Abstract
Background
Diabetes affects 75% of people in low-income countries, where conventional drugs like metformin are available, but newer drugs like alpha-glucosidase inhibitors are not accessible to most Southern African patients.
Aim
To evaluate the α-glucosidase and α-amylase inhibitory activities of fractionated aqueous extracts of Kigelia africana fruit (KAFE) and their phytochemical fingerprints using gas chromatography-mass spectrometry (GC–MS).
Materials and methods
We studied K. africana fruit fractions' inhibitory effects on alpha-glucosidase and alpha-amylase using bioassay-guided fractionation, and analyzed their phytochemical profiles with GC–MS.
Key findings
Both the aqueous extract and ethyl acetate fraction of the aqueous extract exhibited a low dose-dependent inhibition of alpha-amylase activity (p < 0.0001). At a concentration of 500 μg/mL, the aqueous extract caused an alpha-glucosidase inhibition of 64.10 ± 2.7%, with an estimated IC50 of 193.7 μg/mL, while the ethyl acetate fraction had an inhibition of 89.82 ± 0.8% and an estimated IC50 of 10.41 μg/mL. The subfraction G, which had the highest alpha-glucosidase inhibitory activity at 85.10 ± 0.7%, had significantly lower activity than the ethyl acetate fraction. The most bioactive fraction was found to contain 11"(2-cyclopenten-1-yl) undecanoic acid, ( +)- and cyclopentane undecanoic acid as well as the indole alkaloids Akuammilan-17-ol-10-methoxy, N-nitroso-2-methyl-oxazolidine and epoxide Oxirane2.2″ -(1.4-butanediyl) bis-.
Conclusion
The K. africana fruit fraction demonstrated significant alpha-glucosidase inhibitory activity, while its alpha-amylase inhibitory activity was limited. This study suggests a potential natural alpha-glucosidase inhibitor and phytocompounds that could serve as leads for developing antidiabetic agents.
Highlights of the study
1. The study highlights the in vitro alpha-glucosidase inhibitory activity of Kigelia fruit extract and its fractions with estimated IC50 of aqueous crude extract at 193.7 μg/mL and ethyl acetate fraction 10.41μg/mL.
2. The study has highlighted rare cyclic fatty acids 11"(2-cyclopenten-1-yl) undecanoic acid, (+)- and cyclopentane undecanoic acid; indole alkaloids akuammilan-17-ol- 10-methoxy, N-nitroso-2-methyl-oxazolidine and epoxide oxirane2.2” -(1.4-butanediyl) bis- and a naturally occurring phthalic acid ester in the bioactive subfractions.
Similar content being viewed by others
Introduction
The burden of diabetes mellitus is substantial in low-income countries, with three of the four individuals with diabetes residing in low- and middle-income countries [1, 2]. In Zambia, it is estimated that 726,300 adults live with diabetes [2]. A significant number of these patients also experience complications such as vision impairment, sexual dysfunction, and fatigue, as well as comorbidities such as obesity, stroke, and hypertension [3, 4]. While conventional drugs such as metformin are available for management, recent drug groups such as alpha-glucosidase inhibitors that may be used effectively in prediabetes and to alleviate metabolic syndrome of diabetes are not readily available to most Southern African patients (Rossiter, 2014). This warrants the need to investigate the possible sources of these drug groups to provide a local solution. Furthermore, in cases where alpha-glucosidase inhibitors are available, these drugs are not devoid of adverse drug reactions [5]. Therefore, there is a need to find more alpha-glucosidase inhibitors with fewer adverse drug reactions.
Kigelia africana (Lam) Benth. (Mupolota in Silozi or Muzungula in Tonga) is a medicinal plant that has attracted the attention of researchers because it is traditionally used to lower blood sugar levels [6]. Previous studies have shown that crude extracts from multiple parts of the plant (leaves, flowers, and fruits) can lower blood sugar levels in diabetes-induced mouse models [7, 8]. However, very few studies have highlighted the compounds responsible for the observed antidiabetic activity and bioactivity of the fruit extracts concerning their blood glucose-lowering effect. Although studies have determined the basic phytochemistry of Kigelia concerning diabetes, it has often been associated with the presence of alkaloids, iridoids, and phenolic compounds [9,10,11]. Furthermore, while some studies have demonstrated the plant extracts’ ability to enhance glucose utilization in muscle, as well as improve insulin secretion [12], there is scanty information that discusses the postprandial benefits of Kigelia extracts. Literature has also highlighted plants that are potential alpha-glucosidase inhibitors [13, 14].
