Abstract
Background
Chronic kidney disease (CKD), a major public health problem with differing disease etiologies, leads to complications, comorbidities, polypharmacy, and mortality. Monitoring disease progression and personalized treatment efforts are crucial for long-term patient outcomes. Physicians need to integrate different data levels, e.g., clinical parameters, biomarkers, and drug information, with medical knowledge. Clinical decision support systems (CDSS) can tackle these issues and improve patient management. Knowledge about the awareness and implementation of CDSS in Germany within the field of nephrology is scarce.
Purpose
Nephrologists’ attitude towards any CDSS and potential CDSS features of interest, like adverse event prediction algorithms, is important for a successful implementation. This survey investigates nephrologists’ experiences with and expectations towards a useful CDSS for daily medical routine in the outpatient setting.
Methods
The 38-item questionnaire survey was conducted either by telephone or as a do-it-yourself online interview amongst nephrologists across all of Germany. Answers were collected and analysed using the Electronic Data Capture System REDCap, as well as Stata SE 15.1, and Excel. The survey consisted of four modules: experiences with CDSS (M1), expectations towards a helpful CDSS (M2), evaluation of adverse event prediction algorithms (M3), and ethical aspects of CDSS (M4). Descriptive statistical analyses of all questions were conducted.
Results
The study population comprised 54 physicians, with a response rate of about 80–100% per question. Most participants were aged between 51–60 years (45.1%), 64% were male, and most participants had been working in nephrology out-patient clinics for a median of 10.5 years. Overall, CDSS use was poor (81.2%), often due to lack of knowledge about existing CDSS. Most participants (79%) believed CDSS to be helpful in the management of CKD patients with a high willingness to try out a CDSS. Of all adverse event prediction algorithms, prediction of CKD progression (97.8%) and in-silico simulations of disease progression when changing, e. g., lifestyle or medication (97.7%) were rated most important. The spectrum of answers on ethical aspects of CDSS was diverse.
Conclusion
This survey provides insights into experience with and expectations of out-patient nephrologists on CDSS. Despite the current lack of knowledge on CDSS, the willingness to integrate CDSS into daily patient care, and the need for adverse event prediction algorithms was high.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) is a major public health problem with a prevalence of about 10% in high-income countries [1,2,3]. The prevalence of CKD is rising, especially in older patients [2, 4] and will continue to increase in an ageing society, leading to a higher proportion of multi-morbid patients and higher complexity in patient treatment.
CKD constitutes a complex disease due to differing underlying disease etiologies in each patient that can in turn lead to many complications [3], comorbidities [1, 5], and polypharmacy [6, 7]. CKD is associated with a worse prognosis of adverse long-term patient outcomes due to reduced kidney function itself and due to comorbidities, particularly cardiovascular diseases (CVD) [1, 8], as well as major risk factors associated with CKD progression and increased mortality [9, 10]. A clear overview and differentiated risk calculation of an individual patient’s diseases and possible complications are necessary to avoid adverse events, such as CVD or acute kidney injury (AKI). Optimized treatment requires the consideration of all data levels, from clinical parameters, biomarkers, lifestyle factors and disease history to medication use. Mathematical models can assist in integrating all mentioned data levels. These models recognize underlying data dependencies and their associations with disease progression or risk for complications. Many clinical decision support systems (CDSS) integrate mathematical models based on machine-learning methods to predict different adverse events and can thereby improve management of CKD patients [11,12,13,14]. Here, supervised machine-learning algorithms use training data, i.e., pairs of predictor and known outcome variables, to deduce mathematical models which can then be employed to predict an unknown outcome for new data.
Several prediction algorithms in nephrology already exist. The most prominent algorithms have focused on prediction of CKD progression to kidney failure. One of them has been developed in a German CKD cohort (Z6 risk equation [15]) including 4915 CKD stage 1–5 patients with a mean observation time of 3.71 years. The Z6 has been successfully validated in three independent cohorts, including a total of 3063 patients. The other one has been developed within a Canadian cohort of 3004 CKD patients of whom 344 reached kidney failure (KF) during a follow-up of three years and validated within the CKD prognosis consortium (kidney failure risk equation [16, 17]). Here, the developed equation was validated in 31 cohorts from 30 countries in 721,355 CKD patients with 23,829 KF cases during 4 years of follow-up with high discrimination and adequate calibration of the equation concerning development of KF. The former selected six, whereas the latter selected four clinical routine patient parameters as predictors in order to predict the outcome “kidney failure requiring kidney replacement therapy” for CKD patients. Two very specific prediction algorithms can be used in special settings only: 1) A prediction algorithm for IgA nephropathy that can be used in multiple ethnic groups at the time of kidney biopsy [18] developed and validated in 3927 patients with biopsy-proven IgA nephropathy from Europe, North America, China, and Japan over seven years of follow-up; 2) a prediction algorithm for AKI [19], which is based on a retrospective analysis of data from a large number of adult intensive care patients of the EPaNIC clinical trial [20] using 4640 patients to build and validate the predictive models. So far, these algorithms are individual efforts and no overarching framework has been developed making the use of these CDSS somewhat tedious for nephrologists with restricted time on their hands that is bound by acute patient care.
One of two initiatives trying to remedy this issue is a junior consortium in the field of systems medicine (CKDNapp [21]), the other one an effort from the Hannover region (NEPHRO-DIGITAL; [22]), Germany. While CKDNapp (CKD Nephrologists’ app) is aiming at predicting adverse events and disease progression, as well as delivering comprehensive literature support as an easy-to-use software for cell phones, tablets, and desktop computers, NEPHRO-DIGITAL will be an eHealth platform for data sharing between outpatient nephrology, primary care, pediatricians and nephrology clinics and will also incorporate an interoperable CDSS.
