Abstract
Background
Breast cancer is the most common type of cancer diagnosed in women. Finding novel therapeutic agents with significant cytotoxic action and minimal adverse impact on normal cells becomes crucial. Today, natural anticancer agents present an unconventional method of treating cancer, either as a curative or preventative agent, with considerable concern for marine organisms.
Methods
The anticancer effect of the alcoholic extract of different Red Sea Seagrasses on MCF-7 human breast cancer cell line has been investigated. Seagrasses were collected from Wadi El Gamal, Red Sea and extracted. Qualitative HPLC analysis was performed on the extracts for the identification of their active biomarkers. This study was aimed to explore the cytotoxic impact of Thalassia hemprichii (Ehren.) and Enhalus acoroides (L.f.) Royle on MCF-7 and their mode of action. Their anti-proliferative effects on cancer cells were performed using Neutral red assay. On the other hand, their apoptotic effect and their capacity to induce cell cycle arrest were investigated by flow cytometry assay. The effect of Seagrasses on the mitochondrial membrane potential (ΔψM) was studied by using JC-1 mitochondrial membrane potential assay kit in Seagrasses treated cancer cells to Δψ Caspases 3/7activity was examined using the colorimetric method. Gene expression analysis and quantitative real time RT-PCR for the sea grasses on MCF-7 was performed. Immune-blotting technique for Bcl-2 and p53 was investigated.
Results
HPLC analysis demonstrated that the extracts contained mainly flavonoids and polyphenols such as Caffeic acid, Chlorogenic acids, catechin and kaempferol that might be responsible for these anticancer effects. Seagrasses alcoholic crude extract markedly suppressed the growth and expansion of MCF-7 cells concentration-dependently with no toxicity against normal human skin fibroblast HSF. Thalassia hemprichii and Enhalus acoroides trigger mode of cell death primarily via apoptosis as confirmed by the flow cytometry. Additionally, they have ability to induce G0/S cell cycle arrest in MCF-7. The data showed the depletion in mitochondrial membrane potential (ΔψM) in the treated cells dose-dependently Caspases 3/7activities markedly increased following 24 h treatment. Finally, Gene expression analysis showed a marked reduction in Bcl-2, Survivin and CDC2 gene expression levels and a significant increase in the expression of p53 and CC2D1A as compared to control cells.
Conclusion
In summary, the Methanolic extract of seagrass, Thalassia hemperchii and Enhalus ocoroides are able to induce concentration-dependent cytotoxic effects in human MCF-7 cells through intrinsic pathway of apoptosis in MCF-7 cells. This study reveals the beneficial importance of sea grasses as a source of anticancer agents. Further in vivo study is recommended for the active isolated biomolecules.
Similar content being viewed by others
Introduction
Cancer is a fatal condition that arises from unregulated cell growth and expansion with a high potential to spread to other body organs [1]. Breast cancer is thought to be the main reason for cancer-associated death. Chemotherapy, radiation, and surgery are frequently used in the treatment of cancer [2]. The risk of cancer recurrence is still extremely significant, despite the high cure rate associated with surgical treatment [3]. In addition to having undesirable, severe side effects like hair loss, nausea, and vomiting, it has been reported that the majority of chemotherapeutic medicines cause toxicity to normal cells and can even lead to the development of resistance to the treatment itself [4]. Consequently, it becomes an imperative need to find new therapeutic agents with potent cytotoxic effect and no negative influence on normal cells. Nowadays, the natural anticancer agent represents a novel approach to cancer therapy either as a therapeutic or protective factor with a great concern for marine organisms [5]. Numerous chemically distinct substances originating from the sea have been studied and are being developed as novel nutraceuticals. One such example is Plitidepsin, which has been shown to be useful in treating a variety of malignancies, including melanoma, bladder, and small and non-small cell lung cancers [6, 7].
According to Kennedy and Björk [8], seagrass is the smallest taxonomic category of marine angiosperms, consisting of just around 60 species divided among four families and found worldwide, with the exception of the Polar Regions. The most fruitful meadow, which contains roughly 24 species, is made up of tropical seagrass [9]. They were historically ingested as supplements by the coastal people and utilized in the treatment of different remedies [10].
Marine seagrasses are a rich source for secondary metabolites, mainly, phenolic compounds [11]. Thalassia hemperchii is a widespread species of seagrass in the genus Thalassia that is indigenous to the shore of the Red Sea [12].
Yondelis® is the first marine-derived anticancer drug that received the European approval after clinical studies for the treatment of soft tissue sarcoma and ovarian carcinoma in 2007 and in 2009, respectively [13]. Phenolic compounds are one of the secondary metabolites of plants that play a role in their defense mechanism against different environmental conditions [14]. Marine organisms are a rich source of different classes of phenolic compounds [15]. Seagrasses are poorly studied widely spread marine plants that have been reported to have their capacity to induce cytotoxicity in various cancer cells via apoptosis as well as oxidative stress-dependent cell death [16, 17]. Based on this previous background, we decided to obtain and characterize crude MeOH extract from two different Seagrass to study their cytotoxic potential in breast cancer cells and their effect on mitochondrial membrane potential and caspases enzyme activities.
Materials and methods
Collection of seagrass material
Seagrass samples were gathered from the Red Sea, Egypt’s Wadi El Gemal National Park. Dr. Amgad ElShaffai (The Egyptian Environmental Affairs Agency (EEAA) carefully collected and identified the samples. Enhalus acoroides (Linn.F.) Royle was collected from Abu Ghoson Mangrove-North, Wadi El Gemal National Park, Marsa Alam, Egypt in February 2019. It is encompassing a segment of the Red Sea coastal plain (about 70 km of coastline, including the ecotourism development areas) and mountains extending roughly between 24°22’ N in the north and 24°05’ N in the south. The voucher specimens of the plant have been deposited in the Wadi El Gemal National Park herbarium under specimen number #WGAE0013. Thalassia hemprichii (Ehren.) Asch. was gathered from Sharm El Fakeri, Wadi El Gemal National Park, Marsa Alam, Egypt (Latitude 24°45 17.84” and Longitude 35°03 58.36”) in April 2018. Voucher specimen was deposited in the Wadi El Gemal National Park with specimen number # WGAE0012. Collection of living plants material from the wild was complied with Seagrass Net protocol and as per standards for seagrass collection, identification and sample design. Samples were thoroughly washed with sea water and epiphytes were carefully removed. Clean fresh samples were kept in freezer for further analysis.
Method of extraction
Fresh Enhalus acoroides (Linn.F.) Royle and Thalassia hemprichii (Ehren.) Asch. were extracted as previously mentioned by [17]. Briefly, seagrass was extracted by methanol using electric blender, then the mixture was subjected for sonication for 20 min at room temp. Then, the mixture of each seagrass was filtered, and the solvent was evaporated under reduced pressure at 45 °C. The crude methanol extracts of Thalassia hemprichii, Enhalus acoroides were stored for further analysis.
Qualitative HPLC analysis of phenolic
Phenolic acids and flavonoids were qualitatively analyzed using HPLC analysis. 10 µl of the crude MeOH extract (50 mg/ml, MeOH) was injected into HPLC (waters 2690 Alliance) with a photodiode array detector. The Column, C18 Intertsil ODS 3 (4.6 × 250 mm, 5 μm), was gradient eluted with a buffer of 0.1% phosphoric acid in water and methanol at a flow rate of 1 ml/min, ambient temperature and wave length 280 nm. Ten µl of each of the standard samples were injected into the HPLC under the same conditions after being dissolved in methanol.
