Abstract
Background
Ginsenoside Rg3 is a component of ginseng that protects against myocardial ischemia/reperfusion (MI/R) injury. Ferroptosis is a new form of cell death characterized by oxidative damage to phospholipids. The purpose of this study was to examine the role and of ginsenoside Rg3 in MI/R and the mechanism.
Methods
A mouse model of left anterior descending (LAD) ligation-induced myocardial ischemia/reperfusion (MI/R) injury and oxygen-glucose deprivation/reperfusion (OGD/R) were used as in vitro and in vivo models, respectively. Echocardiographic analysis, 2,3,5-triphenyltetrazolium chloride (TTC) staining and hematoxylin-eosin (H&E) staining were used to assess the cardioprotective effects of ginsenoside Rg3. Western blotting, biochemical analysis, small interfering RNA analysis and molecular docking were performed to examine the underlying mechanism.
Results
Ginsenoside Rg3 improved cardiac function and infarct size in mice with MI/R injury. Moreover, ginsenoside Rg3 increased the expression of the ferroptosis-related protein GPX4 and inhibited iron deposition in mice with MI/R injury. Ginsenoside Rg3 also activated the Nrf2 signaling pathway. Ginsenoside Rg3 attenuated myocardial ischemia/reperfusion-induced ferroptosis via the Nrf2 signaling pathway. Notably, ginsenoside Rg3 regulated the keap1/Nrf2 signaling pathway to attenuate OGD/R-induced ferroptosis in H9C2 cells. Taken together, ginsenoside Rg3 attenuated myocardial ischemia/reperfusion-induced ferroptosis via the keap1/Nrf2/GPX4 signaling pathway.
Conclusions
Our findings demonstrated that ginsenoside Rg3 ameliorate MI/R-induced ferroptosis via the keap1/Nrf2/GPX4 signaling pathway.
Similar content being viewed by others
Background
Myocardial ischemia is the root cause of heart failure due to myocardial infarction and inflammatory changes, which often lead to irreversible heart damage [1]. Timely and effective myocardial reperfusion, such as via percutaneous coronary interventional therapy (PPCI) or thrombolytic therapy, is an effective strategy for alleviating myocardial ischemic injury [2, 3]. However, myocardial ischemia/reperfusion (MI/R) can damage the heart muscle, which can contribute to further cardiomyocyte death.
Ferroptosis is a novel iron-dependent form of cell death characterized by oxidative damage to phospholipids and is related to iron ion metabolism, lipid oxidative metabolism and glutathione metabolic pathways [4, 5]. Previous studies have shown that oxidative stress and metabolic disorders are associated with the progression of MI/R [6]. In addition, increased levels of reactive oxygen species (ROS) have been observed in MI/R, and oxidative stress triggered by excessive ROS accumulation is an essential initiator of ferroptosis [7]. Under physiological conditions, cellular iron uptake is mainly controlled by transferrin receptor (TFR), which imports iron bound to transferrin from extracellular to intracellular via receptor-mediated endocytosis [8]. Whereas excess iron is stored in ferritin, an iron storage protein complex that includes ferritin light chain (FTL) and ferritin Heavy Chain 1 (FTH1) [9]. FTH1/FTL can increase iron levels through autophagic degradation, whereas a decrease in ferritin expression, especially its heavy chain, facilitates myocardial ferroptosis in myocardial ischemia/reperfusion injury [10]. Notably, glutathione peroxidase 4 (GPX4), which plays a central enzymatic role in ferroptosis, catalyzes the conversion of lipid hydroperoxides to nontoxic lipohydrols to prevent ferroptosis [4]. In myocardial ischemia reperfusion injury, increasing of GPX4 expression inhibits the accumulation of lipid peroxides, which in turn inhibits cardiomyocyte ferroptosis, thereby ameliorating MI/R injury [11].
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that protects the myocardium from oxidative damage by activating antioxidant proteins [12]. Kelch-like ECH-associated protein 1 (keap1) is a chaperone protein that plays an essential role in maintaining redox homeostasis in vivo [13]. Under physiological conditions, Nrf2 binds to Keap1 in the cytoplasm and is continuously degraded by ubiquitination, as a result, it is unable to enter the nucleus to mediate transcriptional activity [14]. When oxidative stress occurs, conformational changes in keap1 result in the inability of Nrf2 to be degraded by ubiquitination [15]. Subsequently, Nrf2 translocates to the nucleus to activate various antioxidant genes, such as GPX4, HO-1 and NQO1, by binding to antioxidant response elements, ultimately reducing ROS production in vivo [16, 17]. A reduction in ROS ameliorates oxidative damage, which reduces lipid peroxidation and ferroptosis [18]. Thus, activating Nrf2 to reduce lipid peroxidation and ferroptosis may be an effective strategy to ameliorate MI/R.