Taking these efforts into account, it is of prime interest to assess nephrologists’ attitudes towards a CDSS in order to successfully implement CDSS frameworks into daily medical routine. We therefore aimed to investigate the following user aspects via a uniquely designed survey for nephrologists: 1) experiences with CDSS (module M1), and 2) expectations of a CDSS in general (M2), 3) evaluation of potential features of interest, like adverse event prediction algorithms within a CDSS in particular (M3), as well as 4) opinions on ethical aspects of CDSS (M4).
Methods
Survey sample population
The study was approved by the ethics committee of the University of Freiburg and registered at the German registry for clinical studies (DRKS00025054) and reporting was standardized using the modified STROBE Statement whenever possible [23]. Nephrologists were recruited via three different ways: (i) the German Chronic Kidney Disease (GCKD) study coordinating center by using contact information of collaborating nephrologists, (ii) a public presentation at the German society of nephrology conference, (iii) published articles in two magazines for nephrologists involved in CKD out-patient care [24, 25]. Nephrologists collaborating with the GCKD study were contacted via telephone by study personal and interviewed directly, if they were willing to do so. Conference attendees of the German society of nephrology conference were able to directly take the survey by a provided QR code on one of the slides and were also encouraged to do so. Nephrologists reading the provided articles on the in two magazines were able to take the survey via a provided link or QR code at their leisure or could also contact the study center for a telephone interview. Nephrologists from all regions across Germany were thereby recruited for the survey, since the GCKD study is a study working with nephrologist all over Germany, the conference of the German Society of Nephrology adheres to nephrologists in all of Germany and both magazines are available to any nephrologist in Germany. Any nephrologist with experience in CKD out-patient management was therefore able to participate after giving informed consent. There were no decided exclusion criteria. All participants gave informed consent prior to taking the survey. All methods were carried out in accordance with relevant guidelines and regulations following the Declaration of Helsinki.
Survey questionnaire
The survey was conducted either by telephone or as a do-it-yourself online interview from 08/2021 to 03/2022. The telephone interview was conducted by trained interviewers. The survey was developed as a 38-item questionnaire with a processing time of approximately ten minutes and included a short introduction giving a definition of CDSS in general and prediction algorithms in particular (Additional file 1: Supplemental Table 1). First, information on socio-demographics, e.g., age, gender, years of ambulatory work experience, or number of CKD patients treated per day on average, was collected. Subsequently, questions from four modules were asked: (M1) the physician’s experience with CDSS in general; (M2) nephrologists’ expectations toward a helpful CDSS; (M3) evaluation of the importance/usefulness of prediction algorithms and literature support within a CDSS; (M4) ethical aspects with regard to CDSS. The very last question asked for the willingness of nephrologists to participate in a testing phase of a uniquely designed CDSS, once a first version of the application has been developed.
The survey was available in German language only since the survey was conducted in Germany. (Additional file 1: Supplemental Table 1). All questions were included based on suitability after a literature search on the topic of CDSS. Here, previous studies have identified the “perceived usefulness” and “perceived ease-of-use” to be particularly important for the acceptance or declination of innovations [26, 27] as part of the frequently used extended Technology Acceptance Model (TAM2) [28,29,30]. Additionally, the physician’s willingness to change his/her workflow and the future compatibility of the CDSS to daily work practice were included in the questionnaire [26, 28, 31]. Response formats included the Likert scale (five-point: 1: does not apply at all; 2: does rather not apply; 3: partly applies; 4: largely applies; 5: fully applies; or 1: unimportant – 5: very important, respectively) or multiple choice with preselected options for closed questions.
Data collection with REDCap and data privacy
Development of the questionnaire, study data collection and data management were carried out using the Electronic Data Capture System (REDCap) [32, 33]. REDCap is a well-established system for translational research and has been described before. Participants’ answers were collected anonymously. In order to preserve data protection, no location of activity was requested. Furthermore, the collection of socioeconomic data was categorized to prohibit recognizability of participants.
Statistical analysis
Descriptive analyses of all queried items were conducted. Visualizations of answer profiles per question included histograms, pie charts, bubble plots, and radar charts accessible at (www.ckdn.app/survey). Software tools included, on the one hand, pre-established workflows within REDCap, giving out basic barplots, scatterplots, pie charts, and basic statistics on missingness of variables, on the other hand, Stata SE 15.1 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). A radar chart, bubble plot as well as modified pie chart were created for Modules 1, 2, and 3 using the software Excel (Microsoft Office, Release 16).
Results
Demographics
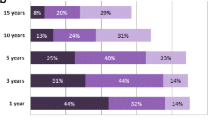
Our study population comprised 54 nephrologists from all across Germany. All survey questions were fully completed by 30 nephrologists (56.6%). Missing responses ranged from N = 0 to N = 11, resulting in a response rate of about 80–100% per question (Additional file 2: Supplemental Fig. 1). Participants were mostly aged between 51–60 years (45.1%), followed by the age group of 40–50 year olds (27.5%). Age groups of participants being older than 60 years or younger than 40 years were almost equally present (13.7% and 11.8% respectively; Table 1). About one third of all participants were female. Participating nephrologists had been specialists for a median of 14 years, while 3 participants did not further specify this question. Years spent practicing as a nephrologist in CKD out-patient care was indicated with a median of 10.5 years and years spent treating out-patients in general with 15.0 years. Participants indicated that they were seeing a median of 30 patients per day of which a median of 11 were CKD pre-dialysis patients. The study population included physicians with both fewer and many years of experience in patient care, indicated by the range of being a specialist (0–35 years) and treating out-patients (0–40 years; Additional file 2: Supplemental Fig. 2), reducing bias through a selection of physicians representing only one level of work experience.
Module 1: nephrologists’ experiences with CDSS in general
Overall, current CDSS use was poor (81.2% never/rarely, N = 39; Table 2). The most frequently indicated reason (Table 2) was missing knowledge about CDSS (43.2%, N = 19), followed by no need for systems tied with other reasons (both 20.5%, N = 9), and lack of availability of good systems (15.9%, N = 7).