Fourier transform infrared (FT-IR) analysis
Potassium bromide (KBr) was mixed with a few mg of crude extract before being compressed into a disc with a 10 mm diameter. The Jasco FT/IR-6100 type A was used for the FT-IR analysis, with wave numbers ranging from 400 cm− 1 to 4000 cm− 1.
Biological activities
Cell propagation and maintenance
The appropriate conditions were used to culture MCF-7 (cancer cell line) and HSF (normal human skin fibroblasts) cell lines obtained from the ATCC (American Type Culture Collection). The cells were propagated in DMEM (Lonza, Beligium) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 g/ml streptomycin sulphate at 37 °C in a humid incubator with 5% CO2. The cells were collected after being trypsinized with 0.025% trypsin and 0.02% EDTA, followed by two washes in DPBS. Cells were divided for continued cultivation when the cell density reached about 80%. When the cells were in the logarithmic growth phase, the experiments were started.
Anti- proliferation assay
Neutral red uptake assay was used to measure the cell viability [18]. The amount of viable cells in the culture can be quantitatively estimated using the neutral red uptake assay. It is predicated on how well-functioning cells can bind and integrate the supravital dye neutral red in lysosomes. At a cell density of 104 cells/well in a 96-well plate, MCF-7 and HSF (normal cell line) were treated for 48 h with various concentrations of the crude extracts. 20 mg of both Methanolic extracts was dissolved in DMSO (Dimethylsulfoxide) and a serial dilution in culture media was prepared of different concentrations (0, 25, 50, 100, 200, 400, 800, and 1000 µg/ml). Similar to how the treated cells were cultured, a neutral red working solution (0.4 µg/ml) (Sigma-Aldrich) was incubated at 37 °C overnight. Culture media were removed from the cultured cells’ wells and neutral red medium (100 µl) was added to each well and incubated for 2 h to allow the vital dye to be incorporated into the viable cells. The Dulbecco’s PBS buffer (150 µl) was used to rinse the neutral red medium after removing them. By adding extraction buffer (150 µl, 1% acetic acid, 50% ethanol (96%) and 49% deionized water) and shaking the cells for at least 10 min on a micrometer plate shaker, dye was removed from the cells. In a micro-titer plate reader spectrophotometer (Sorin, Bio-medica S.p.A., Milan, Italy), the intensity of the extract’s neutral red color was measured at 530 and 645 nm as the excitation and emission wavelengths. Using the relationship between the neutral red and the used log concentrations, the IC50 of the tested crude extracts was determined. For the untreated cells (negative control), medium was added instead of the test crude extract. A positive control Doxorubicin (Dox, Mr = 543.5) was used (at concentrations 0.37, 0.75, 1.5, 3 and 6 µg/ml) as a cytotoxic agent giving 100% inhibition. Dimethyl sulfoxide (DMSO), the vehicle used for dissolving the tested crude extracts, was used and its final concentration on the cells was less than 0.2%. All experiments were done in triplicates and repeated 3 times under the same conditions. The data was statistically analyzed by graph prism V5 and presented as mean values with their standard error means (mean SEM).
cell viability % was calculated as follows:
Determination of cell death- annexin V / propidium iodide double staining apoptosis assay
The capacity of the tested extracts to induce necrosis and apoptosis was evaluated by using Annexin V-FITC apoptosis detection kit and two fluorescent channels flowcytometry (Abcam Inc., Cambridge Science Park, Cambridge, UK). MCF-7 cells were treated using three different concentrations 101.19 (IC50), 200 and 400 µg/ml for Thalassia hemperchii and 604.2 (IC50), 800 and 1000 µg/ml for Enhalus acoroides as well as Doxorubicin (1.2 µg/ml) as a positive control for 48 h. Following that, cells (1 × 105 cells) were collected by trypsinization and washed twice with ice-cold PBS (pH 7.4). Cells were then incubated for 30 min at room temperature in the dark with 0.5 ml of Annexin V-FITC/PI solution, as directed by the manufacturer [19]. Cells were injected into the ACEA NovocyteTM flow-cytometer (ACEA Biosciences Inc., San Diego, CA, USA) after staining, and FITC and PI fluorescence signals were analyzed using FL1 and FL2 signal detectors, respectively (λex/em 488/530 nm for FITC and λex/em 535/617 nm for PI). For each test sample, 12,000 events were recorded, and the number of positive FITC and/or PI cells was determined using the ACEA Novo-Express TM software (ACEA Biosciences Inc., San Diego, CA, USA) and quadrant analysis.
Assessment of cell cycle arrest
MCF-7 cells (5 × 105 cells) were treated for 48 h with doxorubicin (1.2 µg/ml) (as a positive control) and extracts at IC50 concentrations (101.19 µg/ml for TH and 604.2 µg/ml for EA) for the measurement of cell cycle on a six-well plate at 37 °C and 5% CO2. Cells were collected and washed after trypsinization, centrifuged at 2000 ×g for 10 min., and the pellet was then re-suspended in 0.5 ml PBS. After adding 1.2 ml of 70% cold ethanol for two hours, fixation was finished. The fixed cells were then centrifuged at 2000x g for 10 min after being washed with PBS. 8 µl of DNAase free RNAse (10 mg/ml) was added and incubated for 1 h after cells had been suspended in 0.3 mL of PBS. Cells were incubated at 4 °C for 30 min after adding 15 µl of Propidium iodide (0.5 mg/ml). Using a flow cytometer FACS calibur (Beckman Coulter, Fullerton, CA, USA) with an excitation wavelength of 488 nm and an emission wavelength of 670 nm, the cells were examined for cell cycle [20]. ACEA Novo-ExpressTM software (ACEA Biosciences Inc., San Diego, CA, USA) was used to analyze cell cycle distribution and determine DNA content.
Assessment of mitochondrial membrane potential
The mitochondrial membrane potential (ΔψM) in Seagrasses-treated MCF-7 cells was measures using the JC-1 mitochondrial membrane potential assay kit (ab113850, Abcam, Cambridge, United Kingdom) [21]. Tetra-ethylbenzimidazolyl carbocyanine iodide, a cationic dye that accumulates in polarized mitochondria attracted by a high ΔψM, generates red luminous aggregates at high concentrations. JC-1 ceases accumulation inside mitochondria upon ΔψM dissipation, and the low concentration monomers of JC-1 molecules show green fluorescence. We carried out the test in accordance with the manufacturer’s instructions. In a 96-well plate with a black wall, 15,000 MCF-7 cells were sown within each well before 100 µl of the treatment solution was applied. Both extracts were used at various concentrations (0, 100, 200, 400, 800 and 1000 µg/ml) for 48 h. Then, we added 100 µl of 2X JC-1 (40 µM) solution to each well 30 min before the end of the treatment, and we incubated the samples for 30 min at 37 °C in a light-protected environment. With 1X dilution buffer that had been pre-warmed (100 µl/well), the cells were washed twice. The final wash was left on the cells, and the microplate reader was used to detect the fluorescence of the aggregates (excitation: 475 nm, emission: 590 nm) and monomers (excitation: 475 nm, emission: 530 nm). We calculated the fold-change in relation to the negative control (untreated cells) using the aggregate-to-monomer fluorescence ratio (JC-1 ratio), which declines during ΔψM depolarization. Both JC-1 staining and final determination were carried out in the presence of test treatment. Thus, we spiked both staining solution and dilution buffer with the corresponding concentration of extract. The cells were treated with ionophore uncoupled FCCP (carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone, 50 µM) as a positive control for 2 h which decreased the JC-1 ratio to ~ 38% of the control (DMSO 0.1%) levels.