Ginsenoside Rg3, which is the active ingredient in ginseng, has a variety of pharmacological effects, including anti-inflammatory and antioxidant effects [19, 20]. Recent pharmacological studies have shown that ginsenoside Rg3 can inhibit myocardial infarct size, maintain left ventricular function and ameliorate myocardial ischemia‒reperfusion injury [21]. In 2021, it was reported that ginsenoside Rg3 improved transverse aortic constriction (TAC)-induced myocardial hypertrophy in rats [22] and inhibited coronary artery ligation (CAL)-induced heart failure in mice [23]. In 2023, ginsenoside Rg3 was reported to ameliorate myocardial fibrosis after infarction induced by left anterior descending coronary artery ligation in mice [24]. However, it is not clear whether ginsenoside Rg3 ameliorates myocardial ischemia‒reperfusion injury by inhibiting ferroptosis. Therefore, we investigated the pharmacological effects of ginsenoside Rg3 on MI/R and the potential mechanisms.
Materials and methods
Reagents
Ginsenoside Rg3 (purity > 98%, B21059) was obtained from Shenzhen Combe Scientific Instrument Co., Ltd. (Shenzhen, China). Antibodies against keap1 (AF5266), GPX4 (DF6701), HO-1 (AF5393) and β-tubulin (AF7011) were purchased from Affinity Biosciences (Jiangsu, China). FTH1 (#3998S), Lamin B (#13435S) and Nrf2 (#20733S) antibodies were obtained from Cell Signaling Technology (Boston, MA). β-actin (AF2811) was purchased from Beyotime (Shanghai, China). NQO1 (PAB32354) was purchased from Bioswamp (Wuhan, China). HRP-linked anti-mouse IgG (S0002) and HRP-linked anti-rabbit IgG (S0001) were purchased from Affinity Biosciences (Jiangsu, China).
Animals and the MI/R model
The experimental procedures were approved by the Animal Ethical Use Committee of Guangzhou University of Chinese Medicine (No. 00371405). Male C57BL/6 mice (6–8 weeks) were purchased from the Laboratory Animal Center of Guangzhou University of Chinese Medicine. The mice were subjected to MI/R as previously described. Briefly, the mice were anesthetized by i.p. injection of 0.3% sodium pentobarbital and ventilated with a pressure-control ventilator. The left anterior descending coronary artery was ligated with a 7 − 0 silk suture and a PE10 tube for 30 min. After 30 min of ischemia, the suture and the PE10 tube were removed. At the end of 6 h of reperfusion. The mice were pretreated with ginsenoside Rg3 and normal saline for 7 days before ischemia. The mice were randomly divided into five groups: the sham group was given saline, the model group was given saline, and the MI/R + Ginsenoside Rg3 groups were given 5, 10 or 20 mg/kg ginsenoside Rg3. At the end of the experiment, the mice were euthanized by cervical dislocation, which resulted in immediate unconsciousness and cessation of breathing.
2,3,5-Triphenyltetrazolium chloride (TTC) staining
The hearts were removed, frozen, sliced and incubated with 1% TTC at 37 °C for 15 min. The area of myocardial infarction in each section was analyzed by imaging software.
Echocardiographic analysis
The mice were anesthetized with isoflurane ventilation, and an echocardiography system (Visual Sonics Inc., Toronto, Canada) was used to assess cardiac function after MI/R. The left ventricular ejection fraction (LVEF) and left ventricular shortening (LVFS) were calculated.
Hematoxylin-eosin (H&E) staining
The hearts were fixed with 4% paraformaldehyde, embedded in paraffin, cut into 5 μm slices, stained with hematoxylin-eosin (H&E) and observed under a light microscope.
Cell culture and oxygen-glucose deprivation/reperfusion (OGD/R) model
H9C2 cardiomyocytes were obtained from BeiNa Biological Technology Co., Ltd. and cultured in DMEM supplemented with 10% FBS (Invitrogen) at 37 °C in a 5% CO2 incubator. H9C2 cells were subjected to hypoxic conditions (0.1% O2, 5% CO2 and 95% N2) for 6 h at 37 °C. The cells were then reoxygenated for 24 h at 37 °C under normal culture conditions (95% air and 5% CO2). H9C2 cells were treated with the indicated doses of ginsenoside Rg3 (5, 10, 20 µM) and ML334 (50 µM) for 24 h. The cells were treated with erastin (10 µM) to induce ferroptosis.
Biochemical analysis
Iron concentrations were determined with an iron assay kit (Elabscience) according to the manufacturer’s instructions.
Glutathione (GSH) levels were determined by a GSH assay kit (Nanjing Jiancheng Bioengineering Research Institute) according to the manufacturer’s instructions.
Western blot analysis
H9c2 cells and the border zones of the infarcted hearts were lysed with RIPA lysis buffer (Beyotime, China) supplemented with protease and phosphatase inhibitors. The samples were separated by 10% or 12% sodium dodecyl sulfate‒polyacrylamide gel electrophoresis (SDS‒PAGE) and subsequently transferred to polyvinylidene difluoride (PVDF) membranes, which were blocked with TBST buffer containing 5% BSA for 2 h at room temperature and incubated with primary antibodies against β-tubulin, Lamin B, Nrf2, HO-1, FTH1, GPX4, NQO1, β-actin and Keap1 overnight at 4 °C, followed by incubation with the appropriate HRP-conjugated secondary antibodies diluted in 2% BSA in TBST. The bands were detected using ECL reagent. The grayscale of the protein bands was determined by ImageJ software.
siRNA transfection
H9C2 cells were cultured in 6-well plates. Control siRNA and Nrf2 siRNA were transfected into the cells (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. After 24 h of culture, the cells were subjected to OGD/R, treated with ginsenoside Rg3 and then subjected to Western blot analysis.