Only few participants evaluated CDSS as being impractical (9.1%, N = 4), and without advantages for patient care (9.1%, N = 4) or giving out incomprehensible advice (2.3%, N = 1). More than half of all participants did not feel confident in using CDSS (59.6%, N = 28) or were not sure how to apply CDSS in patient care (56.5%, N = 26; Table 2). Nevertheless, there was a high confidence that the implementation of a CDSS would be helpful (personalized information/personalized prognosis: 80.9%, N = 38; personalized treatment: 70.2%, N = 33) and might improve quality of patient care (71.7%, N = 33) and work efficiency (63.1%, N = 29). The lowest ratings were observed for the question regarding fitting to daily routine (44.7%, N = 21). However, most participant were willing to try out an available CDSS (73.9%, N = 34).
Module 2: nephrologists’ expectations towards a helpful CDSS
Next, expectations towards a helpful CDSS were inquired (Additional file 1: Supplemental Table 3; Table 3). The surveyed nephrologists rated as most important CDSS features: the incorporation of guidelines for the treatment of CKD patients (95.6%, N = 43; M2.1) as well as the provision of any other important secondary information needed for patient care (97.8%, N = 44; M2.2), like specific guidelines or information on rarer diseases underlying the etiology of CKD.
Participants were undecided about changing their working routine to incorporate a CDSS into their workday (partially applies 51.1%, N = 23; M2.4). Details for questions 1 to 4 of module 2 (M2.1-M2.4; see above) with percentages for answer possibilities “mostly and completely true” are graphically displayed in Additional file 2: Supplemental Fig. 3. Overall, most participants were in favour of using a desktop computer with internet browser to query a potential CDSS (67%, N = 30, Additional file 1: Supplemental Table 3; Additional file 2: Supplemental Fig. 4).
Module 3: evaluation of the importance/usefulness of prediction algorithms
Table 4 shows numbers and percentages for all answer possibilities concerning possible prediction algorithms incorporated into CDSSs for nephrologists. Here, participants were mostly interested in the prediction of CKD progression and prediction of an AKI event. Prediction of stroke, a peripheral arterial occlusive disease (PAOD) event, and mortality were rated as being less important, but still of high interest (Additional file 1: Supplemental Table 4).
The access to guidelines was rated as being highly important (93.3%, N = 42) as well as an in-silico modification for virtually changing patient features in order to explore their influence on potential future outcomes (97.7%, N = 43; Table 4). Most participant were in favour of testing a CDSS incorporating the evaluated features (84.4%, N = 38; Table 4).
Module 4: ethical aspects with regard to CDSS
Answers to seven questions on ethical aspects of CDSS were mostly diverse (Additional file 1: Supplemental Table 5). Participants agreed that CDSS would not reduce trust of patients into their treating physicians (80.0%, N = 24) and most participants also did not believe that application of CDSS would lead to discrimination of some patient groups (68.9%, N = 24). Statements being assessed as partially or mostly true were that certain parts of a physician’s work will be taken over by CDSS in the future (66.7%, N = 21) and that CDSS are more helpful for inexperienced doctors (57.8%, N = 13), but about 50% of participants did not share this opinion, respectively. About 67% of all participants did not believe that introduction of CDSS will lead to a reduction in a physicians clinical abilities. Mixed answers were given for question 4 of this module with one third of participants believing that CDSS will lead to a shift for doctors towards a more patient-centered medicine in the future, one third of participants partially agreeing and one third of participants not agreeing. Similarly mixed answers were given to the question whether missing transparency of advice given by CDSS is perceived as a problem. Here, about 30% of participants stated that this was mostly not true, but about 44% of participants believed this to be partially or mostly true.
Discussion
Our survey evaluated answers given by nephrologists from Germany regarding their awareness of existing CDSS, their openness to use CDSS, their knowledge of how CDSS can assist them in everyday patient care, and an assessment of prediction algorithms as well as literature support being part of a future nephrologists’ CDSS. The work-up of this survey will help in addressing possible barriers for implementation of CDSS in a nephrologist’s medical routine and will therefore facilitate an optimal development of CDSS in the nephrology field. Past studies have shown that the identification of a specific target group is an important aspect of CDSS design. Notably, specialty physicians reported an increased need for a knowledge source that helps to answer complex context specific clinical questions, which implies that they are more likely to benefit from a specified CDSS [34]. Surveys are a useful and valuable tool in determining needs of researchers developing tools, CDSSs on the one hand and research users/physicians on the other hand [35].
In everyday clinical practice, treating nephrologists are confronted with a large amount of patient data as well as a multitude of research papers and guidelines. Moreover, patient care needs to be individualized as well as based on these newest guidelines and extensive research has been done on the facilitators and barriers of adherence to guidelines in patient care [36, 37]. In recent years, a growing number of mobile-health technology in the management of chronic diseases has been observed [38, 39]. These applications are mainly aimed at patients as end-users and primarily achieve pure data collection [40]. The integration of data accumulated through these apps in clinical nephrology practices is limited by: 1. A diversity of programs, 2. Data entries, that are optimized to the patient perspective and not the physician’s perspective, 3. A lack of compatibility with clinical data/data processing software (adequate infrastructure) [41]. In addition, highly sought after and need of prediction algorithms of possible adverse events and disease progression require models developed and validated in CKD patient cohorts. Moreover, a transparent presentation of outcomes from these prediction models is of high importance for the treating nephrologist. Taken together, these limitations have led to low ratings of CDSS use by nephrologists [42, 43]. Early integration of physicians into the development of a CDSS is therefore essential for later implementation in clinical practice.
Nephrologists’ experiences with CDSS in general
Little is known about the experiences of German nephrologists with CDSS. Our survey showed that 80% of all participants rarely or never use CDSSs, but about 80% of participants would find a personalized CDSS helpful for CKD patient care and over 70% of participants believe that it would improve patient care as well as their work efficiency. In the past, many examples of helpful CDSSs can be found supporting this notion [16, 44,45,46,47]. Taken together with the knowledge that specialty physicians in particular profit from CDSS support [34], the development of a specialized CDSS for nephrologists supporting them in personalized CKD patient care, seems overdue. In our survey we focus on first general aspects of CDSS to then focus on special aspects of possible CDSS in the form of prediction algorithms. Medication management, diagnostic tools, and classification systems can also be a part of CDSS. However, we did not focus on in these aspects in our survey.