Assay of caspase-3/7 activity
According to the manufacturer’s instructions (R&D Systems), a colorimetric assay kit was used to measure the enzymatic activity of the caspase 3/7 triggered by sea grasses [22]. Briefly, Cells 1 × 105/well were treated with doxorubicin (1.2 µg/ml) (as a positive control) and extracts at IC50 concentrations (101.19 µg/ml for TH and 604.2 µg/ml for EA) for 24 h. After the treated cells were lysed in a lysis buffer, the protein was incubated at 37 °C for 2 h with 50 µl of a reaction buffer and 5 µl of the colorimetric tetrapeptide Asp-Glu-Val-Asp (DEVD)-p-nitroaniline (pNA) for caspase-3/7. At a wavelength of 405 nm, the optical density of the reaction mixture was measured spectrophotometrically.
Immunoblotting
In 6 well culture plate, 1 × 105/well cells were treated with doxorubicin (1.2 µg/ml) (as a positive control) and extracts at IC50 concentrations (101.19 µg/ml for TH and 604.2 µg/ml for EA) for 24 h. RIPA buffer was used to obtain the cell lysates from MCF-7 cells that had been pre-treated for 48 h. Thermo-Fisher scientific’s Pierce BCA Protein assay kit was used to calculate the total protein concentration. Following 10% SDS-PAGE (sodium dodecyl sulphate polyacrylamide gel electrophoresis), samples of the same protein content (30 µg/20 µl) were blotted onto polyvinylidene difluoride membranes (PVDF) (Millipore, Bedford, MA, USA). The membrane was first blocked for 1 h at 25 °C with 5% non-fat milk in TBS-T buffer containing 0.1% Tween 20 before being incubated overnight at 4 °C with blocking solution containing primary antibodies for Bcl-2, p53 and GAPDH using rabbit polyclonal anti-Bcl-2 (# PA5-20068, Invitrogen, Thermo Fisher Scientific, USA), mouse monoclonal anti-p53 (# MA5-12557, Invitrogen, Thermo Fisher Scientific, USA) and rabbit monoclonal anti-GAPDH (#2118S, Cell Signaling Technology, USA) at 1:1000 dilutions, respectively. The membrane was then washed three times before being incubated for 1 h at 25 °C with constant agitation in the blocking solution containing anti-mouse and anti-rabbit secondary antibody conjugated to horseradish peroxidase (HRP) at 1:3000 dilutions (Cell Signaling Technology, USA). The positive results were acquired using an enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech) following the washing phase. Using ChemiDoc imaging system of Bio-Rad Co, protein bands were detected. The expression level of protein was quantified using Bio-Rad Image Lab Software. The internal control used was the GAPDH expression.
Gene expression analysis
RNA was extracted and isolated from cells treated with both extracts (101.19 µg/ml for TH and 604.2 µg/ml for EA) and Doxorubicin (1.2 µg/ml) using Qiazol buffer (Qiagen, USA), as directed by the manufacturer. RNA-easy Mini-Kit (Qiagen, USA) was then used to clean up the RNA. Using the easyscript first strand cDNA synthesis supermix (Transbiotech, China), RNA was reverse transcribed. The copy numbers of b-actin, Bcl-2, survivin, CDC2, p53, and CC2D1A were measured using Transbiotech’s perfect start green qpcr supermix (China). The housekeeping gene beta actin was used to normalize the copy numbers to 100,000 copies. Table 1 contains a list of primer sequences. The following were the RT and subsequent PCR cycling conditions: RT: 42 °C for 10 min, 85 °C for 5 s; qPCR: 94 °C for 30 s, 94 °C for 5 s, then 60 °C for 30 s.; there were 40 cycles total. Bio-Rad Real Time Thermal Mini-Opticon TM Thermal Cycler was used to quantify the gene expression.
Statistical analysis
Data from three different trials are represented as mean values with their standard error means (mean SEM). All data were analyzed using Graph Prism v5. One-way analysis of variance (ANOVA) and post-test Dunnett’s multiple comparison tests were used to analyze the data. For all tests, statistical significance was defined as *P < 0.05. All experiments were done in triplicates and repeated under same conditions.
Results
HPLC analysis
HPLC analysis (Table 2) reveals the abundance of Chlorogenic acid, Catechin, rutin and quercetin in both sea grass. On the other hand, Caffeic Acid, Kaempferol and Apigenin were only detected in Thalassia hemprichii (Ehern.) Asch. Seagrass were reported as a valuable source of phenolic compounds [23]. Chlorogenic acid (caffeoylquinic acid) is first to be detected in seagrass while other phenolic acids and flavonoids were previously isolated and/or detected. Caffeic acid was isolated from the alcoholic extract of Thalassia hemprichii (Ehren.) Asch [24, 25] and was detected only in the leaves of Enhalus acoroides (L. f.) Royle [15]. Rutin was previously detected using HPLC in leaves, roots and rhizome of Enhalus acoroides (L. f.) Royle. While Catechin was only in rhizome [17]. Quercetin, Kaempferol and Apigenin, were detected for the first time (Fig. 1). In our study apigenin wasn’t detected in Enhalus acoroides (L. f.) Royle that was in contrary to [26] Qi et al., 2008 findings. Further fractionation and purification is required to illustrate more phenolic compounds.
HPLC analyses of MeOH extract of (A) Enhalus acoroides (L.f.) Royle acoroides (Linnaeus f.) and (B) Thalassia hemprichii (Ehrenberg). a Representative HPLC chromatograms of (C) mixture of standards for identification of phenolic acids mainly (1) Chlorogenic acid (RT 30.608 min), (2) Caffeic acid (RT 32.821 min) and flavonoids mainly, (1) Catechin (RT 30.608), (2) Rutin at (RT 48.985 min), (3) Quercetin at (RT 56.997 min), (4) Kaempferol at (RT 59.212 min), (5) Apignin at (RT 59.667 min)
FTIR analysis
FTIR analysis was performed to detect the functional groups. The spectra (Fig. 2A and B) were interpreted and presented in Table 3 Thalassia hemprichii (Ehren) Asch spectrum illustrates the presence of alkanes, cyclic alcohol, aromatic amine III, hydroxyl, amide, and ketone functional groups that were absent in Enhalus acoroides (L.f.) Royle. Those functional groups are attributed to the abundance of terpenoids and flavonoids (Philip et al., 2011). 2850 and 2919 cm− 1, symmetric and asymmetric stretching vibrations of methylene (-CH2), respectively, 1722, 1380 cm-1 C = O or C-H stretching, and 1460 cm− 1 asymmetric (δ) bending vibration of C-H and the stretching vibration of aromatics corresponding to CH3, CH2 of flavonoids and aromatic rings.
Both spectra show a broad, strong band at 3261 and 3304 cm− 1, revealing OH-stretching vibration corresponding to phenols or alcohol [27]; the latter may also be attributed to lipids [28]. Spectra also illustrate C-N stretching bands that reveal the presence of amines, amides, and proteins. C = C stretching vibration ring (1409.43 cm− 1) attributed to flavonoids and amino acids. Enhalus acoroides (L.f.) Royle spectrum, characterized by a band at 1189.38 cm− 1, reveals the presence of sulfated compounds. The FTIR confirms the HPLC finding that Thalassia hemprichii (Ehren.) Asch has higher phenolic compounds content than Enhalus acoroides (L.f.) Royle, which directly correlated to the difference in their anticancer activity.