Thus, in this study, through active fractionats we aimed to determine the alpha-glucosidase and alpha-amylase inhibitory activity of the fractionated aqueous extracts of Kigelia africana fruit (KAFE). Additionally, we aimed to associate their phytochemical fingerprints using GC–MS to the observed bioactivity.
Materials and methods
Chemicals and reagents
The materials utilized in this study consisted of various analytical standard substances obtained from Merck, South Africa., namely; hexane, chloroform, ethyl acetate, methanol, porcine pancreatin buffer, potassium monobasic anhydrous phosphate, reduced glutathione, p-NP-Gluc, p-Nitrophenyl α-D-glucopyranoside, Na2CO3 sodium carbonate, α–glucosidase from Saccharomyces cerevisiae, Positive control for alpha-glucosidase assay: Epigallocatechin gallate (EGCG), Silica gel.
Plant material collection and authentication
The K. africana fruit was collected with permission in January 2022, in the Kazungula District specifically from the riverine area of Singanga village in Kachola, Chief Sekute area, in the Southern Province of Zambia. The plant material underwent authentication in the Department of Biological Sciences within the School of Natural Sciences at the University of Zambia (UNZA), by Florence Nyirenda (MSc) a scientist. The specimen had receipt number 1793751 and was mounted in the herbarium under specimen number 22, 420.
Plant extraction and fractionation
The fruits of K. africana were subjected to cutting, mincing, and subsequent air drying within a designated shed for one week. The fruits were subsequently pulverized and filtered using a screen with a hole diameter of 0.6 mm to acquire a uniform powder, which was stored at a temperature of 10 °C. A quantity of 500 g of powdered K. africana fruit was utilized in a Soxhlet extraction process, employing 2500 mL of water as the extracting solvent, resulting in the production of an aqueous extract. The extracted sample was subjected to a drying process under decreased pressure utilizing a Rotavapor at a temperature of 45 °C. Subsequently, a liquid–liquid fractionation technique was employed to separate the extract.
About 50 g of ethyl acetate fraction was mixed with 50 g of silica gel, then subjected to the column fraction, using a glass column of diameter 200 mm and 2 m length. The column was packed with 400 g of silica while 1.330 L of hexane was used to form a slurry. Once the sample had settled, the hexane was collected in a conical flask while ensuring that the column did not dry out the sample at the top. The following solvent systems were used to obtain fractions of hexane/ethyl acetate (100%; 97.5:2.5; through to 10:90) and then Ethyl acetate/methanol (100%; 95:5 through to 90:10). Guided by TLC, bands with similar mobility were grouped and a total of thirteen fractions were collected.
In vitro postprandial activity
The collected fractions were tested for in vitro inhibition of alpha-glucosidase and alpha-amylase enzymes according to the methodology given by Pingle et al. (2021).
Test sample preparation
Test samples were reconstituted in dimethyl sulfoxide (DMSO) to a final concentration of 100 mg/mL. Samples were sonicated if insoluble and stored at 4 °C until required. Samples were diluted in each respective assay buffer to concentrations as specified.