Nephrologists’ expectations towards a helpful CDSS
In our survey, most nephrologists generally agreed that the most important topics are accessibility to newest guidelines and other important secondary information essential for CKD patient care, but also management of patient data from previous visits. This is in line with data obtained from other groups working on facilitators for the implementation of CDSSs. Varonen et al. were able to show that physicians are mostly interested in managing the mass and complexity of information available when thinking of CDSSs [48]. It will be important to differentiate the expectations of nephrologists on how guidelines will be implemented into a CDSS. Having access to an uploaded PDF guideline might be less comfortable since one will have to go through the guideline and decide what will fit the patient in question best; alternatively, implementation of guideline recommendations would go along with easier accessibility, but also with restriction to the given recommendation suggested by the CDSS. Another important aspect of this module was the resistance to change of a nephrologist’s workday. Here, 40% of all participants were opposed to any changes, an aspect that has been seen during the implementation process of CDSS before [49], bringing up the necessity to carefully describe and collect contextual factors important for practice changes before CDSS implementation [50, 51]. Overall, nephrologists did recognize the usefulness of CDSS, which is a common theme on the perception of CDSS by health care professionals [52]. But long-term implementation of CDSS may pose a challenge that might be tackled by the implementation of behavior change interventions [53].
Evaluation of the importance/usefulness of prediction algorithms within CDSS
CKD patients constitute a heterogeneous group, making the development of useful prediction models challenging. The development of a clinical prediction model requires a collaborating team of experts in statistics, well qualified in the specific methodology, computer science, researchers and physicians to identify key elements of the model [54]. Predicting outcomes in everyday practice can help guide clinical care, decision making, and resource allocation. In fact, prediction of CKD progression and AKI were rated the highest by participating physicians. Several prediction models have been developed for CKD before, however, are currently infrequently used [55]. Reasons could be that few of them have been appropriately externally validated and out of 23 models only 2 met the proposed criteria [56, 57] for clinical usefulness [55]. The best-known model, the Kidney Failure Risk Equation by Tangri et al. [16], uses 8 routine laboratory parameters. Zacharias et al. [15] established a new risk equation based on data from the GCKD study using six routinely available laboratory parameters and validated the algorithm in 3 independent prospective observational cohort studies. There are tools for useful clinical prediction models to guide development and ensure transparent reporting (TRIPOD [58]: Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis).
Acute kidney injury (AKI) is associated with short- and long-term mortality in hospitalized patients (particularly, if patients are admitted to the intensive care unit [59]) and increased risk of major adverse kidney events including CKD progression [10, 60, 61]. Current international guidelines recommend follow-up of all patients with AKI for 90 days [62]. Recently, a study from Sawhney et al. indicated benefits for re-empting readmissions, death, and subsequent CKD progression for follow-up after AKI [63]. Risk-stratifying CKD patients for AKI has potential to facilitate targeted interventions, thus, physicians were second most interested in an AKI prediction model. Overall, data from large CKD observational studies on the relationship between AKI and CKD are scarce. Identifying patients for increased risk can improve disease management.
The risk of CVD events is higher in patients with CKD than in the general population. However, few models exist to predict the risk of CVD events in patients with CKD to date. Although prediction of CVD in general was not queried in this survey, but stroke and PAOD as secondary complications of CVD were included (M3.3, M3.4). In addition to disease etiology and comorbidities, modifiable lifestyle factors of CKD play an important role in primary prevention [64, 65]. Current CKD management guidelines recommend that patients adhere to a healthy lifestyle including diet with low potassium and low salt intake, physical activity, abstain from tobacco use, and limited alcohol consumption [66, 67]. Adherence to healthy dietary patterns is associated with lower risk for CKD progression and all-cause mortality in CKD [68, 69], however, long-term behaviour change of patients remains a challenge in practice. CKD is a silent disease, i.e., it causes few symptoms, which negatively affects the motivation of patients to change their behaviour. Successfully addressing this problem involves ensuring adequate time spent on conceptualization and planning, as well as visualization of goals with regular adjustment involving the patients.
Ethical aspects with regard to CDSS
CDSS and usage of machine learning techniques will lead to profound changes within healthcare. This also gives rise to many ethical questions, since high-quality patient treatment does not only depend on the correct prediction, prevention or improved decision making by CDSS containing a better selection of individual treatments, but also on experience, empathy, and the concrete judgement of physicians [70]. In this survey, nephrologists were not worried about a reduction in trust from patients when applying and using CDSS. This might be due to the fact, that both patients and physicians share the view that the final act of decision making rests with the human actors. CDSS cannot carry out the full process of clinical care, making CDSS supportive systems to be used by physicians not vice versa also in the future [71]. Moreover, advice by CDSS does not necessarily have to be followed but there is a complex interaction between expert system advice and physicians’ own judgment and discretion which affects physicians’ epistemic authority, particularly in situations of “peer disagreement” [72]. Nonetheless, past research endeavors were able to show that the adoption of CDSS based on clinical practice guidelines (= systematically developed statements to assist practitioners and patient decisions about appropriate health care for specific clinical circumstances) promoted adherence to these guidelines and led to improved patient management [73]. In this survey, most nephrologists did not believe in a reduction in clinical abilities by applying CDSS. Whether CDSS will open up more time for physicians to actually talk to their patients by freeing them from time-consuming decision making seems to be less clear and nephrologists were ambivalent in their answers. Indeed past studies were able to show, that rigidity of CDSS can lead to time-consuming data entry processes that physicians will come to resent leading to rejection of the system [74] and potentially affecting the patient-physician-relationship and the quality of clinical care in a negative way. Moreover, manual data entry can lead to erroneous data entries resulting in inaccurate CDSS responses [75]. An optimal CDSS for nephrologists would therefore entail an internal data entry check, which would help in recognizing false data entries and give feedback to the physician entering the data into the application. Other challenges that need to be faced are, amongst others, poor data sets and poorly optimized algorithms leading to possible discrimination of population fractions or erroneous decision making for underrepresented patient groups. In our survey, only about 11% of participants were worried that this might be a problem of CDSS. A study by Mitchell et al., by contrast, could show that using CDSS in high-minority hospitals had higher quality improvements when focusing on CDSS adoption [76]. Nonetheless, underrepresentation of minority groups in clinical trials is a well-known problem [77] and may lead to maladjusted algorithms for these groups concerning CDSS-based prediction of adverse events.