Biological assays
In vitro evaluation of cytotoxic activity
In this experiment, we investigated the cytotoxicity of two different Seagrasses including Enhalus acoroides (Linnaeus f.) Royle and Thalassia hemprichii. Using the neutral red assay, which is based on the ability of viable cells to incorporate and bind the supravital dye neutral red in the lysosomes, the cytotoxic activity of the crude methanolic extracts was investigated individually as anti-cancer agents against human breast cancer (MCF-7) cells at different concentrations (0, 50, 100, 200, 400, 800, and 1000 µg/ml). The reference drug, doxorubicin, has IC50 values of 1.2 µg/ml against the tested cell line MCF-7. After being exposed to DMSO for 48 h, MCF-7 vitality was not significantly affected. The studied extracts markedly inhibited cell proliferation as compared to negative control cells.
Data in Table 4 and Fig. 3 (A, B) indicated that the cytotoxic activity of the tested crude extracts was against MCF-7 cancer cells. The data showed that the cell viability of the treated cell line decreases dose-dependently. At 48 h, Thalassia hemperchii (101.19 µg/ml) showed more inhibitory effect. On the other hand, Enhalus acoroides revealed inhibition on the growth of MCF-7 with IC50 values of 604.2 µg/ml. Both extracts are safe and don’t exhibit any cytotoxicity on normal human skin fibroblast (HSF).
Thalassia Hemperchii and Enhalus acoroides induce programmed cell death
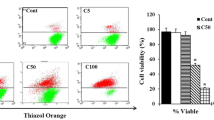
To explore the capability of Thalassia hemperchii and Enhalus acoroides to induce cell death via apoptosis rather than necrosis, MCF-7 cells were pre-treated using three different concentrations 101.19 (IC50), 200 and 400 µg/ml for Thalassia hemperchii and 604.2 (IC50), 800 and 1000 µg/ml for Enhalus acoroides as well as Doxorubicin as a positive control. The percentage of early and late apoptosis was determined by Annexin V/PI dual staining kit following 48 h of treatment. In Thalassia hemperchii treated MCF-7 the percentage of early apoptosis increased from 14.7% at IC50 value up to 60.7% by treatment with 400 µg/ml (Fig. 4A & C). Furthermore, the percentage of late apoptosis increased significantly up to 22%. On the other hand, Enhalus acoroides induced apoptosis in MCF-7 treated cells with marked increasing in early and late apoptosis up to 56.4% and 59.4%, respectively (Fig. 4B & D). The data shows that both extracts are able to induce programmed cell death after treatment with IC50 concentration rather than necrosis.
Representative FACS analyses of Annexin V and PI staining. MCF-7 cells were treated with (A) Thalassia hemperchii and (B) Enhalus acoroides for 48 h. Percentage of early and late apoptosis increased after treatment with 3 different concentrations (Thal 1 = 101.19, Thal 2 = 200 and Thal 3 = 400 µg/ml and Enhalus 1 = 604.2, Enhalus 2 = 800 and 1000 µg/ml) in compare with untreated cells. Bar graphs represent the percentage of viable, early apoptosis, late apoptosis and necrosis cells after treatment with (C) Thalassia hemperchii and (D) Enhalus acoroides for 48 h. Each histogram represents the mean ± SD from three independent experiments. * P < 0.05 and **P < 0.01 ***P < 0.001 compared with the control, as determined by one-way ANOVA
Effect of Thalassia hemperchii and Enhalus acoroides on cell cycle distribution
The capacity of sea grasses extracts to inhibit cells proliferation was further evaluated by determination of cell cycle distribution. After 48 h of treatment using the methanol extract of Thalassia hemperchii and Enhalus acoroides (101.19 and 604.2 µg/ml, respectively) and Doxorubicin (1.2 µg/ml) or control cells with vehicle (< 0.5% DMSO), an increase in S phase concomitant with a significant decrease in G0/G1 phase (*** P < 0.001) was observed as compared with untreated cells (Fig. 5A& B). The G2/M phase did not significantly increase following extract treatment. The data suggests that G0/S phase cell cycle arrest is related to the growth suppression of MCF-7 cells by methanol extract. The expression levels of pro-apoptotic Bcl-2 and anti-apoptotic p53 were measured utilizing western blots and gene expression patterns to further analyze these findings [37].
(A) Cell cycle analysis by flow cytometry. MCF-7 cell line was treated with Enhalus acoroides (Sample 1), Thalassia hemperchii (Sample 2) and Doxorubicin as a positive control. (B) Percentage of cell populations in MCF-7 treated cells with Enhalus acoroides (Sample 1), Thalassia hemperchii (Sample 2) and Doxorubicin as a positive control. Data are expressed as mean ± SD. (%). The data show a significant decrease *** P < 0.001, * * P < 0.01 in the percentage of cells at G0/G1 phase in treated sample in compare with negative control (untreated cells)
Mitochondrial membrane potential
Thalassia hemperchii and Enhalus acoroides were used at various concentrations (0, 100, 200, 400, 800 and 1000 µg/ml) to treat the MCF-7 cell line in order to measure the mitochondrial membrane potential. The result demonstrated that there was a drop in mitochondrial membrane potential (ΔψM) in the treated cells in dose-dependent manner. The results showed that Thalassia hemperchii was more significant in induction of mitochondrial membrane potential depletion in MCF-7 than Enhalus acoroides. Treatment of the MCF-7 cells with the Thalassia hemperchii and Enhalus acoroides for 48 h induced a reduction in mitochondrial membrane potential (depolarization), expressed as the reductions in JC-1 590/530 nm fluorescence ratios (in control versus in cells treated with the extract) (Fig. 6), which is a crucial indicator of mitochondrial-dependent apoptosis. We calculated the fold-change compared to the negative control (untreated). The ratio between aggregate and monomer fluorescence (JC-1 ratio) was determined, which declines with ΔψM depolarization.
Effect of (A) Thalassia hemperchii (B) Enhalus acoroides on mitochondrial membrane potential of MCF-7. Mitochondrial membrane potential (ΔψM) was measured by JC-1 fluorescence [fluorescence of JC-1 monomers (em 535 nm)/aggregates (em 590 nm)] in MCF-7 cells treated with (100, 200, 400, 800 and 1000 µg/mL) for 48 h. Thalassia hemperchii and Enhalus acoroides showed significant decrease in MCF-7 cells. Data are expressed as mean ± SD. *** P < 0.001, * P < 0.05
Induction of caspase 3/7 activity
Caspases are activated at the last stage of apoptosis [38], and understanding the active pathway is crucial for developing more effective treatment strategies [39]. In the current investigation, the activation of caspase-3/7 was examined on the MCF-7 cell line at concentrations of 101.19 (IC50) for Thalassia hemperchii and 604.2 (IC50) for Enhalus acoroides extracts after 24 h. At IC50 values, the MCF-7 cells’ caspase-3 activity was considerably higher than that of the control (untreated) cells (Fig. 7). The cleavage of various caspases results in the execution of apoptosis. We gain insight into cell death and other biological processes by better understanding the effects of caspase cleavage [40]. According to Lim et al. [41], the caspase activity in treated MCF-7 and disturbance in the mitochondrial membrane potential indicate that the extracts cause apoptosis via an intrinsic mechanism.
