad journalgranskning - Markörer med definitioner för att identifiera och mäta skador i vården) [https://skr.se/download/18.4829a209177db4e31aa39782/1615555088200/Markorer_definitioner_journalgranskning_inom_somatisk%20vard.pdf].
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9. https://doi.org/10.1016/j.ijsu.2014.07.013.
Parry D, Salsberg J, Macaulay AC, Fcpc C. A guide to researcher and knowledge-user collaboration in health research. Ottawa: Canadian Institutes of Health Research; 2009.
Isaacs WN: Creating a shared field of meaning: an action theory of dialogue. In: The Transformative Power of Dialogue edn; 2002: 203–241.
Schein E. Organizational culture and leadership. 4th ed: Wiley; 2010.
Harvey G, Loftus-Hills A, Rycroft-Malone J, Titchen A, Kitson A, McCormack B, et al. Getting evidence into practice: the role and function of facilitation. J Adv Nurs. 2002;37(6):577–88. https://doi.org/10.1046/j.1365-2648.2002.02126.x.
Dogherty EJ, Harrison MB, Graham ID. Facilitation as a role and process in achieving evidence-based practice in nursing: a focused review of concept and meaning. Worldviews Evid-Based Nurs. 2010;7(2):76–89.
Process evaluation of complex interventions [https://mrc.ukri.org/documents/pdf/mrc-phsrn-process-evaluation-guidance-final/].
Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061. https://doi.org/10.1136/bmj.n2061.
Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. BMJ Qual Safety. 2004;13(suppl 1):i85–90.
Haig KM, Sutton S, Whittington J. SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32(3):167–75.
Situation, Bakgrund, Aktuell bedömning, Rekommendation - SBAR [https://www.vardhandboken.se/arbetssatt-och-ansvar/samverkan-och-kommunikation/teamarbete-och-kommunikation/situation-bakgrund-aktuell-bedomning-rekommendation%2D%2D-sbar/].
National Early Warning Score (NEWS) 2, Standardising the assessment of acute-illness severity in the NHS. Updated report of a working party. [https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2].
Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified early warning score in medical admissions. QJM. 2001;94(10):521–6. https://doi.org/10.1093/qjmed/94.10.521.
Widgren BR, Jourak M. Medical emergency triage and treatment system (METTS): a new protocol in primary triage and secondary priority decision in emergency medicine. J Emerg Med. 2011;40(6):623–8.
Thim T, Krarup NHV, Grove EL, Rohde CV, Løfgren B. Initial assessment and treatment with the airway, breathing, circulation, disability, exposure (ABCDE) approach. Int J Gen Med. 2012;5:117–21. https://doi.org/10.2147/IJGM.S28478.
Andersson C, Cesar K, Einarson E, Guldstand M, Hvarfner A, Lindby Å, Lindgren P, Marklew A, Melby A, Rundgren M et al: ProACT: förebygg och behandla livshotande tillstånd (Eng. prevent and treat life-threatening conditions), 1. uppl. edn. Lund: Lund : Studentlitteratur; 2015.
Flin RH. Safety at the sharp end: a guide to non-technical skills. Aldershot: Ashgate; 2008.
Fore AM, Sculli GL. A concept analysis of situational awareness in nursing. J Adv Nurs. 2013;69(12):2613–21. https://doi.org/10.1111/jan.12130.
Ministry of Health and Social Affairs: Patient law (Patientlag) (2014:821). In., vol. SFS nr: 2014:821: Sveriges Riksdag; 2014.
Blåsövervakning vid sjukhusvård (Eng. Bladder monitoring in-hospital care) [https://www.vardhandboken.se/vard-och-behandling/basal-och-preventiv-omvardnad/blasovervakning-vid-sjukhusvard/schema-for-kontroll-av-blastomning/].
ASA Physical Status Classification System [https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system].
Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied logistic regression, vol. 398: Wiley; 2013.
SAS Institute Inc. SAS 9.4. Sixth edition: Cary; 2016.
Hommel A, Magnéli M, Samuelsson B, Schildmeijer K, Sjöstrand D, Göransson KE, et al. Exploring the incidence and nature of nursing-sensitive orthopaedic adverse events: A multicenter cohort study using Global Trigger Tool. Int J Nurs Stud. 2020;102:103473. https://doi.org/10.1016/j.ijnurstu.2019.103473.
Durant DJ. Nurse-driven protocols and the prevention of catheter-associated urinary tract infections: a systematic review. Am J Infect Control. 2017;45(12):1331–41. https://doi.org/10.1016/j.ajic.2017.07.020.
Laborde E, Hill H, Dukovac TE, Carriere SP, Lata-Arias K, Hebert K, et al. A nurse-driven protocol for Foley catheter utilization decreases the incidence of traumatic Foley catheterization. Ochsner J. 2021;21(1):41–62. https://doi.org/10.31486/toj.20.0004.
Patel P, Gupta A, Vaughn V, Mann J, Ameling J, Meddings J. Review of strategies to reduce central line-associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) in adult ICUs. J Hosp Med. 2018;13(2):105–16. https://doi.org/10.12788/jhm.2856.