Strengths and Limitations
This study has important strengths and limitations. Since we did not have a tracking system to indicate respondents from non-respondents, we could not compare characteristics of those not included in the study. Having two methods to receive answers from participants, namely telephone interview or do-it-yourself, might also have introduced bias as participants might have been less likely to not give answers for some of the questions. We were only able to acquire a small number of nephrologists compared to the number of nephrologists practicing in the outpatient setting in Germany (2021: N = 1125) willing to participate in our study, which potentially introduced bias. Nevertheless, our study population reflected demographics of nephrologists in Germany well with higher number of males mostly in an age range between 51–60 years (https://www.bundesaerztekammer.de/baek/ueber-uns/aerztestatistik/2022; https://www.gbe-bund.de/gbe/pkg_olap_tables.prc_set_hierlevel?p_uid=gasta&p_aid=21479372&p_sprache=D&p_help=2&p_indnr=96&p_ansnr=47431095&p_version=3&p_dim=D.489&p_dw=44485&p_direction=drill.). Our study, however, has multiple strengths. This is the first survey of nephrologists exploring their CDSS knowledge and requirements to contemporary needs across Germany. We examined readiness in terms of infrastructure availability and interest to test the instruments for use in their own practice.
Conclusion
This survey provides insights into experience with and expectations of outpatient nephrologists on CDSS in general and prediction algorithms, literature support, and in-silico-modification possibilities of CDSS in particular. Despite the current lack of knowledge on CDSS, the willingness to integrate CDSS into daily patient care, and trying out specialized CDSS in the future was high. This is underlined by the readiness to test a CDSS prototype focused on CKD patient care.
Availability of data and materials
Public posting of individual level participant data is not covered by the informed patient consent form. All summary statistics necessary to interpret, replicate, and build upon the results reported in the article are provided in anonymized form in the publication.
Abbreviations
- AKI:
-
Acute kidney injury
- BMBF:
-
German Federal Ministry of Education and Research
- CDSS:
-
Clinical decision support systems
- CKD:
-
Chronic kidney disease
- CKDNapp:
-
CKD Nephrologist App
- DESIREE:
-
Decision Support In Routine and Emergency Health
- eGFR:
-
Estimated glomerular filtration rate
- GCKD:
-
German Chronic Kidney Disease study
- M:
-
Module question
- ns:
-
Not specified
- p25:
-
25Th percentile
- p75:
-
75Th percentile
- REDCap:
-
Electronic Data Capture System
- sd:
-
Standard deviation
- SP:
-
Subproject
- TAM2:
-
Technology Acceptance Model
References
Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158–69. https://doi.org/10.1016/S0140-6736(13)60439-0.
Bruck K, Stel VS, Gambaro G, Hallan S, Volzke H, Arnlov J, et al. CKD prevalence varies across the european general population. J Am Soc Nephrol. 2016;27(7):2135–47. https://doi.org/10.1681/ASN.2015050542.
Murton M, Goff-Leggett D, Bobrowska A, Garcia Sanchez JJ, James G, Wittbrodt E, et al. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv Ther. 2021;38(1):180–200. https://doi.org/10.1007/s12325-020-01568-8.
Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47. https://doi.org/10.1001/jama.298.17.2038.
Beck H, Titze SI, Hubner S, Busch M, Schlieper G, Schultheiss UT, et al. Correction: heart failure in a cohort of patients with chronic kidney disease: The GCKD Study. PLoS ONE. 2015;10(6):e0131034. https://doi.org/10.1371/journal.pone.0131034.
Kotsis F, Schultheiss UT, Wuttke M, Schlosser P, Mielke J, Becker MS, et al. Self-reported medication use and urinary drug metabolites in the German Chronic Kidney Disease (GCKD) Study. J Am Soc Nephrol. 2021;32(9):2315–29. https://doi.org/10.1681/asn.2021010063.
Schmidt IM, Hubner S, Nadal J, Titze S, Schmid M, Barthlein B, et al. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: the German Chronic Kidney Disease study. Clin Kidney J. 2019;12(5):663–72. https://doi.org/10.1093/ckj/sfz046.
Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80(6):572–86. https://doi.org/10.1038/ki.2011.223.
Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–66. https://doi.org/10.1016/s0140-6736(11)61454-2.
Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–8. https://doi.org/10.1038/ki.2011.379.
Ali SI, Jung SW, Bilal HSM, Lee SH, Hussain J, Afzal M et al. Clinical decision support system based on hybrid knowledge modeling: a case study of chronic kidney disease-mineral and bone disorder treatment. Int J Environ Res Public Health. 2021;19(1). https://doi.org/10.3390/ijerph19010226.
Khoong EC, Karliner L, Lo L, Stebbins M, Robinson A, Pathak S, et al. A pragmatic cluster randomized trial of an electronic clinical decision support system to improve chronic kidney disease management in primary care: design, rationale, and implementation experience. JMIR Res Protoc. 2019;8(6):e14022. https://doi.org/10.2196/14022.
Litvin CB, Hyer JM, Ornstein SM. Use of clinical decision support to improve primary care identification and management of Chronic Kidney Disease (CKD). J Am Board Fam Med. 2016;29(5):604–12. https://doi.org/10.3122/jabfm.2016.05.160020.
