Abstract
Background
Oxidative stress and inflammation can lead to apoptosis of ovarian granulosa cells (GCs), resulting in ovulation disorders and infertility. Baicalin (BAI) promotes cell proliferation and reduces inflammation and oxidative stress. However, the mechanisms by which BAI treatment affects oxidative stress and inflammation in GCs remain incompletely understood.
Methods
KGN cells were treated with hydrogen peroxide (H2O2) to analyze the effect of oxidative stress on GCs in vitro. Subsequently, H2O2-stimulated KGN cells were treated with BAI. The levels of GSH-Px, CAT, and SOD were measured using an activity assay kit. The levels of MDA, IL-1β, IL-6, IL-8, and TNF-α were measured by ELISA. Proliferation, apoptosis, and mRNA and protein levels were measured using the CCK8, flow cytometry, qRT-PCR, and western blotting.
Results
H2O2 treatment inhibited KGN cell proliferation and promoted apoptosis, accompanied by increased oxidative stress and inflammation. BAI promoted proliferation, inhibited apoptosis, and reduced oxidative stress and inflammation in H2O2-stimulated KGN cells. BAI treatment promoted USP48 protein expression, and USP48 knockdown abrogated the protective effects of BAI, indicating that USP48 is a downstream mediator of BAI.
Conclusion
BAI treatment enhanced cell proliferation and ameliorated oxidative stress and inflammation by enhancing USP48 protein expression. BAI, which is used clinically and as a dietary supplement, may alleviate oxidative stress-induced GC injury and ovarian disorders.
Similar content being viewed by others
Background
Follicles are the structural and functional units of the ovary and include oocytes and granulosa cells (GCs). GCs synthesize various hormones and growth factors, including estrogen and progesterone, to regulate the growth, differentiation, and maturation of oocytes, thereby regulating follicular development [1]. GC apoptosis leads to oocyte dysfunction and reduction in ovarian reserve [2, 3]. For example, GC apoptosis and dysfunction can induce follicular dysplasia in childbearing women with polycystic ovarian syndrome (PCOS) [4]. Therefore, GSs are essential for the initiation and development of follicles, and their dysfunction or apoptosis is a key factor in follicular atresia. Studying apoptosis and GC dysfunction is crucial to improve ovarian function and oocyte development. Abnormal oxidative stress is an important cause of abnormal function and apoptosis in GCs. Oxidative stress in the ovarian microenvironment can cause GC apoptosis and dysfunction, thereby affecting normal oocyte development and ovarian functions [5, 6]. Oxidative stress and chronic inflammation have been observed in patients with PCOS owing to the influence of obesity and hyperinsulinemia, which induce apoptosis and dysfunction of GCs, leading to a decline in oocyte quality or capacity. Oxidative stress in GCs can lead to reproductive system disorders in women such as PCOS and premature ovarian failure [7]. Therefore, reducing inflammation and oxidative stress in GCs may improve oocyte quality and function.
Baicalin (BAI) is a flavonoid extracted from the radix of Scutellaria baicalensis [8]. In many diseases, BAI enhances cell proliferation while dampening inflammation and oxidative stress. BIA can effectively alleviate infection-associated lung injury by inhibiting inflammation, oxidative stress, immune responses, and cell apoptotic pathways [9]. BAI alleviates central nervous system disorders via its anti-neuroinflammatory and anti-neuronal apoptotic properties and mitigates metabolic disorders via anti-inflammatory and antioxidant mechanisms [10]. It also regulates the production of the gut microbiota and short-chain fatty acids, thereby relieving the symptoms of gastrointestinal disorders by improving chronic inflammation, immune imbalance, lipid metabolism disorders, cell apoptosis, and oxidative stress [11]. Hence, BAI exhibits potent anti-inflammatory and antioxidant properties. In addition, BAI treatment can enhance the secretion of estradiol and progesterone and the activity of GCs and improve the estrous cycle and oocyte quality via the mTOR pathway, thereby promoting the improvement of ovarian function [12]. BAI improves hormone imbalance, prolongs the estrous cycle, insulin resistance, and inflammation in mice with PCOS by activating the AMPK pathway and improving ovarian histological changes and follicular development [13]. BAI treatment in a rat model of hyperandrogenic PCOS significantly reversed elevated serum androgen levels and ovarian abnormalities and restored the estrous cycle via the GATA1/HSD3B2 axis [14]. BAI treatment reduces inflammation and testosterone concentrations and improves hormone secretion and follicular development in rats with PCOS via the miR-874-3p/FOXO3 and miR-144/FOXO1 axes [15]. However, the mechanisms by which BAI treatment affects oxidative stress and inflammation in GC remain incompletely understood.