Molecular docking
The molecular structure of ginsenoside Rg3 was retrieved from PubChem Compound. The 3D coordinates of Keap1 (PDB ID, 7C60; resolution, 1.95 Å) were downloaded from the PDB database. For docking analysis, all protein and molecular files were converted into the PDBQT format; all water molecules were excluded, and polar hydrogen atoms were added. The grid box was centered to cover the domain of each protein and accommodate free molecular movement. The grid length was set to 30 Å × 30 Å × 30 Å, and the grid point distance was 0.05 nm. Molecular docking studies were performed by Autodock Vina 1.2.2 (http://autodock.scripps.edu/).
Statistical analysis
Quantitative data are shown as the means ± SDs. Comparisons of among groups were performed using one-way analysis of variance with Dunnett’s test. For two independent variables, two-way ANOVA was used. The statistical data were analyzed using SPSS 21.0. P values < 0.05 indicated statistical significance.
Results
Ginsenoside Rg3 attenuated myocardial ischemia/reperfusion injury-induced damage to cardiac function
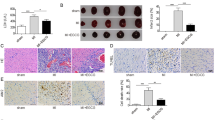
To verify the protective effect of ginsenoside Rg3 on cardiac tissues, we induced myocardial ischemia/reperfusion injury in mice and administered ginsenoside Rg3 by gavage. Cardiac function was impaired by MI/R injury, as evidenced by a decrease in the left ventricular ejection reduction fraction (LVEF) and left ventricular shortening reduction (LVFS). However, 10 and 20 mg/kg ginsenoside Rg3 ameliorated myocardial ischemia/reperfusion injury (Fig. 1A-C). As shown in Fig. 1D-E, ginsenoside Rg3 significantly reduced the MI/R-induced myocardial infarction area. H&E staining revealed that MI/R disrupted myofiber structure and led to inflammatory cell infiltration. However, ginsenoside Rg3 not only prevented changes in myofiber structure but also reduced the recruitment of inflammatory cells (Fig. 1F). Overall, ginsenoside Rg3 exerted cardioprotective effects.
Ginsenoside Rg3 improved cardiac function and protected against myocardial ischemia/reperfusion (MI/R) injury in mice. (A) M-mode images of each group. (B) Ejection fraction (EF%). (C) Fractional shortening (FS%). (D) TTC results in each group of hearts. (E) Statistical analysis of the TTC results. (F) H&E-stained heart sections in each group. The data are shown as the means ± SDs (n = 5). Statistical comparisons between the different groups were performed by one-way ANOVA followed by Dunnett’s test. *p < 0.05, **p < 0.01, ***p < 0.001, the sham group or the I/R + Rg3 group vs. the I/R group
Ginsenoside Rg3 ameliorated ferroptosis induced by myocardial ischemia/reperfusion injury
Myocardial ischemia/reperfusion injury is accompanied by ferroptosis, which is characterized by iron accumulation, lipid peroxidation, and glutathione depletion [25]. In addition, studies have reported that ginsenoside Rg3 ameliorates acute pancreatitis by inhibiting ferroptosis [26]. The levels of iron and GSH in H9C2 cells were examined. As shown in Fig. 2A-B, ginsenoside Rg3 significantly reduced iron levels and increased GSH levels in H9C2 cells exposed to OGD/R conditions. In addition, GPX4 catalyzes the conversion of lipid peroxides to the lipohydrol form, which plays a central role in ferroptosis [4]. Ferritin heavy chain 1 (FTH1) is an iron storage protein that is essential for iron metabolism [9]. Accordingly, we examined the expression levels of ferroptosis-related proteins. The Western blot results showed that ginsenoside Rg3 promoted the expression of GPX4 and FTH1 in H9C2 cells exposed to OGD/R conditions (Fig. 2C-E). The changes in GPX4 and FTH1 protein expression in mice with myocardial ischemia/reperfusion injury was consistent with the results in cells (Fig. 2F-H). These results indicated that ginsenoside Rg3 inhibited MI/R-induced ferroptosis.
Effect of ginsenoside Rg3 on ferroptosis. (A, B) Determination of ferrous iron (Fe2+) and glutathione (GSH) levels in H9C2 cells. (C–E) The protein levels of GPX4 and FTH1 in H9C2 cells. The data are shown as the means ± SDs (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, the Ctrl group or OGD/R + Rg3 groups vs. the OGD/R group. (F-H) The protein levels of GPX4 and FTH1 in the heart tissue of the mice. The data are shown as the means ± SDs (n = 5). Statistical comparisons between the different groups were performed by one-way ANOVA followed by Dunnett’s test. *p < 0.05, **p < 0.01, ***p < 0.001, the sham group or the I/R + Rg3 group vs. the I/R group
The ferroptosis inducer erastin reversed the protective effect of ginsenoside Rg3 against OGD/R-induced injury in H9C2 cardiomyocytes
To further validate the role of ferroptosis in myocardial ischemia/reperfusion injury, we used erastin to induce ferroptosis in H9C2 cells exposed to OGD/R conditions. As shown in Fig. 3A-B, ginsenoside Rg3 decreased the iron content and increased the GSH level in H9C2 cells exposed to OGD/R. However, erastin partially reversed these changes. Western blot analysis indicated that ginsenoside Rg3 promoted the expression of GPX4 and FTH1 in H9C2 cells exposed to OGD/R conditions. However, the promotion of ferroptosis-related protein expression by ginsenoside Rg3 was partially inhibited by erastin (Fig. 3C-E). These results showed that erastin reversed the inhibitory effect of ginsenoside Rg3 on OGD/R-induced ferroptosis in H9C2 cells.