Mendu ML, Schneider LI, Aizer AA, Singh K, Leaf DE, Lee TH, et al. Implementation of a CKD checklist for primary care providers. Clin J Am Soc Nephrol. 2014;9(9):1526–35. https://doi.org/10.2215/cjn.01660214.
Zacharias HU, Altenbuchinger M, Schultheiss UT, Raffler J, Kotsis F, Ghasemi S, et al. A predictive model for progression of CKD to kidney failure based on routine laboratory tests. Am J Kidney Dis. 2022;79(2):217–30.e1. https://doi.org/10.1053/j.ajkd.2021.05.018.
Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553–9. https://doi.org/10.1001/jama.2011.451.
Tangri N, Grams ME, Levey AS, Coresh J, Appel LJ, Astor BC, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164–74. https://doi.org/10.1001/jama.2015.18202.
Barbour SJ, Coppo R, Zhang H, Liu ZH, Suzuki Y, Matsuzaki K, et al. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179(7):942–52. https://doi.org/10.1001/jamainternmed.2019.0600.
Katholieke Universiteit Leuven DLoICM. Acute Kidney Injury Predictor. https://www.akipredictor.com/en/. Accessed Apr 2022.
Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506–17. https://doi.org/10.1056/NEJMoa1102662.
CKDNapp Junior Konsortium. https://www.sys-med.de/en/junior-research-alliances/ckdnapp/. Accessed Apr 2022.
Pape L, Schneider N, Schleef T, Junius-Walker U, Haller H, Brunkhorst R, et al. The nephrology eHealth-system of the metropolitan region of Hannover for digitalization of care, establishment of decision support systems and analysis of health care quality. BMC Med Inform Decis Mak. 2019;19(1):176. https://doi.org/10.1186/s12911-019-0902-0.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. https://doi.org/10.1016/j.jclinepi.2007.11.008.
Schultheiss UT, Doenitz J, Kotsis F, Altenbuchinger M, Zacharias HU. Apps in der Betreuung von CKD-Patienten: Die Chronic Kidney Disease Nephrologist’s App. Nephro-News. 2021;5:18–25.
Schultheiss UT, Kotsis F, Doenitz J, Kosch R, Altenbuchinger M, Zacharias HU. Entwicklung eines Entscheidungsunterstüztungssystems für die ärztliche Behandlung in der niedergelassenen Nephrologie. ConnexiPlus. 2021;4:18–25.
Sedlmayr B, Patapovas A, Kirchner M, Sonst A, Müller F, Pfistermeister B, et al. Comparative evaluation of different medication safety measures for the emergency department: physicians’ usage and acceptance of training, poster, checklist and computerized decision support. BMC Med Inform Decis Mak. 2013;13:79. https://doi.org/10.1186/1472-6947-13-79.
Zhang H, Cocosila M, Archer N. Factors of adoption of mobile information technology by homecare nurses: a technology acceptance model 2 approach. Comput Informatics, Nurs. 2010;28(1):49–56. https://doi.org/10.1097/NCN.0b013e3181c0474a.
Venkatesh V, Davis F. A theoretical extension of the technology acceptance model: four longitudinal field studies. Manage Sci. 2000;46:186–204. https://doi.org/10.1287/mnsc.46.2.186.11926.
Chismar WG, Wiley-Patton S. Test of the technology acceptance model for the internet in pediatrics. Proc AMIA Symp. 2002:155–9.
Yu P, Li H, Gagnon MP. Health IT acceptance factors in long-term care facilities: a cross-sectional survey. Int J Med Informatics. 2009;78(4):219–29. https://doi.org/10.1016/j.ijmedinf.2008.07.006.
Bhattacherjee A, Hikmet N. Physicians’ resistance toward healthcare information technology: a theoretical model and empirical test. Eur J Inf Syst. 2007;16:725–37. https://doi.org/10.1057/palgrave.ejis.3000717.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. https://doi.org/10.1016/j.jbi.2019.103208.
Ostropolets A, Chen R, Zhang L, Hripcsak G. Characterizing physicians’ information needs related to a gap in knowledge unmet by current evidence. JAMIA Open. 2020;3(2):281–9. https://doi.org/10.1093/jamiaopen/ooaa012.
Holmes BJ, Schellenberg M, Schell K, Scarrow G. How funding agencies can support research use in healthcare: an online province-wide survey to determine knowledge translation training needs. Implement Sci. 2014;9:71. https://doi.org/10.1186/1748-5908-9-71.
Bierbaum M, Rapport F, Arnolda G, Nic Giolla Easpaig B, Lamprell K, Hutchinson K, et al. Clinicians’ attitudes and perceived barriers and facilitators to cancer treatment clinical practice guideline adherence: a systematic review of qualitative and quantitative literature. Implement Sci. 2020;15(1):39. https://doi.org/10.1186/s13012-020-00991-3.
Sasaki N, Yamaguchi N, Okumura A, Yoshida M, Sugawara H, Shin JH, et al. Factors affecting the use of clinical practice guidelines by hospital physicians: the interplay of IT infrastructure and physician attitudes. Implement Sci. 2020;15(1):101. https://doi.org/10.1186/s13012-020-01056-1.
Thangada ND, Garg N, Pandey A, Kumar N. The emerging role of mobile-health applications in the management of hypertension. Curr Cardiol Rep. 2018;20(9):78. https://doi.org/10.1007/s11886-018-1022-7.
Hussein WF, Bennett PN, Pace S, Chen S, Legg V, Atwal J, et al. The mobile health readiness of people receiving in-center hemodialysis and home dialysis. Clin J Am Soc Nephrol. 2020;16(1):98–106. https://doi.org/10.2215/cjn.11690720.
Salloum RG, Bilello L, Bian J, Diiulio J, Paz LG, Gurka MJ, et al. Study protocol for a type III hybrid effectiveness-implementation trial to evaluate scaling interoperable clinical decision support for patient-centered chronic pain management in primary care. Implement Sci. 2022;17(1):44. https://doi.org/10.1186/s13012-022-01217-4.