Alpha amylase inhibitory activity
The method of Xiao et al. (2006), modified as described by Pringle et al. (2021) was used. In a 96-well microtiter plate, 15 μL of the test sample (aqueous crude extract, ethyl acetate fraction or acarbose as positive control) was incubated for 10 min at 37 °C with 5 μL of 1 mg/mL porcine pancreatin in phosphate-buffered saline. To initiate the reaction, 20 μL of 2 mg/mL starch solution was added followed by incubation for 30 min at 37 °C. The 2 mg/mL starch solution was prepared by boiling with continuous stirring for 15 min until the solution turned clear; it was then cooled to room temperature with continuous stirring and the volume of evaporated water was replaced. At the end of the 30-min incubation, the reaction was halted by adding 10 μL 1 M HCl and 75 μL iodine reagent (0.127 g iodine and 0.083 g potassium iodide in 100 mL distilled water). The absorbance was measured at 580 nm. Controls containing no enzyme or substrate were included for each sample to account for the absorbance of the extracts. The absorbance of the enzyme- and substrate-free wells was subtracted from the absorbance readings of the wells containing enzyme and substrate, and the percentage of α-amylase inhibition was calculated using the following formula:
where: amylase activity = A 580 nm without enzyme—A 580 nm with enzyme:
Alpha-glucosidase activity
The method of Akinloye et al. (2012) as modified by Pringle et al. (2021) was used. In a 96-well plate, 15 μL of the sample (aqueous crude extract, ethyl acetate fraction or epigallocatechin gallate (ECGC) as positive control), 20 μL of 50 µg/mL enzyme and 60 μL reaction buffer (67 mM potassium phosphate, pH 6.8 to which 3 mM reduced glutathione was added directly before use) was pre-incubated for 5 min at 37 °C. Ten microlitres of 10 mM p-nitrophenyl α-D-glucopyranoside substrate was added, followed by incubation for 30 min at 37 °C. The reaction was halted by adding 25 μL of 100 mM Na2CO3. Controls containing no enzyme or substrate were included for each sample to account for the absorbance of the extracts. The amount of p-nitrophenol released was determined spectrophotometrically at 405 nm. The percentage of α-glucosidase inhibition was calculated as follows:
The absorbance of the enzyme and substrate-free wells was subtracted from the absorbance readings of the wells containing enzyme and substrate.
GC–MS analysis
GCMS analysis of the bioactive subfractions was performed on the Scion 436 GC–MS Single Quadruple equipped with a low bleed, high inertness SCION 5MS column (equivalent to 5% phenyl/95% dimethyl polysiloxane). Capillary column (30 m × 250 m × 0.25 m). Pure helium gas (99.99%) with a constant flow rate of 1 ml/min was used as the carrier gas. For spectral detection, an electron ionization energy method was used with a high ionization energy of 70 eV (electron volts) with a scan time of 0.2 s and fragments in the range of 40 to 600 m/z. The injection quantity of 1µL was used with a split ratio of 10:1 and an injection temperature of 250 °C (constant). The column oven temperature was set at 50 °C for 3 min, increased at 10 °C per minute to 280 °C and the final temperature increased to 300 °C for 10 min. The phytochemicals present in the fractions were identified by comparing their retention time (min), peak area, peak height, and mass spectral pattern with the spectral database of authentic compounds stored in the National Institute of Standards and Technology (NIST) library.
Results
Alpha amylase inhibitory activity
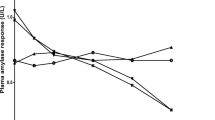
The aqueous crude extract did not display any alpha-amylase inhibitory activity. The ethyl acetate fraction of the aqueous extract displayed a very low dose-dependent alpha-amylase inhibitory activity. However, this activity was significantly less than that of the positive control, acarbose(p < 0.0001; Fig. 1).
Alpha-glucosidase inhibitory activity
The aqueous extract showed a dose-dependent increase in the alpha-glucosidase inhibitory activity. At the highest concentration of 500 μg/mL, there was 64.10 ± 2.7% inhibition. In contrast, the study observed a statistically significant increase in the alpha-glucosidase inhibitory activity of the ethyl acetate fraction (p < 0.0001) compared to the crude extract. At 500 μg/mL, there was an 89.82 ± 0.8% inhibition of the fractions. However, this was statistically significantly lower than the % inhibition of the positive control which had 94.88 ± 0.1% inhibition at 200 μg/mL, see Fig. 2.
An estimated IC50 for the aqueous crude extract was determined to be IC50 – 193.7 μg/mL while that of ethyl acetate fraction was determined to be IC50 – 10.41 μg/mL.
Thirteen subfractions collected from the ethyl acetate fraction were tested for alpha-glucosidase inhibitory activity. Consequently, subfractions F, G, H and J had the best activity. Subfraction G had the highest inhibitory activity at 85.10 ± 0.7%. as shown in Fig. 3.
GC–MS analysis of bioactive fractions
The GC–MS analysis of fractions F, G, H, and J showed the presence of fatty acids, phenolic compounds as well as nitrogen-containing compounds as indicated in Tables 1, 2, 3 and 4 below. The GC–MS analysis of subfractions F, G, H, and J had several peaks that were distributed among the sub-fractions.
Subfraction F.