Erastin reversed the protective effect of ginsenoside Rg3 against OGD/R-induced injury in H9C2 cardiomyocytes. (A, B) Ferrous iron (Fe2+) and glutathione (GSH) levels in H9C2 cells were examined by kits. (C–E) The protein levels of GPX4 and FTH1 in H9C2 cells were examined by Western blotting. The data are shown as the means ± SDs (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, the Ctrl group, the OGD/R + Rg3 group or the OGD/R + Rg3 + Erastin group vs. the OGD/R group
Ginsenoside Rg3 regulated the Nrf2 signaling pathway to protect against OGD/R-induced injury in H9C2 cardiomyocytes
Myocardial ischemia/reperfusion injury is closely related to oxidative stress [27]. When oxidative stress occurs, Nrf2 translocates to the nucleus and activates various antioxidant genes, such as HO-1 and NQO1, ultimately exerting antioxidant effects [28]. Previously, ginsenoside Rg3 was reported to attenuate cerebral ischemia/reperfusion injury by mitigating mitochondrial oxidative stress via the Nrf2/HO-1 signaling pathway [29]. Therefore, we examined the expression of the antioxidant proteins HO-1 and NQO1. As shown in Fig. 4A-C, the expression of HO-1 and NQO1 was reduced in the OGD/R group; however, ginsenoside Rg3 increased the expression of these proteins. Moreover, we determined the effect of ginsenoside Rg3 on Nrf2 nuclear translocation. Western blot analysis revealed that ginsenoside Rg3 promoted Nrf2 translocation into the nucleus (Fig. 4D-F). Taken together, ginsenoside Rg3 modulated the Nrf2 signaling pathway during MI/R injury.
Ginsenoside Rg3 regulates the Nrf2 signaling pathway. (A-C) The protein levels of HO-1 and NQO1 in H9C2 cells were examined by Western blotting. (D) The proteins were isolated from the cytoplasm and nucleus for Western blot analysis. (E, F) Histogram of Nrf2 protein expression in the nucleus and cytoplasm. The data are shown as the means ± SDs (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, the Ctrl group or the OGD/R + Rg3 group vs. the OGD/R group
Ginsenoside Rg3 attenuated OGD/R-induced ferroptosis in H9C2 cardiomyocytes via Nrf2
Nrf2 can alleviate ferroptosis by activating GPX4 [16]. Thus, we investigated whether the cardioprotective effect of ginsenoside Rg3 was related to the regulation of ferroptosis by the Nrf2 signaling pathway, and we used Nrf2-specific small interfering RNAs to knock down Nrf2 expression. As shown in Fig. 5A, Nrf2 was knocked down in H9c2 cells. In addition, ginsenoside Rg3-mediated promotion of the expression of ferroptosis-related proteins in H9C2 cells exposed to OGD/R conditions was partially blocked by Nrf2 knockdown (Fig. 5B-D). Moreover, we observed that knockdown of Nrf2 reduced the relative abundance of the antioxidant proteins HO-1 and NQO1 (Fig. 5E-G). These data demonstrated that Nrf2 was involved in the cardioprotective and anti-ferroptotic effects of ginsenoside Rg3.
Ginsenoside Rg3 attenuates ferroptosis via Nrf2. (A) Western blot analysis of Nrf2 in H9C2 cells. (B-D) The protein levels of GPX4 and FTH1 in H9C2 cells after Nrf2 silencing were examined by Western blotting. (E-G) The protein levels of HO-1 and NQO1 in H9C2 cells after Nrf2 silencing were examined by Western blotting. The data are shown as the means ± SDs (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001.) The OGD/R + NC siRNA group vs. the NC siRNA group or the OGD/R + Rg3 + NC siRNA group; the OGD/R + Nrf2 siRNA group vs. the Nrf2 siRNA group or the OGD/R + Rg3 + Nrf2 siRNA group; and the OGD/R + Rg3 + Nrf2 siRNA group versus the OGD/R + Rg3 + NC siRNA group
Ginsenoside Rg3 regulated the keap1/Nrf2 signaling pathway to attenuate OGD/R-induced ferroptosis in H9C2 cardiomyocytes
Under physiological conditions, Nrf2 binds to Keap1 in the cytoplasm resulting in the inability to enter the nucleus to exert transcriptional activity [30]. However, it is not clear whether ginsenoside Rg3 can target keap1, causing a conformational change in keap1 that leads to the translocation of Nrf2 to the nucleus. We first examined Keap1 expression in H9C2 cells exposed to OGD/R conditions. The results revealed that ginsenoside Rg3 inhibited keap1 expression (Fig. 6A, B). To evaluate the affinity of ginsenoside Rg3 for keap1, we performed molecular docking analysis. The results showed that ginsenoside Rg3 bound to Keap1 through hydrogen bonds and strong hydrophobic interactions and had a low binding energy of -15.587 kcal/mol, indicating highly stable binding (Fig. 6C). To further clarify whether ginsenoside Rg3 ameliorated ferroptosis induced by myocardial ischemia/reperfusion injury via the keap1/Nrf2 signaling pathway, we used ML334, a keap1/Nrf2 interaction inhibitor. As shown in Fig. 6D-F, treatment with ginsenoside Rg3 and ML334 more robustly promoted ferroptosis-related protein expression than treatment with ginsenoside Rg3 or ML334 alone. Similarly, treatment with ginsenoside Rg3 and ML334 promoted antioxidant-related protein expression (Fig. 6G-I). Overall, ginsenoside Rg3 regulates the keap1/Nrf2 signaling pathway to attenuate OGD/R-induced ferroptosis in H9C2 cells.