KGN is a human ovarian granulosa-like tumor cell line. KGN cell exhibits a steroidogenesis pattern similar and the physiological regulation of apoptosis similar to that observed in normal human GCs, therefore, this cell line is considered a useful model for studying the steroidogenesis, growth, and apoptotic regulation of human GCs in vitro [16]. In addition, exposure to H2O2 increases the inflammatory factors, free radicals, ROS levels, and oxidative stress in GCs, thereby promoting GC apoptosis [17, 18]. Hence, H2O2-stimulated KGN cells were used to analyze the effects of oxidative stress on GCs in vitro. Proliferation, apoptosis, inflammation, and oxidative stress were measured after BAI treatment of H2O2-stimulated KGN cells. In addition, potential target genes of BAI in H2O2-stimulated KGN cells were screened and verified. Here, using Gene Expression Omnibus (GEO) data, a differentially expressed gene UPS48 related to polycystic ovary syndrome (PCOS) was discovered. USP48 is a deubiquitinating enzyme with a catalytic core ubiquitin C-terminal hydrolase domain located at the N-terminus [19]. USP48 possesses an ubiquitin-like (UBL) domain at its C-terminal end, which adopts a three-dimensional structure similar to ubiquitin and provides a regulatory mechanism for its catalytic activity [19]. USP48 is involved in various cellular processes, including DNA repair, cell cycle regulation, immune responses, and tumorigenesis [20,21,22,23]. These results revealed the key regulatory gene, USP48, by which BAI affects apoptosis in GCs.
Materials and methods
Compound
BAI (C21H18O11; molecular weight: 446.37 g/mol; purity: HPLC ≥ 98%) was purchased from Taian Biotechnology Co., Ltd. (Shanxi, China) and dissolved in DMSO.
Cell culture, transfection, and treatment
KGN cells (AnWei-Sci, Shanghai, China) were cultured in DMEM/HamF12 + 10% FBS at 37 ℃ in an atmosphere containing 5% CO2. After three passages, KGN cells were treated with different concentrations of H2O2 for 24 h to mimic the oxidative stress microenvironment (H2O2 group). H2O2 concentrations ranging from 100 to 500 µmol/L significantly inhibit the proliferation of GCs [17, 18]. Therefore, in this study, we selected a concentration range of 50–800 µmol/L of H2O2 to analyze its effects on KGN cell proliferation. The untreated KGN cells served as controls (control group). Subsequently, the H2O2-stimulated KGN cells were treated with different concentrations of BAI (0–80 µmol/L, H2O2 + BAI group) 24 h to analyze the effects of BAI. In the BAI + 0 µmol/L BAI group, a concentration of 0 µmol/L signified the introduction of solely dimethyl sulfoxide (DMSO). To identify the impact of USP48 on BAI-treated cells, 20 µM siRNA negative control (siNC group, RIBOBIO, Guangzhou, China) and 20 µM siUSP48 (siUSP48 group, RIBOBIO) were transfected into KGN cells, which were then treated with H2O2 and 20 µmol/L of BAI. Untransfected KGN cells served as blank controls and were treated with H2O2 and 20 µmol/L BAI (Blank group). The siUSP48 sequences were as follows: siUSP48-1, 5ʹ-GCATATTTGGTTAGGAGAA-3ʹ; and siUSP48-2, 5ʹ-GGTGAATGGTATAAGTTTA-3ʹ.