Western-blot analysis for the expression of Bcl-2 and p53 in MCF-7
The expression levels of the apoptotic markers, p53 and Bcl‑2 protein, in MCF‑7 cells were evaluated using western blot technique. MCF-7 cells were treated with both sea grasses Thalassia hemperchii and Enhalus acoroides with IC50 values (101.19 and 604.2 µg/ml, respectively). The results indicated that the expression of Bcl‑2 was significantly decreased in the treated cells (*** P < 0.001) (Fig. 8a & b). On the other hand, extracts increased the expression levels of p53 compared with the untreated control cells (Fig. 8a & b). Doxorubicin was used as a positive control with IC50 value of 1.2 µg/ml. Natural products rich in kaempferol and caffeic acid induce cytotoxic effect against breast cancer MCF-7 via induction of apoptosis [42, 43].
(a) Western blot analysis and (b) quantification of protein expression level of Bcl-2 and p53 in MCF-7 after treatment with 2 (Thalassia hemperchii at 101.19 µg/ml), 3 (Enhalus ocoroides at 604.2 µg/ml) and 4 (Doxorubicin at 1.2 µg/ml) for 48 h relative to GAPDH and compared with (1) untreated cells. All samples were derived from the same experiment and the blots were processed in parallel. Data are expressed as mean ± SD. ***P < 0.001. The expression level of protein was quantified using Image Lab Software Bio-Rad
Gene expression patterns
As illustrated in Fig. 9, the effect of Doxorubicin, Thalassia hemperchii (extract 1) and Enhalus ocoroides (extract 2) on Bcl-2, survivin, CDC2, CC2D1A, and p53 genes expression levels were evaluated by normalization of their expression to that of β-Actin and in comparison to negative control cells. According to Yahya et al. [44]. It is well known that p53 genes are down-regulated whereas Bcl-2, survivin, and CDC2 expression levels are up-regulated in breast cancer cell lines. In the current study, in MCF-7, Doxorubicin, Thalassia hemperchii (extract 1), Enhalus ocoroides (extract 2) exhibited a marked reduction in Bcl-2, Survivin and CDC2 gene expression levels but significantly increased the expression of p53 and CC2D1A as compared to control cells (Fig. 9A, B, C, D &E). These finding may introduce these extracts as a promising regulators of apoptosis and cell cycle.
On the other side, Enhalus ocoroides produced significant increase in p53 as compared to control cells which is considered as an important tumor suppressor and transcription factors. p53 has an important role in various cellular processes, such as cellular response to DNA damage, cell signal transduction, genomic stability, apoptosis and cell cycle arrest [45].
Discussion
Natural remedies have been extensively used to cure a number of illnesses and are now a significant field of study for the development of new cancer drugs [46,47,48]. This growing attention was due to the high curative rate and as well as the minimal side effect.
Valuable medicinal plants are under pressure and their biodiversity is at risk due to the growing demand for medications generated from plants [49]. In developing nations, the endangerment of species is a result of growing populations and deforestation. Plant part replacement, tissue cultures, cryopreservation, and germplasm conservation must all be implemented in order to support the conservation of these species [50]. Conservation may also be aided by the mass production of therapeutic plant species and the industrial use of their raw byproducts [51]. Seagrass were reported as a valuable source of phenolic compounds and are of ecological importance [17]. The sustainability of any marine organism can’t be granted because their abundance is based on different environmental factors. However, seagrass can be grown in tissue culture labs. The cytotoxic effect of phenolic compounds is induced through the modulation of various mechanisms, such as cellular proliferation, differentiation, apoptosis, angiogenesis, and metastasis. These products have demonstrated anti-carcinogenic activities by obstructing the onset, development, and progression of cancer [52]. Because it is a more affordable option than traditional cancer therapy, this idea is gaining more attention. Diverse groups of phenolic acids are abundant in marine seagrasses [15]. Caffeic acid and kaempferol, in particular, are phenolic acids that have anticancer, anti-inflammatory, antibacterial, immune-regulatory, and antioxidant properties [53, 54]. Several investigations have verified their biological impact on cancerous cells. The cytotoxicity activity and the capacity to prevent migration in the colorectal cancer cell lines, SW480 and SW620, were demonstrated by Villota et al. [55]. Coffee extract containing Caffeic and Chlorogenic acid has also been observed to induce apoptosis and regulate the cell cycle in prostate cancer cells [56] and K562 chronic myeloid leukemia cells [57].
The phytochemical results show the difference in the chemical composition of Enhalus acoroides and Thalassia hemprichii. with predominance of more phenolic compounds functional groups (carboxylic acid, phenols, alcohols) in Thalassia hemprichii (Ehren.) Asch. explaining the difference in their biological activity against cancer cell lines. Thalassia hemperchii is more efficient with IC50 value 101.19 µg/ml. On the other hand, Enhalus acoroides showed proliferation inhibition on the growth of MCF-7 with IC50 values of 604.2 µg/ml. Apoptosis (programmed cell death) is triggered via both extrinsic and intrinsic pathways in mammalian cells. The mitochondrial (intrinsic) apoptotic pathway is mainly regulated by the Bcl‑2 family proteins (pro‑apoptotic and anti‑apoptotic proteins) such as Bax and Bcl‑2 [58]. Our data showed that both extracts TH and EA induce apoptosis and cell cycle arrest at G0/S phase. The result was aligned with the capacity of the extracts to induce disruption in the mitochondrial membrane potential suggesting that sea grasses extracts induces apoptosis via the mitochondrial apoptotic pathway. The apoptotic cascade in the treated cells was presumably set off by this decline in mitochondrial membrane potential [59, 60].
Various studies referred to the apoptotic effect of phytonutrients including flavonoids, phenolic acids and carotenoids. The apoptotic activity is mainly associated with the down-regulation of Bcl-2 expression in breast cancer cell lines [61, 62]. Additionally, the immunoblotting results shown revealed that Thalassia hemperchii and Enhalus acoroides significantly up-regulated the expression level of p53 but down-regulated the expression of Bcl2. p53 play an important role in the transition from normal cell to abnormal tumor cell and it has been known as a common mutated cancer suppressor in human tumorigenesis [63]. The increase of p53 and p21 gene expression and the reduction of CDK2 gene expression were the mechanisms of action, which may result in G0/G1 cell cycle arrest [64].
Gene expression analysis showed that p53 gene was down-regulated whereas Bcl-2, survivin, and CDC2 expression levels were up-regulated in breast cancer cell lines. Bcl-2 is a well-established anti-apoptotic protein; Bcl-2 was involved in caspase-3 initiated apoptosis. Besides, the increase in the Bax/Bcl-2 ratio promotes apoptotic events including the up-regulation of caspase 3/9 and the consequent degradation of intracellular substrates [65]. Survivin is a vital member of the inhibitor of apoptosis protein family (IAP) where it suppresses apoptosis and enhances cell division. The knockdown of survivin protein was found to suppress proliferation and enhances cell cycle arrest in Hela cells [66]. CDC2 is implicated in the progression cell cycle from the G2/M phase and consequently, anticancer agents can initiate G2/M arrest in cancer cells by inhibiting CDC2 protein levels [67]. The blocking of this step is a vital target in cancer treatment. P53 is an important player in many cellular processes, such as cellular response to DNA damage, cell signal transduction, genomic stability, apoptosis and cell cycle arrest. In addition to, its activation of p21WAF1 and Bax transcription resulting in promoting apoptosis [68]. CC2D1A, also known as Akt kinase–interacting protein 1 (Aki1) or Freud1 was reported as a scaffold protein in the PI3K–PDK1–Akt pathway. Its role in cancer biology has many controversies. It was reported that CC2D1A contributes to cell survival and proliferation through the formation of a complex with EGFR and Akt upon EGF stimulation. In Diffuse Malignant Mesothelioma, CC2D1A silencing resulted in tumor growth inhibition in mice models [69]. CDC2 protein complex is one of the kinases responsible for Aki1 phosphorylation during mitosis [70]. However, recently, Reiff et al. [71], introduced CC2D1A as tumor Suppressor gene working with the aid of Notch signaling.