Ginsenoside Rg3 regulates the keap1/Nrf2 signaling pathway to attenuate OGD/R-induced ferroptosis. (A, B) The protein level of keap1 in H9C2 cells was examined by Western blotting. (C) Molecular docking analysis of ginsenoside Rg3 with the Kelch domain of Keap1. (D-F) The protein levels of GPX4 and FTH1 in H9C2 cells treated with ML334 were examined by Western blotting. (G-I) The protein levels of HO-1 and NQO1 in H9C2 cells treated with ML334 were examined by Western blotting. The data are shown as the means ± SDs (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001. The Ctrl group, the OGD/R + Rg3 group, the OGD/R + ML334 group or the OGD/R + ML334 + Rg3 group vs. the OGD/R group. The OGD/R + Rg3 group or the OGD/R + ML334 group vs. the OGD/R + ML334 + Rg3 group
Discussion
Myocardial ischemia/reperfusion injury (MI/R) poses a serious threat to human health, exhibits a high mortality rate in critical patients and imposes a heavy burden on the public [31], and it is imperative to develop treatment strategies and examine its pathogenesis. Ginsenoside Rg3 is a well-studied ginsenoside with high pharmacological activity that has anticancer and antiangiogenic effects [32, 33], but its role in MI/R has rarely been reported. We showed that ginsenoside Rg3 significantly improved cardiac function and infarct size in mice with myocardial ischemia/reperfusion injury.
Ferroptosis is an iron-dependent form of cell death that is implicated in myocardial ischemia/reperfusion injury [4]. Ferroptosis is associated with the metabolism of iron, glutathione, and ROS [34]. Extracellular Fe3+ is transported into the cell by transferrin, reduced to Fe2+, and reacts with excess H2O2 in the cell to produce large amounts of ROS, thereby triggering ferroptosis [35]. Multiple studies have shown that ROS production is increased during ischemia/reperfusion injury [36]. During the ischemic phase, ROS accumulation in cells decreases the effect of antioxidants [37]. After the blood supply to ischemic tissues is restored, oxidative stress contributes to cell damage and death, depending on the severity of ROS levels [7]. In contrast, cellular resistance to ROS is mediated by a variety of antioxidant molecules and enzymes, including the glutathione (GSH)-dependent antioxidant system [1, 38]. Glutathione (GSH), which is an antioxidant, scavenges oxygen free radicals in the body, which reduces the damage caused by oxidative stress [39]. Consequently, the upregulation of GSH during myocardial ischemia/reperfusion injury may attenuate the damage caused by oxygen free radicals to cardiomyocytes and reduce the extent of reperfusion injury [7]. Notably, our results revealed that ginsenoside Rg3 promoted GSH expression. GPX4 plays a critical regulatory role in ferroptosis by converting glutathione to oxidized glutathione and reducing lipid peroxides to nontoxic alcohols [5]. It has been shown that inhibiting GPX4 activity causes the accumulation of lipid peroxides, leading to ferroptosis [16]. In addition, studies have reported that ginsenoside Rg3 ameliorates acute pancreatitis by inhibiting ferroptosis [26]. In contrast, Hu et al. reported that ginsenoside Rg3 promoted hepatic stellate cell ferroptosis to suppress liver fibrosis progression [40]. However, how ginsenoside Rg3 affects ferroptosis to ameliorate myocardial ischemia‒reperfusion injury is unknown. Accordingly, we examined the effect of ginsenoside Rg3 on cardiomyocyte ferroptosis. We found that Ginsenoside Rg3 ginsenoside inhibited iron deposition and promoted the expression of the ferroptosis-associated proteins GPX4 and FTH1, thereby exerting a protective effect on cardiomyocytes. Furthermore, the ferroptosis inducer erastin partially reversed the protective effect of ginsenoside Rg3 on OGD/R-induced H9C2 cardiomyocytes.