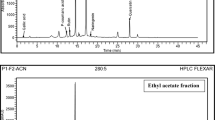
Subfraction F had the largest number of peaks. However, there were 11 peaks of interest which have been indicated in Fig. 4 and Table 1 below the highest peak corresponded to n-hexadecenoic acid, followed by undecanoic acid at 40.8 and 40.4 min, respectively.
GC–MS chromatogram of subfraction F. The numbers indicated in the spectrogram correspond with the peaks of compounds indicated in Table 1 below
Subfraction G
Under subfraction G, we recorded 13 peaks with the highest peak corresponding to n-decanoic acid at 39.95 min. At 30.06 min, the peak corresponded to 11"(2-cyclopenten-1-yl) undecanoic acid, ( +)-. Other peaks of interest at 24.08 and 27.4 min corresponded to cyclopentane undecanoic acid and tridecane, 4-methyl- respectively. The highest peak corresponded to 1,2-benzene dicarboxylic acid and butyl octyl ester. Data is shown in Fig. 5 and Table 2.
GC–MS chromatogram for sub-fraction G. The numbers indicated in the spectrogram correspond with the peaks of compounds indicated in Table 2 below
Subfraction H
Subfraction H had 30 peaks, most of which corresponded to 11"(2-cyclopenten-1-yl) undecanoic acid, ( +)-. However, the highest peaks were observed around 38.11 min to 41.2 min. The phytocompounds observed here included akuammilan-17-ol- 10-methoxy 11-(2-cyciopenten-1-yl) undecanoic acid. ( +)-, cyclopentane undecanoic acid methyl ester and cyclopentane undecanoic acid. Significant peaks were also observed at 26.3 and 26.5 min which corresponded to oxirane 2.2″ -(1.4-butanediyl) bis- and cyclopentane undecanoic acid. At 23.05 min, we also see ( +)-2- hydroxy octanoic acid acetate. One more peak of interest was observed at 11.49 min and corresponded to N-nitroso-2-methyl-oxazolidine. Data is shown in Fig. 6 and Table 3.
GC–MS chromatogram for sub-fraction H. The numbers indicated in the spectrogram correspond with the peaks of compounds indicated in Table 3 below
Subfraction J
Subfraction J had 15 peaks, the peaks of interest were seen at 39.9 min to 41.3 min and corresponded to the following phytoconstituents 1-ethenyl-3 (1-hexenyl) -4- trimethylsilyl cyclopentane, epoxy hexanol and n-decanoic acid. in this subfraction also we see the continued appearance of 11-(2-cyclopenten-1-yl) undecanoic acid at different at 36–37 min. We also see some significant peaks at 11.22 min corresponding to butane nitrile, 2,3-dioxo-, dioxime, O, O" diacetyl-. At 8.9 min propane nitrile, 2-hydroxy- was seen. Data is shown in Fig. 7 and Table 4.
GC–MS chromatogram for sub-fraction J. The numbers indicated in the spectrogram correspond with the peaks of compounds indicated in Table 4 below
Discussion
Studies have shown that alpha-glucosidase inhibitors can prevent the development of diabetes in prediabetics at risk of developing diabetes and can also prevent disease progression and the emergence of complications of diabetes [15]. Alpha-glucosidase inhibition has proved to be beneficial not only for postprandial hyperglycemia but also for the management of weight and reduction of insulin resistance [16]. However, the reported gastrointestinal-related adverse effects [13, 15] have highlighted the need for natural alpha-glucosidase inhibitors. Although there was a significant difference in the alpha-glucosidase inhibitory activity of the fruit fractions, the observed bioactivity of Kigelia subfractions implies that the phytocompounds of Kigelia fruit extract has great potential to prevent the development of diabetes among patients at risk of T2DM and the development of complications of diabetes. The findings in this study also suggest a possible alpha-glucosidase inhibitor that is readily available to Southern African patients considering that conventional alpha-glucosidase inhibitors are not readily available to diabetic patients in most Southern African countries (MOH, 2013; Rossiter,2014). Furthermore, the results of this study suggest an added new natural source of alpha-glucosidase inhibitors that are needed as a safe alternative to existing conventional therapies.