Conclusion
In conclusion, the Methanolic extract of seagrass, Thalassia hemperchii and Enhalus ocoroides collected from Wadi El Gemal National Park, Red Sea, Egypt are able to induce the intrinsic pathway of apoptosis in MCF-7 cells. Our study aims to compare between two different extracts and correlate their anticancer activity to their chemical profile. This growth inhibitory effect of the Methanolic extracts was studied by apoptosis and cell cycle assay by flowcytotmry. The data showed that extracts induced programmed cell death primarily via apoptosis as confirmed by the exteriorization of phosphatidylserine. Moreover, they have ability to induce G0/S cell cycle arrest in MCF-7. The data showed the depletion in mitochondrial membrane potential (ΔψM) in the treated cells dose-dependently. Caspases 3/7activities increased following 24 h treatment. Finally, Gene expression analysis for both of them exhibited a marked reduction in Bcl-2, Survivin and CDC2 gene expression levels but significantly increased the expression of p53 and CC2D1A as compared to control cells. This study reveals the beneficial importance of sea grasses as a source of anticancer agents. It is confirmed from our current study that these sea grasses possess active molecules such as Caffeic acid, Chlorogenic acids, catechin and kaempferol in considerable amount that might be responsible for these anticancer effects, which offering potential therapeutics and a starting point for further research using isolated active compounds against breast cancer cells as well as in vivo study.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- HPLC:
-
High performance liquid chromatography.
- TH:
-
Thalassia hemperchii
- EA:
-
Enhalus acoroides (L.f.) Royle.
- MeOH:
-
Methanol
- KBr:
-
Potassium bromide.
- MeOH:
-
Methanol
- FT-IR:
-
Fourier transform infrared.
- DPBS:
-
Dulbecco′s Phosphate Buffered Saline.
- EEAA:
-
Egyptian Environmental Affairs Agency.
- ΔψM:
-
Mitochondrial Membrane Potential.
- Bcl-2:
-
B-cell lymphoma 2 protein family.
- DOX:
-
Doxorubicin.
- DMSO:
-
Dimethyl sulfoxide, Mr; Molecular mass
- OD:
-
Optical density.
- FBS:
-
Fetal bovine serum.
- MCF-7:
-
Human breast cancer.
- ATCC:
-
American Type Culture Collection.
- DMEM:
-
Dulbecco’s modified Eagle’s Medium.
- PI:
-
Propidium iodide.
- FITC:
-
Fluorescein isothiocyanate.
- pNA:
-
p-nitroaniline.
- Aki 1:
-
kinase–interacting protein 1.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics. CA Cancer J Clin. 2023;73(1):17–48. https://doi.org/10.3322/caac.21763. PMID: 36633525.
Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193–9. https://doi.org/10.7150/ijms.3635. Epub 2012 Feb 27. PMID: 22408567; PMCID: PMC3298009.
Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3(4):242–9. https://doi.org/10.3978/j.issn.2218-6751.2013.12.05. PMID: 25806307; PMCID: PMC4367696.
Rao KV, Faso A. Chemotherapy-induced nausea and vomiting: optimizing prevention and management. Am Health Drug Benefits. 2012;5(4):232–40. PMID: 24991322; PMCID: PMC4046471.
Pradhan P, Sharpe L, Menzies RE. Towards a stepped care model for managing fear of Cancer recurrence or progression in Cancer survivors. Cancer Manag Res. 2021;13:8953–65. https://doi.org/10.2147/CMAR.S294114. PMID: 34880676; PMCID: PMC8645945.
Malve H. Exploring the ocean for new drug developments: Marine pharmacology. J Pharm Bioallied Sci. 2016;8:83.
Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–36.
Kennedy H, Björk M. Seagrass meadows. The management of natural coastal carbon sinks.2009;23.
Short F, Carruthers T, Dennison W, Waycott M. Global seagrass distribution and diversity: a bioregional model. J Exp Mar Biol Ecol. 2007;350(1–2):3–20.
Menajang FS, Mahmudi M, Yanuhar U, Herawati EY. Evaluation of phytochemical and superoxide dismutase activities of Enhalus acoroides (lf) Royle from coastal waters of North Sulawesi. Indonesia Vet World. 2020;13(4):676.
Regalado E, et al. Photoprotecting action and phytochemical analysis of a multiple radical scavenger lipophilic fraction obtained from the leaf of the sea grass Thalassia testudinum. Photochem Photobiol. 2011;87:1058–66.
Jiang Z, Liu S, Cui L, et al. Sand supplementation favors tropical seagrass Thalassia hemprichii in eutrophic bay: implications for seagrass restoration and management. BMC Plant Biol. 2022;22:296. https://doi.org/10.1186/s12870-022-03647-0.
Mayer AM, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts BC, Shuster DE. 2010. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol. Sci. 2010; 31(6), pp.255–265.
Mohamed SIA, Jantan I, Nafiah MA, Seyed MA, Chan KM. Lignans and Polyphenols of Phyllanthus amarus Schumach and Thonn Induce Apoptosis in HCT116 Human Colon Cancer Cells through Caspases-Dependent Pathway. Curr Pharm Biotechnol. 2021;22(2):262–273. https://doi.org/10.2174/1389201021666200612173029. PMID: 32532192.
Mateos R, Pérez-Correa JR, Domínguez H. Bioactive properties of Marine Phenolics. Mar Drugs. 2020;18(10):501. https://doi.org/10.3390/md18100501. PMID: 33007997; PMCID: PMC7601137.
Hamdy AHA, El-Fiky NM, El-Beih AA, Mohammed MM, Mettwally WS. Egyptian red sea seagrass as a source of biologically active secondary metabolites. Egypt Pharm J. 2020;19(3):224.
Shaffai AE, Mettwall WSA, Mohamed SIA. A comparative study of the bioavailability of Red Sea seagrass, Enhalus acoroides (L.f.) Royle (leaves, roots, and rhizomes) as anticancer and antioxidant with preliminary phytochemical characterization using HPLC, FT-IR, and UPLC-ESI-TOF-MS spectroscopic analysis. Beni-Suef Univ J Basic Appl Sci. 2023;12:41.
Repetto G, Del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3(7):1125.
Wang J, Ma L, Tang X, Zhang X, Qiao Y, Shi Y, Xu Y, Wang Z, Yu Y, Sun F. Doxorubicin induces apoptosis by targeting Madcam1 and AKT and inhibiting protein translation initiation in hepatocellular carcinoma cells. Oncotarget. 2015;6(27):24075–91. https://doi.org/10.18632/oncotarget.4373.
Boroumand Moghaddam A, Moniri M, Azizi S, Abdul Rahim R, Bin Ariff A, Navaderi M, Mohamad R. Eco-friendly formulated zinc oxide nanoparticles: induction of cell cycle arrest and apoptosis in the MCF-7 Cancer Cell line. Genes (Basel). 2017;8(10):281. https://doi.org/10.3390/genes8100281.
Li X, Kang B, Eom Y, Zhong J, Lee HK, Kim HM, Song JS. Comparison of cytotoxicity effects induced by four different types of nanoparticles in human corneal and conjunctival epithelial cells. Sci Rep. 2022;12(1):155. https://doi.org/10.1038/s41598-021-04199-3.