Wang CS, Ku E. eHealth in kidney care. Nat Rev Nephrol. 2020;16(7):368–70. https://doi.org/10.1038/s41581-020-0271-z.
Singh K, Diamantidis CJ, Ramani S, Bhavsar NA, Mara P, Warner J, et al. Patients’ and nephrologists’ evaluation of patient-facing smartphone apps for CKD. Clin J Am Soc Nephrol. 2019;14(4):523–9. https://doi.org/10.2215/cjn.10370818.
Lee YL, Cui YY, Tu MH, Chen YC, Chang P. Mobile health to maintain continuity of patient-centered care for chronic kidney disease: content analysis of apps. JMIR Mhealth Uhealth. 2018;6(4):e10173. https://doi.org/10.2196/10173.
Flechet M, Güiza F, Schetz M, Wouters P, Vanhorebeek I, Derese I, et al. AKIpredictor, an online prognostic calculator for acute kidney injury in adult critically ill patients: development, validation and comparison to serum neutrophil gelatinase-associated lipocalin. Intensive Care Med. 2017;43(6):764–73. https://doi.org/10.1007/s00134-017-4678-3.
Zazzi M, Cozzi-Lepri A, Prosperi MC. Computer-aided optimization of combined anti-retroviral therapy for HIV: new drugs, new drug targets and drug resistance. Curr HIV Res. 2016;14(2):101–9. https://doi.org/10.2174/1570162x13666151029102254.
Lindsey R, Daluiski A, Chopra S, Lachapelle A, Mozer M, Sicular S, et al. Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci USA. 2018;115(45):11591–6. https://doi.org/10.1073/pnas.1806905115.
Kucher N, Koo S, Quiroz R, Cooper JM, Paterno MD, Soukonnikov B, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–77. https://doi.org/10.1056/NEJMoa041533.
Varonen H, Kortteisto T, Kaila M. What may help or hinder the implementation of computerized decision support systems (CDSSs): a focus group study with physicians. Fam Pract. 2008;25(3):162–7. https://doi.org/10.1093/fampra/cmn020.
Toth-Pal E, Wårdh I, Strender LE, Nilsson G. Implementing a clinical decision-support system in practice: a qualitative analysis of influencing attitudes and characteristics among general practitioners. Inform Health Soc Care. 2008;33(1):39–54. https://doi.org/10.1080/17538150801956754.
Grossi A, Hoxhaj I, Gabutti I, Specchia ML, Cicchetti A, Boccia S, et al. Hospital contextual factors affecting the implementation of health technologies: a systematic review. BMC Health Serv Res. 2021;21(1):407. https://doi.org/10.1186/s12913-021-06423-2.
Tomoaia-Cotisel A, Scammon DL, Waitzman NJ, Cronholm PF, Halladay JR, Driscoll DL et al. Context matters: the experience of 14 research teams in systematically reporting contextual factors important for practice change. Ann Fam Med. 2013;11(Suppl 1):S115–23. https://doi.org/10.1370/afm.1549.
Jansen-Kosterink S, van Velsen L, Cabrita M. Clinician acceptance of complex clinical decision support systems for treatment allocation of patients with chronic low back pain. BMC Med Inform Decis Mak. 2021;21(1):137. https://doi.org/10.1186/s12911-021-01502-0.
Michie S, Thomas J, Johnston M, Aonghusa PM, Shawe-Taylor J, Kelly MP, et al. The Human Behaviour-Change Project: harnessing the power of artificial intelligence and machine learning for evidence synthesis and interpretation. Implement Sci. 2017;12(1):121. https://doi.org/10.1186/s13012-017-0641-5.
Schena FP, Anelli VW, Abbrescia DI, Di Noia T. Prediction of chronic kidney disease and its progression by artificial intelligence algorithms. J Nephrol. 2022. https://doi.org/10.1007/s40620-022-01302-3.
Kadatz MJ, Lee ES, Levin A. Predicting progression in CKD: perspectives and precautions. Am J Kidney Dis. 2016;67(5):779–86. https://doi.org/10.1053/j.ajkd.2015.11.007.
Johnson ES, Thorp ML, Platt RW, Smith DH. Predicting the risk of dialysis and transplant among patients with CKD: a retrospective cohort study. Am J Kidney Dis. 2008;52(4):653–60. https://doi.org/10.1053/j.ajkd.2008.04.026.
Tangri N, Kitsios GD, Inker LA, Griffith J, Naimark DM, Walker S, et al. Risk prediction models for patients with chronic kidney disease: a systematic review. Ann Intern Med. 2013;158(8):596–603. https://doi.org/10.7326/0003-4819-158-8-201304160-00004.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350: g7594. https://doi.org/10.1136/bmj.g7594.
Geri G, Stengel B, Jacquelinet C, Aegerter P, Massy ZA, Vieillard-Baron A, et al. Prediction of chronic kidney disease after acute kidney injury in ICU patients: study protocol for the PREDICT multicenter prospective observational study. Ann Intensive Care. 2018;8(1):77. https://doi.org/10.1186/s13613-018-0421-7.
Hsu C-y, Hsu RK, Liu KD, Yang J, Anderson A, Chen J, et al. Impact of AKI on urinary protein excretion: Analysis of two prospective cohorts. J Am Soc Nephrol. 2019;30(7):1271–81.
See EJ, Toussaint ND, Bailey M, Johnson DW, Polkinghorne KR, Robbins R, et al. Risk factors for major adverse kidney events in the first year after acute kidney injury. Clin Kidney J. 2021;14(2):556–63. https://doi.org/10.1093/ckj/sfz169.
Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138.
Sawhney S, Tan Z, Black C, Marks A, McLernon DJ, Ronksley P, et al. Validation of risk prediction models to inform clinical decisions after acute kidney injury. Am J Kidney Dis. 2021;78(1):28–37. https://doi.org/10.1053/j.ajkd.2020.12.008.