The keap1/Nrf2 signaling pathway is a major defense mechanism against oxidative and electrophilic stress [18]. Keap1 modulates the ubiquitination and translocation of Nrf2 into the nucleus [41, 42]. Previous studies have shown that the structures of chemicals that induce oxidative stress have an electrophilic center that allows them to react with cysteine residues in proteins [18, 43]. In the current study, molecular docking suggested that ginsenosides Rg3 and Keap1 have high affinity. Nrf2, which is a transcriptional regulator, activates adaptive responses like anti-oxidative stress and ferroptosis by transcriptionally inducing a multitude of antioxidant enzymes such as GPX4 and HO-1 [44]. In addition, activation of the Nrf2 signaling pathway is a major mechanism of the cellular defense against OGD/R induction [45]. Previously, ginsenoside Rg3 was reported to attenuate cerebral ischemia‒reperfusion injury by mitigating mitochondrial oxidative stress via the Nrf2/HO-1 signaling pathway [29]. However, whether ginsenoside Rg3 alleviates myocardial ischemia/reperfusion injury via the Nrf2 signaling pathway is not clear. Our results indicated that ginsenoside Rg3 activated the Nrf2 signaling pathway to protect against MI/R- and OGD/R-induced oxidative stress and ferroptosis. Silencing Nrf2 significantly partially blocked the inhibitory effect of ginsenoside Rg3 on OGD/R-induced ferroptosis in cardiomyocytes. Interestingly, when ML334 was used to inhibit the interaction between keap1 and Nrf2, ginsenoside Rg3 robustly promoted the expression of ferroptosis- and antioxidative proteins. Ginsenoside Rg3 regulates the keap1/Nrf2 signaling pathway to inhibit ferroptosis. In this study, we confirmed the protective effect of ginsenoside Rg3 on H9C2 cardiomyocytes. We will further verify this finding in other cardiac cells in addition to H9C2 cells at a later stage. In addition, ginsenoside Rg3 inhibits ferroptosis induced by other pathways during myocardial ischemia‒reperfusion injury, which we will also further validate. As mentioned previously, ginsenoside Rg3 protect the heart from ischemia/reperfusion injury via the Keap1/Nrf2/GPX4 pathway both in vivo and in vitro.
Conclusions
In conclusion, we demonstrated that ginsenoside Rg3 ameliorates myocardial ischemia/reperfusion injury in mice by suppressing ferroptosis. Furthermore, our results demonstrated that ginsenoside Rg3 attenuates myocardial ischemia/reperfusion-induced ferroptosis via the keap1/Nrf2/GPX4 signaling pathway (Fig. 7).
Data availability
All relevant study data are included in the article and the supplementary materials.
Abbreviations
- FTH1:
-
Ferritin heavy chain 1
- GPX4:
-
Glutathione peroxidase 4
- GSH:
-
Glutathione
- H&E:
-
Hematoxylin-eosin
- Keap1:
-
Kelch-like ECH-associated protein 1
- LAD:
-
Left anterior descending
- LVEF:
-
Left ventricular ejection fraction
- LVFS:
-
Left ventricular shortening
- MI/R:
-
Myocardial ischemia/reperfusion
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- OGD/R:
-
Oxygen-glucose deprivation/reperfusion
- PPCI:
-
Percutaneous coronary interventional therapy
- PVDF:
-
Polyvinylidene difluoride
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TTC:
-
2,3,5-triphenyltetrazolium chloride
References
Yao H, Xie Q, He Q, Zeng L, Long J, Gong Y, Li X, Li X, Liu W, Xu Z, et al. Pretreatment with Panaxatriol Saponin attenuates mitochondrial apoptosis and oxidative stress to Facilitate Treatment of Myocardial Ischemia-Reperfusion Injury via the regulation of Keap1/Nrf2 activity. Oxidative Med Cell Longev. 2022;2022:1–20.
Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100.
Zeng J-j, Shi H-q, Ren F-f, Zhao X-s, Chen Q-y, Wang D-j, Wu L-p, Chu M-p, Lai T-f, Li L. Notoginsenoside R1 protects against myocardial ischemia/reperfusion injury in mice via suppressing TAK1-JNK/p38 signaling. Acta Pharmacol Sin. 2023;44(7):1366–79.
Zhao W-k, Zhou Y, Xu T-t, Wu Q, Li H. Ferroptosis: opportunities and challenges in Myocardial Ischemia-Reperfusion Injury. Oxidative Med Cell Longev. 2021;2021:1–12.
Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–92.
Wang Z, Yao M, Jiang L, Wang L, Yang Y, Wang Q, Qian X, Zhao Y, Qian J. Dexmedetomidine attenuates myocardial ischemia/reperfusion-induced ferroptosis via AMPK/GSK-3β/Nrf2 axis. Biomed Pharmacother 2022, 154.
Xu S, Wu B, Zhong B, Lin L, Ding Y, Jin X, Huang Z, Lin M, Wu H, Xu D. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2) /System xc-/ glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered. 2021;12(2):10924–34.
Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Reviews Clin Oncol. 2021;18(5):280–96.
Chen P-H, Wu J, Ding C-KC, Lin C-C, Pan S, Bossa N, Xu Y, Yang W-H, Mathey-Prevot B, Chi J-T. Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ. 2019;27(3):1008–22.
Ju J, Li X-M, Zhao X-M, Li F-H, Wang S-C, Wang K, Li R-F, Zhou L-Y, Liang L, Wang Y et al. Circular RNA FEACR inhibits ferroptosis and alleviates myocardial ischemia/reperfusion injury by interacting with NAMPT. J Biomed Sci 2023, 30(1).
Yu P, Zhang J, Ding Y, Chen D, Sun H, Yuan F, Li S, Li X, Yang P, Fu L, et al. Dexmedetomidine post-conditioning alleviates myocardial ischemia–reperfusion injury in rats by ferroptosis inhibition via SLC7A11/GPX4 axis activation. Hum Cell. 2022;35(3):836–48.