John A, Raza H, Azadirachtin Attenuates Lipopolysaccharide-Induced ROS, Production DNA, Damage. Apoptosis by regulating JNK/Akt and AMPK/mTOR-Dependent pathways in Rin-5F pancreatic Beta cells. Biomedicines. 2021;9(12):1943. https://doi.org/10.3390/biomedicines9121943.
McMillan G, Zapata O, Escobar L. Sulphated phenolic compounds in seagrasses. Aquat Bot. 1980;8:267–78.
Qi SH, Li-Si Huang F, He, Zhang S, Jun-De, Dong. Phytochemical and chemotaxonomic investigation of seagrass Thalassia hemprichii (Ehrenb.) Aschers (Hydrocharitaceae). Biochem Syst Eco. 2012;43:12–131.
Hawas UW. A new 8-hydroxy flavone O-xyloside sulfate and antibacterial activity from the Egyptian seagrass Thalassia hemprichii. Chem Nat Comp. 2014;50:629–32.
Qi SH, Zhang S, Qian PY, Wang BG. Antifeedant, antibacterial and anti-larval compounds from the South China Sea Seagrass Enhalus acoroides. Bot Mar. 2008;51:441–7.
Al-Otibi FO, Alrumaizan GI, Alharbi RI. Evaluation of anticandidal activities and phytochemical examination of extracts prepared from Vitex agnus-castus: a possible alternative in treating candidiasis infections. BMC Complement Med Ther. 2022;22(1):69. https://doi.org/10.1186/s12906-022-03552-x. PMID: 35292012; PMCID: PMC8925216.
Skotti E, Kountouri S, Bouchagier P, Tsitsigiannis DI, Polissiou M, Tarantilis PA. FTIR spectroscopic evaluation of changes in the cellular biochemical composition of the phytopathogenic fungus Alternaria alternata induced by extracts of some Greek medicinal and aromatic plants. Spectrochim Acta Mol Biomol Spectrosc. 2014;127:463–72. https://doi.org/10.1016/j.saa.2014.02.113.
Lingegowda DC, Kumar JK, Prasad AD, Zarei M, Gopal S. FTIR spectroscopic studies on cleome gynandra-comparative analysis of functional group before and after extraction. Rom J Biophys. 2021;22(3–4):137–43.
Pharmawati M, Wrasiati LP. Phytochemical screening and FTIR spectroscopy on crude extract from Enhalus acoroides leaves. Malaysian J Anal Sci. 2020;24(1):70–7.
Ragupathi RK, Arumugam RR, Anantharaman P. Fourier transform infrared spectroscopy analysis of seagrass polyphenols. Curr Bioact Compd. 2011;7(2):118–25.
Janakiraman N, Johnson M. Functional groups of tree ferns (Cyathea) using FTIR: chemotaxonomic implications. Rom J Biophys. 2015;25(2):131–41.
Yuan C, Hung CH, Yuan CS, Li HW. Preparation and Application of immobilized surfactant-modified PANi-CNT/TiO2 under visible-light irradiation. Mater (Basel). 2017;10(8):877. https://doi.org/10.3390/ma10080877. PMID: 28773238; PMCID: PMC5578243.
Djebara M, Stoquert J, Abdesselam M, Muller D, Chami A. FTIR analysis of polyethylene terephthalate irradiated by MeV He+. Nucl Instrum Methods Phys Res B: Beam Interact Mater at. 2012;274:70–7.
Tsai JC, Lo YL, Lin CY, Sheu HM, Lin JC. Feasibility of rapid quantitation of stratum corneum lipid content by Fourier transform infrared spectrometry. Spectrosc. 2004;18(3):423–31.
Algotiml R, Gab-Alla A, Seoudi R, Abulreesh HH, El-Readi MZ, Elbanna K. Anticancer and antimicrobial activity of biosynthesized Red Sea Marine algal silver nanoparticles. Sci Rep. 2022;12(1):1–18.
Nur G, Caylak E, Kilicle PA, Sandayuk S, Celebi OO. Immunohistochemical distribution of Bcl-2 and p53 apoptotic markers in acetamiprid-induced nephrotoxicity. Open Med (Wars). 2022;17(1):1788–96. https://doi.org/10.1515/med-2022-0603. PMID: 36457797; PMCID: PMC9679552.
Soung YH, Jeong EG, Ahn CH, Kim SS, Song SY, Yoo NJ, et al. Mutational analysis of caspase 1, 4, and 5 genes in common human cancers. Hum Pathol. 2008;39:895–900. https://doi.org/10.1016/j.humpath.2007.10.015.
Tee TT, Cheah YH, Hawariah LPA. F16, a fraction from Eurycoma longifolia jack extract, induces apoptosis via a caspase-9-independent manner in MCF-7 cells. Anticancer Res. 2007;27:3425–30.
Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–9. https://doi.org/10.1074/jbc.M708182200.
Lim SW, Loh HS, Ting KN, Bradshaw TD, Zeenathul NA. Antiproliferation and induction of caspase-8-dependent mitochondriamediated apoptosis by β-tocotrienol. Hum lung Brain cancer cell Lines Biomed Pharmacother. 2014;68:1105–15. https://doi.org/10.1016/j.biopha.2014.10.006.
Rezaei-Seresht H, Cheshomi H, Falanji F, Movahedi-Motlagh F, Hashemian M, Mireskandari E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: an in silico and in vitro study. Avicenna J Phytomed. 2019 Nov-Dec;9(6):574–86. PMID: 31763216; PMCID: PMC6823530.
Sharma H, Stephen NM, Gopal SS, Udayawara Rudresh D, Kavalappa YP, Haranahalli Shivarudrappa A, Gavirangappa H, Ponesakki G. Phenolic extract of Seagrass, Halophila ovalis activates intrinsic pathway of apoptosis in human breast Cancer (MCF-7) cells. Nutr Cancer. 2021;73(2):307–17. Epub 2020 Apr 2. PMID: 32238022.
Yahya SM, Abd-Elhalim MM, Abdelhamid AO, Eskander EF, Elsayed GH. (2021). Could miR-34a Inhibition be Used as a Tool to Overcome Drug Resistance in MCF-7 Cells Treated with Synthesized Steroidal Heterocycles? Asian Pac. J. Cancer Prev: APJCP 2021; 22(3): 819.
Shen Y, White E. p53-dependent apoptosis pathways. Adv Cancer Res. 2001;82:55–84. https://doi.org/10.1016/s0065-230x(01)82002-9. PMID: 11447765.
Mohamed SIA, Jantan I, Haque MA. Naturally occurring immunomodulators with antitumor activity: An insight on their mechanisms of action. Int Immunopharmacol. 2017;50:291–304. https://doi.org/10.1016/j.intimp.2017.07.010. PMID: 28734166.
Ling T, Lang WH, Maier J, Quintana Centurion M, Rivas F. Cytostatic and cytotoxic Natural products against Cancer Cell models. Molecules. 2019;24(10):2012. https://doi.org/10.3390/molecules24102012. PMID: 31130671; PMCID: PMC6571673.
Sauvaget C, Nagano J, Hayashi M, Spencer E, Shimizu Y, Allen N. Vegetables and fruit intake and cancer mortality in the Hiroshima/Nagasaki Life Span Study. Br J Cancer. 2003;88:689–94.
Faisal S, Tariq MH, Ullah R, et al. Exploring the antibacterial, antidiabetic, and anticancer potential of Mentha arvensis extract through in-silico and in-vitro analysis. BMC Complement Med Ther. 2023;23:267. https://doi.org/10.1186/s12906-023-04072-y.