The subfractions of KAFE that demonstrated the best in vitro antidiabetic activity had the indole alkaloids akuammilan-17-ol- 10-methoxy and N-nitroso-2-methyl-oxazolidine. Recent studies have demonstrated the significance that indole alkaloids play in the management of diabetes. Goboza and colleagues reported that they have significant alpha-glucosidase inhibitory activity among other antidiabetic effects [17]. Other studies have shown that oxazolidines have a positive effect on body weight, insulin secretion, and a generally positive outcome in the management of diabetes [18]. Oxirane 2.2″ -(1.4-butanediyl) bis- which contains epoxide was observed. These rings have demonstrated the ability to improve the antidiabetic effects including alpha-glucosidase inhibitory activity of extracts that contain them [19, 20]. Subfraction J, which had the best alpha-glucosidase inhibitory activity also had 1,2-benzanedicarboxylic acid, butyl octyl ester, a naturally occurring phthalic acid ester. These compounds have been reported to possess possible alpha-glucosidase inhibitory activity [21].
We further observed the presence of cyclic fatty acids like 11"(2-cyclopenten-1-yl) undecanoic acid, ( +)- and cyclopentane undecanoic acid. Literature has demonstrated the significant role that fatty acids play in the management of diabetes and other metabolic syndromes as well as their beneficial effect on coronary artery disease [22, 23]. A few studies have demonstrated that some fatty acids like octadecadienoic acid and n-hexadecanoic acid may inhibit alpha-amylase and alpha-glucosidase activity (Go and R, 2019; [24]),however, there is scanty information associating some of the phytocompounds identified in this study to probable antidiabetic activity and particular bioactivity observed in this study. The fatty acid 11"(2-cyclopenten-1-yl) undecanoic acid, ( +)- and cyclopentane undecanoic acid is a cyclic fatty acid that is naturally found in a few plants belonging to the Hydnocarpus species and has been associated with its inhibitory activity on Mycobacterium lepra [25, 26]. Although there is scanty literature available that highlights its activity in diabetes, we observed the presence of this rare fatty acid across all bioactive subfractions. Other phytocompounds observed in this study have previously been associated with antidiabetic activity. For example, decanoic acid has demonstrated that when it is added to chitosan hydrogels, their antidiabetic activity significantly improves [22]. Moreover, nitrile derivatives were able to enhance the effect of metformin in Rabbit-induced diabetes [27]. The fact that the fruit of Kigelia is crushed together with its seeds could have contributed to the significant presence of fatty acids. This suggests that this fruit and its extracts have the potential to be used as nutraceuticals or pharmaceuticals in the management of metabolic and cardiovascular-related conditions. The fruit would be significant in the lowering of postprandial blood glucose. This study also highlights several compounds that may serve as pharmacophores under natural alpha-glucosidase inhibitors considering the narrow range of alpha-glucosidase inhibitors which are also arrayed with several adverse drug reactions.
Conclusion
Through activefractions Kigelia africana fruit (KAFE) ethyl acetate fraction and its active subfractions rich in fatty acids, indole alkaloids and phenolic compounds have been associated with alpha-glucosidase inhibitory activity. The ethyl acetate fraction had an estimated IC50 10.41 μg/mL. We suggest the use of identified phytocompounds, for the development of lead compounds for inhibition of alpha-glucosidase enzymes and the development of pharmaceutical formulations for cheaper alternative treatments.
Availability of data and materials
All the data that supports the findings of this study are presented in this manuscript or supplementary information files.
References
Kibirige D, Lumu W, Jones AG, Smeeth L, Hattersley AT, Nyirenda MJ. Understanding the manifestation of diabetes in sub-Saharan Africa to inform therapeutic approaches and preventive strategies: A narrative review. Clin Diabetes Endocrinol. 2019;5(1):2. https://doi.org/10.1186/s40842-019-0077-8.
International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels; 2021. Available at: https://www.diabetesatlas.org.
Mwila KF, Bwembya PA, Jacobs C. Experiences and challenges of adults living with type 2 diabetes mellitus presenting at the University Teaching Hospital in Lusaka, Zambia. BMJ Open Diabetes Res Care. 2019;7(1):e000497.
Nutakki A, Chomba M, Chishimba L, Zimba S, Gottesman RF, Bahouth MN, Saylor D. Risk factors and outcomes of hospitalized stroke patients in Lusaka. Zambia J Neurol Sci. 2021;424:117404. https://doi.org/10.1016/j.jns.2021.117404.