Chen L, Geng N, Chen T, Xiao Q, Zhang H, Huo H, Jiang L, Shao Q, He B. Ginsenoside Rb1 improves Post-cardiac arrest myocardial stunning and cerebral outcomes by regulating the Keap1/Nrf2 pathway. Int J Mol Sci 2023, 24(5).
Gao G, Xie Z, Li E-w, Yuan Y, Fu Y, Wang P, Zhang X, Qiao Y, Xu J, Hölscher C, et al. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J Nat Med. 2021;75(3):540–52.
Galvan-Alvarez V, Gallego-Selles A, Martinez-Canton M, Garcia-Gonzalez E, Gelabert-Rebato M, Ponce-Gonzalez JG, Larsen S, Morales-Alamo D, Losa-Reyna J, Perez-Suarez I, et al. Antioxidant enzymes and Nrf2/Keap1 in human skeletal muscle: influence of age, sex, adiposity and aerobic fitness. Free Radic Biol Med. 2023;209(Pt 2):282–91.
Cheng K, Huang Y, Wang C. 1,25(OH)2D3 inhibited ferroptosis in zebrafish liver cells (ZFL) by regulating Keap1-Nrf2-GPx4 and NF-κB-hepcidin Axis. Int J Mol Sci 2021, 22(21).
Wang Y-M, Gong F-C, Qi X, Zheng Y-J, Zheng X-T, Chen Y, Yang Z-T, Qing Y, Mao E-Q, Chen E-Z, et al. Mucin 1 inhibits ferroptosis and sensitizes vitamin E to Alleviate Sepsis-Induced Acute Lung Injury through GSK3β/Keap1-Nrf2-GPX4 pathway. Oxidative Med Cell Longev. 2022;2022:1–29.
Iyori M, Ogawa R, Emran TB, Tanbo S, Yoshida S. Characterization of the Gene expression patterns in the Murine Liver following intramuscular administration of Baculovirus. Gene Expr. 2021;20(3):147–55.
Yao H, He Q, Huang C, Wei S, Gong Y, Li X, Liu W, Xu Z, Wu H, Zheng C, et al. Panaxatriol saponin ameliorates myocardial infarction-induced cardiac fibrosis by targeting Keap1/Nrf2 to regulate oxidative stress and inhibit cardiac-fibroblast activation and proliferation. Free Radic Biol Med. 2022;190:264–75.
Wang J, Zeng L, Zhang Y, Qi W, Wang Z, Tian L, Zhao D, Wu Q, Li X, Wang T. Pharmacological properties, molecular mechanisms and therapeutic potential of ginsenoside Rg3 as an antioxidant and anti-inflammatory agent. Front Pharmacol 2022, 13.
Xia J, Chen C, Dong M, Zhu Y, Wang A, Li S, Zhang R, Feng C, Jiang X, Xu X, et al. Ginsenoside Rg3 endows liposomes with prolonged blood circulation and reduced accelerated blood clearance. J Control Release. 2023;364:23–36.
Li L, Wang Y, Guo R, Li S, Ni J, Gao S, Gao X, Mao J, Zhu Y, Wu P, et al. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J Controlled Release. 2020;317:259–72.
Ren B, Feng J, Yang N, Guo Y, Chen C, Qin Q. Ginsenoside Rg3 attenuates angiotensin II-induced myocardial hypertrophy through repressing NLRP3 inflammasome and oxidative stress via modulating SIRT1/NF-κB pathway. Int Immunopharmacol 2021, 98.
Lai Q, Liu F-m, Rao W-l, Yuan G-y, Fan Z-y, Zhang L, Fu F, Kou J-p, Yu B-y, Li F. Aminoacylase-1 plays a key role in myocardial fibrosis and the therapeutic effects of 20(S)-ginsenoside Rg3 in mouse heart failure. Acta Pharmacol Sin. 2021;43(8):2003–15.
Xu H, Miao H, Chen G, Zhang G, Hua Y, Wu Y, Xu T, Han X, Hu C, Pang M, et al. 20(S)-ginsenoside Rg3 exerts anti-fibrotic effect after myocardial infarction by alleviation of fibroblasts proliferation and collagen deposition through TGFBR1 signaling pathways. J Ginseng Res. 2023;47(6):743–54.
Ye J, Lyu T-J, Li L-Y, Liu Y, Zhang H, Wang X, Xi X, Liu Z-J, Gao J-Q. Ginsenoside Re attenuates myocardial ischemia/reperfusion induced ferroptosis via miR-144-3p/SLC7A11. Phytomedicine 2023, 113.
!!!. INVALID CITATION !!!.
Tian L, Cao W, Yue R, Yuan Y, Guo X, Qin D, Xing J, Wang X. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J Pharmacol Sci. 2019;139(4):352–60.
Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865(5):721–33.
Chen D, Duan H, Zou C, Yang R, Zhang X, Sun Y, Luo X, lv D, Chen P, Shen Z et al. 20(R)-ginsenoside Rg3 attenuates cerebral ischemia–reperfusion injury by mitigating mitochondrial oxidative stress via the Nrf2/HO‐1 signaling pathway. Phytother Res 2024.