Kelly JT, Su G, Zhang, Qin X, Marshall S, Gonzalez-Ortiz A, et al. Modifiable lifestyle factors for primary prevention of CKD: a systematic review and meta-analysis. J Am Soc Nephrol. 2021;32(1):239–53. https://doi.org/10.1681/ASN.2020030384.
Kelly JT, Su G, Carrero JJ. Lifestyle interventions for preventing and ameliorating CKD in primary and secondary care. Curr Opin Nephrol Hypertens. 2021;30(6):538–46. https://doi.org/10.1097/MNH.0000000000000745.
Schrauben SJ, Apple BJ, Chang AR. Modifiable lifestyle behaviors and CKD progression: a narrative review. Kidney360. 2022;3(4):752–78. https://doi.org/10.34067/KID.0003122021.
Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150.
Hu EA, Coresh J, Anderson CAM, Appel LJ, Grams ME, Crews DC, et al. Adherence to healthy dietary patterns and risk of CKD progression and all-cause mortality: findings from the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2021;77(2):235–44. https://doi.org/10.1053/j.ajkd.2020.04.019.
Heindel J, Baid-Agrawal S, Rebholz CM, Nadal J, Schmid M, Schaeffner E, et al. Association between dietary patterns and kidney function in patients with chronic kidney disease: a cross-sectional analysis of the German chronic kidney disease study. J Ren Nutr. 2020;30(4):296–304. https://doi.org/10.1053/j.jrn.2019.09.008.
Decety J. Empathy in medicine: what it is, and how much we really need it. Am J Med. 2020;133(5):561–6. https://doi.org/10.1016/j.amjmed.2019.12.012.
Miller RA. Why the standard view is standard: people, not machines, understand patients’ problems. J Med Philos. 1990;15(6):581–91. https://doi.org/10.1093/jmp/15.6.581.
Grote T, Berens P. On the ethics of algorithmic decision-making in healthcare. J Med Ethics. 2020;46(3):205–11. https://doi.org/10.1136/medethics-2019-105586.
Kwok R, Dinh M, Dinh D, Chu M. Improving adherence to asthma clinical guidelines and discharge documentation from emergency departments: implementation of a dynamic and integrated electronic decision support system. Emerg Med Australas. 2009;21(1):31–7. https://doi.org/10.1111/j.1742-6723.2008.01149.x.
Ash JS, Sittig DF, Campbell EM, Guappone KP, Dykstra RH. Some unintended consequences of clinical decision support systems. AMIA Annu Symp Proc. 2007;2007:26–30.
Berner ES, Kasiraman RK, Yu F, Ray MN, Houston TK. Data quality in the outpatient setting: impact on clinical decision support systems. AMIA Annu Symp Proc. 2005;2005:41–5.
Mitchell J, Probst JC, Bennett KJ, Glover S, Martin AB, Hardin JW. Differences in pneumonia treatment between high-minority and low-minority neighborhoods with clinical decision support system implementation. Inform Health Soc Care. 2016;41(2):128–42. https://doi.org/10.3109/17538157.2014.965304.
Hussain-Gambles M, Atkin K, Leese B. Why ethnic minority groups are under-represented in clinical trials: a review of the literature. Health Soc Care Community. 2004;12(5):382–8. https://doi.org/10.1111/j.1365-2524.2004.00507.x.
Acknowledgements
We thank you survey participants for their valuable input and help in improving this study. We thank the large number of nephrologists who provide routine care for the patients and collaborate with the GCKD study. A list of nephrologists currently collaborating with the GCKD study is available at http://www.gckd.org.
Funding
Open Access funding enabled and organized by Projekt DEAL. The work of HUZ, UTS and FK, MA, and JD was supported by the German Ministry of Education and Research (Bundesministerium für Bildung und Forschung; BMBF) within the framework of the e:Med research and funding concept (grants 01ZX1912A, 01ZX1912B, 01ZX1912C, 01ZX1912D). The work of SS and TB was funded by the German Federal Ministry of Education and Research (Project DESIREE, Grant ID 01GP1911A-D).
Author information
Authors and Affiliations
Contributions
Research idea and study design: FK, HUZ, UTS; questionnaire design: FK, HB, SS, TB, UTS; critical questionnaire review: FK, MA, RK, SS, TB, HUZ, UTS; data acquisition: FK, HB, UTS; data analysis/interpretation: FK, UTS; statistical analysis: FK, UTS; supervision or mentorship: FK, UTS; critical review of the manuscript: FK, HB, MA, HM, RK, SS, TB, HUZ, UTS, JD, YANN. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the University of Freiburg and registered at the German registry for clinical studies (DRKS00025054). All participants gave informed consent prior to taking the survey. All methods were carried out in accordance with relevant guidelines and regulations following the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table 1.
CKDNapp survey questionnaire questions and answer possibilities. Supplemental Table 2. Complete numbers and percentages to questions on experiences with clinical decision support software of module 1. Supplemental Table 3. Complete numbers and percentages to questions on expectations of a clinical decision support software in general of module 2. Supplemental Table 4. Complete numbers and percentages to questions on prediction algorithms of module 3. Supplemental Table 5. Complete overview of questions on ethical aspects of CDSS from module 4.
Additional file 2: Supplemental Fig. 1.
CKDNapp survey analysis set and number of missingness per participant across all questions. Supplemental Fig. 2. Scatterplots of participants concerning their work experience. Supplemental Fig. 3. Expectations of a clinical decision support software in general of module 2 – details on questions M2.1 to M2.4. Supplemental Fig. 4. Preferred device for querying a CDSS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kotsis, F., Bächle, H., Altenbuchinger, M. et al. Expectation of clinical decision support systems: a survey study among nephrologist end-users. BMC Med Inform Decis Mak 23, 239 (2023). https://doi.org/10.1186/s12911-023-02317-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-023-02317-x