Zschocke S, Rabe T, Taylor JLS, Jäger AK, van Staden J. Plant part substitution – a way to conserve endangered medicinal plants? J Ethnopharmacol. 2000;71:281–92.
Kasagana VN, Karumuri SS. Conservation of Medicinal plants (past, Present & Future trends). J Pharm Sci Res. 2011;3(8):1378–86.
Sahpazidou D, Geromichalos GD, Stagos D, Apostolou A, Haroutouian SA, Tsarsakis AM, Tzanakakis NG, Hayes AW, Kouretas D. Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells. Toxicol Lett. 2014;230:218–24.
Indap M, Radhika S, Motiwale L, Rao K. Anticancer activity of phenolic antioxidants against breast cancer cells and a spontaneous mammary tumor. Indian J Pharm Sci, 2006, 68.
Pham HNT, Sakoff JA, Vuong QV, Bowyer MC, Scarlett CJ. Comparative cytotoxic activity between kaempferol and gallic acid against various cancer cell lines. Data Brief. 2018;21:1033–6. https://doi.org/10.1016/j.dib.2018.10.121. PMID: 30450396; PMCID: PMC6226582.
Villota H, Moreno-Ceballos M, Santa-González GA, Uribe D, Castañeda I C H, Preciado LM, Pedroza-Díaz J. Biological Impact of Phenolic compounds from Coffee on Colorectal Cancer. Pharmaceuticals. 2021;14:761. https://doi.org/10.3390/ph14080761.
Montenegro J, Dos Santos LS, de Souza R, Lima L, Mattos DS, Viana B, da Fonseca Bastos A, Muzzi L, Conte-Júnior CA, Gimba E. Bioactive compounds, antioxidant activity and antiproliferative effects in prostate cancer cells of green and roasted coffee extracts obtained by microwave-assisted extraction (MAE) Food Res. Int. 2021;140:110014. https://doi.org/10.1016/j.foodres.2020.110014.
Feriotto G, Tagliati F, Giriolo R, Casciano F, Tabolacci C, Beninati S, Khan MTH, Mischiati C. Caffeic acid enhances the anti-leukemic effect of Imatinib on chronic myeloid leukemia cells and triggers apoptosis in cells sensitive and resistant to Imatinib. Int J Mol Sci. 2021;22:1644. https://doi.org/10.3390/ijms22041644.
Hussar P. 2022. Apoptosis regulators Bcl-2 and Caspase-3 encyclopedia 2, no. 4: 1624–36. https://doi.org/10.3390/encyclopedia2040111.
Ricci JE, Gottlieb RA, Green DR. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol. 2003;160(1):65–75. https://doi.org/10.1083/jcb.200208089. Epub 2003 Jan 6. PMID: 12515825; PMCID: PMC2172744.
Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20(3):175–93. https://doi.org/10.1038/s41580-018-0089-8. PMID: 30655609; PMCID: PMC7325303.
Thangapazham RL, Passi N, Maheshwari RK. Green tea polyphenol and epigallocatechin gallate induce apoptosis and inhibit invasion in human breast cancer cells. Cancer Biol Ther. 2007;6(12):1938–43. https://doi.org/10.4161/cbt.6.12.497419.
Choi EJ, Bae SM, Ahn WS. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch Pharm Res. 2008;31(10):1281–5. https://doi.org/10.1007/s12272-001-2107-0.
Rivlin N, Brosh R, Oren M, Rotter V. Mutations in the p53 tumor suppressor gene: important milestones at the various steps of Tumorigenesis. Genes Cancer. 2011;2(4):466–74. https://doi.org/10.1177/1947601911408889. PMID: 21779514; PMCID: PMC3135636.
Nabavi SF, Nabavi SM, Habtemariam S, Moghaddam AH, Sureda A, Jafari M, Latifi AM. Hepatoprotective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress. Ind Crops Prod. 2013;44:50–5.
Kaloni D, Diepstraten ST, Strasser A, et al. BCL-2 protein family: attractive targets for cancer therapy. Apoptosis. 2023;28:20–38. https://doi.org/10.1007/s10495-022-01780-7.
Chen X, Duan N, Zhang C, Zhang W. Survivin and Tumorigenesis: Molecular mechanisms and therapeutic strategies. J Cancer. 2016;7(3):314–23. https://doi.org/10.7150/jca.13332. PMID: 26918045; PMCID: PMC4747886.
Zhu X, Li R, Wang C et al. Pinocembrin Inhibits the Proliferation and Metastasis of Breast Cancer via Suppression of the PI3K/AKT Signaling Pathway. Front Oncol. 2021;11:661184. Published 2021 Jul 16. https://doi.org/10.3389/fonc.2021.661184.
Yahya SMM, Abdelhamid AO, Abd-Elhalim MM, Elsayed GH, Eskander EF. The effect of newly synthesized progesterone derivatives on apoptotic and angiogenic pathway in MCF-7 breast cancer cells. Steroids. 2017;126:15–23. Epub 2017 Aug 8. PMID: 28797724.
Yamada T, Amann JM, Fukuda K, et al. Akt kinase-interacting protein 1 signals through CREB to drive diffuse malignant mesothelioma. Cancer Res. 2015;75(19):4188–97. https://doi.org/10.1158/0008-5472.CAN-15-0858.
Nakamura A, Naito M, Arai H, Fujita N. Mitotic phosphorylation of Aki1 at Ser208 by cyclin B1-Cdk1 complex. Biochem Biophys Res Commun. 2010;393(4):872–6. Epub 2010 Feb 18. PMID: 20171170.
Reiff T, Baeumers M, Tibbe C, Klein T. Unravelling of Hidden Secrets: The Tumour Suppressor Lethal (2) Giant Discs (Lgd)/CC2D1, Notch Signalling and Cancer. Adv Exp Med Biol. 2021;1287:31–46. https://doi.org/10.1007/978-3-030-55031-8_3. PMID: 33034024.
Acknowledgements
The authors would like to express their appreciation to the National Research Centre, Egypt for facilitating this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). All experimental protocols were approved by the projects research committee at National Research Centre, Cairo, Egypt (approval number, 12060107, 2019–2022).
Author information
Authors and Affiliations
Contributions
Shimaa I. A. Mohamed: Conducted the cytotoxicity, mitochondrial membrane potential assay, immunoblotting and apoptotic activity analysis. Walaa S.A. Mettwally: Extraction and Phytochemical screening.Ghada H. Elsayed and Shaymaa M.M. Yahya: Conducted the cytotoxicity and genetics analysis. Amgad El Shaffaib: Collection, identification, and authentication of Seagrass.All authors analyzed the data, wrote the draft, reviewed the manuscript, and approved the final form for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The wild plant has been collected in accordance to the guidelines: https://portals.iucn.org/library/sites/library/files/documents/2011-057-2nd%20ed.pdf. Permissions and licenses were obtained by Dr. Amgad El Shaffai (authorized officially to conduct the research and collect samples). All research experiments were approved by NRC Medical Research Ethics Committee (MREC): Registration number: 19267.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mohamed, S.I.A., Elsayed, G.H., El Shaffai, A. et al. In-vitro study of cytotoxic and apoptotic potential of Thalassia hemprichii (Ehren.) Asch. And Enhalus acoroides (L.f.) Royle against human breast cancer cell line (MCF-7) with correlation to their chemical profile. BMC Complement Med Ther 24, 244 (2024). https://doi.org/10.1186/s12906-024-04512-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-024-04512-3