Yao Y, Hu S, Zhang C, Zhou Q, Wang H, Yang Y, Liu C, Ding H. Ginsenoside Rd attenuates cerebral ischemia/reperfusion injury by exerting an anti-pyroptotic effect via the miR-139-5p/FoxO1/Keap1/Nrf2 axis. Int Immunopharmacol 2022, 105.
Burn BR, Varner KJ. Environmentally persistent free radicals compromise left ventricular function during ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2015;308(9):H998–1006.
Sun M, Ye Y, Xiao L, Duan X, Zhang Y, Zhang H. Anticancer effects of ginsenoside Rg3 (review). Int J Mol Med. 2017;39(3):507–18.
Nakhjavani M, Smith E, Townsend AR, Price TJ, Hardingham JE. Anti-angiogenic properties of Ginsenoside Rg3. Molecules 2020, 25(21).
Ma S, Sun L, Wu W, Wu J, Sun Z, Ren J. USP22 protects against myocardial ischemia-reperfusion Injury via the SIRT1-p53/SLC7A11-Dependent inhibition of Ferroptosis-Induced Cardiomyocyte Death. Front Physiol. 2020;11:551318.
Li X, Ma N, Xu J, Zhang Y, Yang P, Su X, Xing Y, An N, Yang F, Zhang G et al. Targeting Ferroptosis: Pathological Mechanism and Treatment of Ischemia-Reperfusion Injury. Oxid Med Cell Longev 2021, 2021:1587922.
Hu H, Qi L, Ren C, Yan S. M2 macrophage-derived Exosomes Regulate Myocardial Ischemia-Reperfusion and Pyroptosis Via ROS/NLRP3 pathway. Heart Surg Forum. 2022;25(5):E698–708.
Chen Y, Chen H, Chen Y, Yang Z, Zhou T, Xu W. Dexmedetomidine Preconditioning attenuates myocardial Ischemia/Reperfusion Injury in rats by suppressing Mitophagy Via activating Alpha2-Adrenergic receptor. Arq Bras Cardiol. 2023;120(10):e20220750.
Bulbul MRH, Rahman MA, Rahman MZ, Emran TB, Afroze M, Khan M, Chowdhury MAH, Ibrahim MA, Chowdhury MS. Leea Macrophylla (Roxb.) Root extract reverses CCl4 induced liver injury through upregulation of antioxidative gene expression: a molecular interaction for therapeutic inception. Adv Traditional Med. 2019;20(1):35–52.
Wang X, Shen T, Lian J, Deng K, Qu C, Li E, Li G, Ren Y, Wang Z, Jiang Z, et al. Resveratrol reduces ROS-induced ferroptosis by activating SIRT3 and compensating the GSH/GPX4 pathway. Mol Med. 2023;29(1):137.
Hu Y, Lang Z, Li X, Lin L, Li Y, Zhang R, Zheng J, Yu Z. Ginsenoside Rg3 promotes hepatic stellate cell ferroptosis by epigenetically regulating ACSL4 to suppress liver fibrosis progression. Phytomedicine 2024, 124.
Baird L, Yamamoto M. The Molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol 2020, 40(13).
Fonseca Lopez F, Miao J, Damjanovic J, Bischof L, Braun MB, Ling Y, Hartmann MD, Lin YS, Kritzer JA. Computational prediction of cyclic peptide structural ensembles and application to the design of Keap1 binders. J Chem Inf Model 2023.
Asano W, Hantani R, Uhara T, Debaene F, Nomura A, Yamaguchi K, Adachi T, Otake K, Harada K, Hantani Y. Screening approaches for the identification of Nrf2-Keap1 protein-protein interaction inhibitors targeting hot spot residues. SLAS Discov 2023.
Wang Z, Wang L, Jiang R, Li C, Chen X, Xiao H, Hou J, Hu L, Huang C, Wang Y. Ginsenoside Rg1 prevents bone marrow mesenchymal stem cell senescence via NRF2 and PI3K/Akt signaling. Free Radic Biol Med. 2021;174:182–94.
Yuan Y, Zhai Y, Chen J, Xu X, Wang H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11(7).
Funding
This work was supported by grants from Shenzhen Traditional Chinese Medicine Hospital “3030 Program” Chinese Medicine Clinical Research Project (No. G3030202128), Shenzhen Science and Technology Program (JCYJ20230807094503006) and the Scientific Research Project of Traditional Chinese Medicine Bureau of Guangdong Province (No.20231284).
Author information
Authors and Affiliations
Contributions
LW, XC, GFZ, JTC and YTL performed the experiments and analysed the data. YH, MSW and QQN participated in language editing and manuscript revision. MYX and QHZ contributed to the literature review. All authors contributed to the in vivo and in vitro experiments. LW and XC conceived and designed the study. GFZ, JTC and YTL provided the conception and design of the study, participated in the drafting and revision and supervised the study. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the ARRIVE guideline. The experimental procedures and protocols were approved by the Animal Ethical Use Committee of Guangzhou University of Chinese Medicine (No.00371405). Written informed consent was obtained from all participants before any research relevant procedure started, and all were informed that they could withdraw from the study for no reason.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhong, G., Chen, J., Li, Y. et al. Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion-induced ferroptosis via the keap1/Nrf2/GPX4 signaling pathway. BMC Complement Med Ther 24, 247 (2024). https://doi.org/10.1186/s12906-024-04492-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-024-04492-4