Wang B, Liu T, Wu Z, Zhang L, Sun J, Wang X. Synthesis and biological evaluation of stilbene derivatives coupled to NO donors as potential antidiabetic agents. J Enzyme Inhib Med Chem. 2018;33(1):416–23. https://doi.org/10.1080/14756366.2018.1425686.
Muyenga TA, Ezaela CE, Mushabati F, Bamitale SDK, Kibuule D. Commentary on the Antidiabetic Activity of Kigelia Africana. Journal of Preventive and Rehabilitative Medicine. 2021;3(2):Article 2 https://journals.unza.zm/index.php/medicine/article/view/537.
Muyenga T, Prashar L, Bwalya AG, Muungo LTM. The Effect of Kigelia africana Fruit Extract on Blood Glucose in Diabetes-Induced Mice. 2015. https://library.adhl.africa/handle/123456789/11725
Njogu, S. M., Arika, W. M., Machocho, A. K., Ngeranwa, J. J. N., and Njagi, E. N. M. (2018). In Vivo Hypoglycemic Effect of Kigelia africana (Lam): Studies With Alloxan-Induced Diabetic Mice. Journal of Evidence-Based Integrative Medicine, 23, 2515690X18768727. doi: 10.1177/2515690X18768727.
Khan MF, Dixit P, Jaiswal N, Tamrakar AK, Srivastava AK, Maurya R. Chemical constituents of Kigelia pinnata twigs and their GLUT4 translocation modulatory effect in skeletal muscle cells. Fitoterapia. 2012;83(1):125–9. https://doi.org/10.1016/j.fitote.2011.10.002.
Kumar S, Kumar V, Prakash O. Antidiabetic and hypolipidemic activities of Kigelia pinnata flowers extract in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2012;2(7):543–6. https://doi.org/10.1016/S2221-1691(12)60093-8.
Fagbohun, O., Vincent, O., Adekola, M., and Msagati, T. A. M. (2020). Biochemical applications of Kigelia africana (Lam.) Benth. Fruit extracts in diabetes mellitus. Comparative Clinical Pathology, 29. doi: 10.1007/s00580-020-03179-9.
Nabatanzi A, M Nkadimeng S, Lall N, Kabasa JD, J McGaw L. Ethnobotany, Phytochemistry and Pharmacological Activity of Kigelia africana (Lam.) Benth. (Bignoniaceae). Plants. 2020;9(6):753. https://doi.org/10.3390/plants9060753.
Dirir AM, Daou M, Yousef AF, Yousef LF. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem Rev. 2022;21(4):1049–79. https://doi.org/10.1007/s11101-021-09773-1.
Tundis R, Loizzo MR, Menichini F. Natural Products as alpha-Amylase and alpha-Glucosidase Inhibitors and their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini-Rev Med Chem. 2010;10(4):315–31. https://doi.org/10.2174/138955710791331007.
Moelands SV, Lucassen PL, Akkermans RP, De Grauw WJ, Van de Laar FA. Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2018;2018(12):CD005061. https://doi.org/10.1002/14651858.CD005061.pub3.
Sugimoto S, Nakajima H, Kosaka K, Hosoi H. Review: Miglitol has potential as a therapeutic drug against obesity. Nutr Metab. 2015;12(1):51. https://doi.org/10.1186/s12986-015-0048-8.
Goboza M, Meyer M, Aboua YG, Oguntibeju OO. In vitro antidiabetic and antioxidant effects of different extracts of catharanthus roseus and its indole alkaloid, vindoline. Molecules. 2020;25(23):5546. https://doi.org/10.3390/molecules25235546.
Qazi AI, Ahmad B, Sahibzada MUK, Anwar F, Khusro A, Alhumaydhi FA, Mohamed AA-R, Mostafa-Hedeab G, Emran TB. Evaluation of antidiabetic activity of oxadiazole derivative in rats. Evidence-Based Complement Alternat Med. 2023;2023:1141554. https://doi.org/10.1155/2023/1141554.