Schiessler MM, Darwin LM, Phipps AR, Hegemann LR, Heybrock BS, Macfadyen AJ. Don't have a doubt, get the catheter out: a nurse-driven CAUTI prevention protocol. Pediatr Qual Safety. 2019;4(4):e183. https://doi.org/10.1097/pq9.0000000000000183.
Zhang W, Liu A, Hu D, Xue D, Li C, Zhang K, et al. Indwelling versus intermittent urinary catheterization following Total joint arthroplasty: a systematic review and Meta-analysis. PLoS One. 2015;10(7):e0130636. https://doi.org/10.1371/journal.pone.0130636.
Gokula RRM, Hickner JA, Smith MA. Inappropriate use of urinary catheters in elderly patients at a midwestern community teaching hospital. Am J Infect Control. 2004;32(4):196–9. https://doi.org/10.1016/j.ajic.2003.08.007.
Atkins L, Sallis A, Chadborn T, Shaw K, Schneider A, Hopkins S, et al. Reducing catheter-associated urinary tract infections: a systematic review of barriers and facilitators and strategic behavioural analysis of interventions. Implement Sci. 2020;15(1):44. https://doi.org/10.1186/s13012-020-01001-2.
Hälleberg Nyman M, Gustafsson M, Langius-Eklöf A, Johansson J-E, Norlin R, Hagberg L. Intermittent versus indwelling urinary catheterisation in hip surgery patients: a randomised controlled trial with cost-effectiveness analysis. Int J Nurs Stud. 2013;50(12):1589–98. https://doi.org/10.1016/j.ijnurstu.2013.05.007.
Meddings J, Skolarus TA, Fowler KE, Bernstein SJ, Dimick JB, Mann JD, et al. Michigan appropriate perioperative (MAP) criteria for urinary catheter use in common general and orthopaedic surgeries: results obtained using the RAND/UCLA appropriateness method. BMJ Qual Safety. 2019;28(1):56–66. https://doi.org/10.1136/bmjqs-2018-008025.
Balderi T, Mistraletti G, D'angelo E, Carli F. Incidence of postoperative urinary retention (POUR) after joint arthroplasty and management using ultra-sound-guided bladder catheterization. Minerva Anestesiol. 2011;77(11):1050.
Lawrie CM, Ong AC, Hernandez VH, Rosas S, Post ZD, Orozco FR. Incidence and risk factors for postoperative urinary retention in Total hip arthroplasty performed under spinal anesthesia. J Arthroplast. 2017;32(12):3748–51. https://doi.org/10.1016/j.arth.2017.07.009.
World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81(3):14–8.
Acknowledgements
We would like to acknowledge all the participating expert nurses, nurse assistants, physicians, care developers and leaders. Without your valuable input, support and drive, this study would not have been possible. Moreover, we thank Lisbeth Sjöstedt for meticulous data validation and Christopher Bäckström (CB) and Nils-Gunnar Persson (NGP) for statistical advice and analysis.
Funding
Open access funding provided by University of Gothenburg. Funders: Landstingens Ömsesidiga Försäkringsbolag (http://lof.se) and the University of Gothenburg Centre for Person-centred Care (GPCC), Sweden. The GPCC is funded by the Swedish Government’s grant for Strategic Research Areas (Care Sciences) and the University of Gothenburg, Sweden. They played no role in the study design, data collection, analysis, interpretation of data, or in the manuscript writing process.
Author information
Authors and Affiliations
Contributions
Conceptualisation: MF, AEA, BN, CR, EW. Study design: MF, AEA, CR, BN, BG, EW. Data analysis: MF, AEA. Interpretation of data: MF, AEA, BN, CR, BG, EW. Drafting and revising the manuscript: MF, AEA, BN, CR, BG, EW. Received the funding: AEA. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Gothenburg Regional Ethics Review Board, Sweden (Number 166–15, Number 327–17 and amendment Number 2022–00270-02) approved the study. The study was conducted according to the Swedish Ethical Review Act SFS number: 2003:460. Based on this Act, healthcare workers are not required to provide written consent, only verbal consent was obtained as approved by the Regional Ethics Review Board. Healthcare worker were given written and verbal information about the study and who to contact if they did not want to participate in line with the four principal requirements of the Helsinki Declaration: autonomy, non-malfeasance, beneficence, and justice [70]. The hospital’s Chief Executive Officer, the heads of departments, the trauma orthopaedic department and the manager responsible for hip fracture surgery approved the study as a quality improvement project. Informed consent was not required from the patients when using registry data, as approved by The Gothenburg Regional Ethics Review Board. The patients received written information about the quality register, who to contact about their register data if they wanted to withdraw data from the register and/or to be used for research purpose. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
None to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Example of patient case using SBAR pre-intervention.
Additional file 2.
Brief nurse-driven urinary catheterisation protocol.
Additional file 3.
Uni- and multivariable regression in the event of a urine volume of ≥500 ml.
Additional file 4.
Setting for first insertion of indwelling urinary catheter.
Additional file 5.
Identified and documented catheter indications, N = 586.
Additional file 6.
Patient involvement, seeking support and removal plan.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Frödin, M., Nellgård, B., Rogmark, C. et al. A co-created nurse-driven catheterisation protocol can reduce bladder distension in acute hip fracture patients - results from a longitudinal observational study. BMC Nurs 21, 276 (2022). https://doi.org/10.1186/s12912-022-01057-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12912-022-01057-z