Al-Hajj NQM, Algabr M, Sharif HR, Aboshora W, Wang H. In Vitro and in Vivo Evaluation of Antidiabetic Activity of Leaf Essential Oil of Pulicaria inuloides-Asteraceae. J Food Nutri Res. 2016;4(7). https://doi.org/10.12691/jfnr-4-7-8
Liu Y, Zou L, Ma L, Chen W-H, Wang B, Xu Z-L. Synthesis and pharmacological activities of xanthone derivatives as α-glucosidase inhibitors. Bioorg Med Chem. 2006;14(16):5683–90. https://doi.org/10.1016/j.bmc.2006.04.014.
Huang L, Zhu X, Zhou S, Cheng Z, Shi K, Zhang C, Shao H. Phthalic acid esters: natural sources and biological activities. Toxins. 2021;13(7):495.
Lee C, Choi JS, Kim I, Byeon HJ, Kim TH, Oh KT, Lee ES, Lee KC, Youn YS. Decanoic acid-modified glycol chitosan hydrogels containing tightly adsorbed palmityl-acylated exendin-4 as a long-acting sustained-release anti-diabetic system. Acta Biomater. 2014;10(2):812–20. https://doi.org/10.1016/j.actbio.2013.10.009.
Wu Z, Yang W, Li M, Li F, Gong R, Wu Y. Relationship between Dietary Decanoic Acid and Coronary Artery Disease: A Population-Based Cross-Sectional Study. Nutrients. 2023;15(20). https://doi.org/10.3390/nu15204308
Mitri J, Tomah S, Furtado J, Tasabehji MW, Hamdy O. Plasma free fatty acids and metabolic effect in type 2 diabetes, an ancillary study from a randomized clinical trial. Nutrients. 2021;13(4):1145. https://doi.org/10.3390/nu13041145.
Avato P, Tava A. Rare fatty acids and lipids in plant oilseeds: Occurrence and bioactivity. Phytochem Rev. 2022;21(2):401–28. https://doi.org/10.1007/s11101-021-09770-4.
Palyzová A, Řezanka T. Enantiomeric separation of triacylglycerols containing fatty acids with a ring (cyclo fatty acids). J Chromatogr A. 2020;1622:461103.
Algburi F, Al-Tikrity N, Ali O, Taysi S. Effect of a Metformin Derivative Containing Nitrile Group on Some Biochemical Variables in Rabbits Induced by Alloxane. Ann Rom Soc Cell Biol. 2021;25:4726–31. https://www.researchgate.net/publication/349732868_Effect_of_a_Metformin_Derivative_Containing_Nitrile_Group_on_Some_Biochemical_Variables_in_Rabbits_Induced_by_Alloxane.
Acknowledgements
Dr Joey Chifamba.
Funding
The bioactivity study was supported by the South African Council for Scientific and Industrial Research (CSIR) through their African Laser Centre program and the HA Taylor Will Trust.
Author information
Authors and Affiliations
Contributions
Authors (T.A.M., B.K.D.S., K.D., and E.C.) contributed to the conceptualization and appraised the article through the various stages of development. C.R., L.V., A.C.H. and M.v.d.V. did the in vitro studies; while T.A.M. performed the fractionation and phytochemistry. C.R., L.V., A.C.H., M.v.d.V. and T.A.M. conducted the data analysis of the in vitro studies. M.v.d.V., sourced funding for the in vitro bioactivity tests. S.M. and S.S. worked with Tumelo in the fractionation of the extract. All authors reviewed successive drafts and approved the final version of this article.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was approved by the Research Ethics Committee of Mulungushi University School of Medicine [SMHS-MU3-2020–12], the National Health Research Authority of Zambia, and the Research Ethics Committee of the University of Namibia (HG-/162/2021).
The K. africana fruit was collected with permission in January 2022, in the Kazungula District specifically from the riverine area of Singanga village in Kachola, Chief Sekute area, in the Southern Province of Zambia. The plant material underwent authentication in the Department of Biological Sciences within the School of Natural Sciences at the University of Zambia (UNZA), by Florence Nyirenda (MSc) a scientist. The specimen had receipt number 1793751 and was mounted in the herbarium under specimen number 22, 420.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Muyenga, T.A., Bamitale, S.K.D., Kibuule, D. et al. Kigelia africana fruit fractions inhibit in vitro alpha-glucosidase activity: a potential natural alpha-glucosidase inhibitor. BMC Complement Med Ther 24, 230 (2024). https://doi.org/10.1186/s12906-024-04510-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-024-04510-5