CCK8 and flow cytometry assays
KGN cell proliferation was analyzed using a Cell Counting Kit-8 (CCK-8; Dojindo, Japan) after H2O2 and/or treatment with 20 µmol/L BAI. Briefly, KGN (100 µl, 1 × 104 cells) cells were inoculated in a 96-well plate and cultured at 37 ℃ in a conditioned incubator containing 5% CO2. After treatment for 24 h, 10 µL of CCK-8 was added to every well, and the culture plate was incubated in a multiscan MK3 incubator (Thermo Fisher Scientific, Waltham, MA, USA) for 4 h. Absorbance was measured at 450 nm using a microplate reader. KGN cell apoptosis was analyzed using an Annexin V/PI apoptosis kit (BD, Franklin Lakes, NJ, USA). In brief, KGN was washed twice with cold PBS and then resuspended cells in 1X Binding Buffer at a concentration of 1 × 106 cells/ml. Then, 100 µl of the solution (1 × 105 cells) was transferred to a 5 ml culture tube and added 5 µl of FITC Annexin V and 5 µl PI. KGN was gently vortexed and incubate for 15 min at 25 °C in the dark. Finally, 400 µl of 1X Binding Buffer was added to each tube and a FACSCalibur flow cytometer (BD). Reactive oxygen species (ROS) level in KGN cells was analyzed using a DCFH-DA ROS Assay Kit (Beyotime, Shanghai, China) and FACSCalibur flow cytometer (BD). All reactions were performed in triplicate.
ELISA assay
The activities of glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD) were measured using GSH-Px, CAT, and SOD activity assay kits (Catalog No.: E-BC-K096-M, E-BC-K031-M, E-BC-K020-M; Elabscience, Wuhan, China), respectively. Malondialdehyde (MDA), interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) levels were measured using MDA, IL-1β, IL-6, IL-8, and TNF-α ELISA kits (Catalog No.: E-EL-0060c, E-EL-H0149c, E-EL-H6156, E-EL-H6008, E-EL-H0109c, Elabscience). After cell culture at 24 h, the culture supernatant was collected and centrifuged at 1000 × g for 20 min to remove impurities and cellular debris. The supernatant was collected for further analysis. For the detection, 50 µl of the supernatant was taken and analyzed. Following completion of the experiment, the optical density (OD) of each well was measured using a Multiscan MK3 reader (Thermo Fisher Scientific). All reactions were repeated thrice.
qRT-PCR assay
Total RNA was extracted from KGN cells using the Trizol Reagent (Beyotime). The mRNAs were then reverse-transcribed using EasyScript First-Strand cDNA Synthesis SuperMix (TransGen, Beijing, China) at 30˚C for 10 min, 42˚C for 30 min, and then 85˚C for 5 s. The expression levels of SNX29, MDM2, and USP48 were measured using the SYBR Green qPCR SuperMix (Vazyme, Nanjing, China) on an ABI PRISM 7500 Sequence Detection System (Roche, Basel, Switzerland). The reaction system (20 µl) was prepared according to the instructions provided with the reagent kit. The cycling conditions were as follows: Initial denaturation at 95˚C for 5 min, followed by 40 cycles at 95˚C for 15 s and 60˚C for 32 s. 18 S RNA was used for normalization of the expression of SNX29, MDM2, and USP48. All reactions were repeated thrice. The primers used for qRT-PCR are listed in Table 1.
Western blot assay
USP48 and GAPDH protein levels were analyzed by western blotting [24]. Briefly, KNG cells were lysed, and 30 µg of protein was separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes. Subsequently, all membranes were blocked with 5% skimmed milk diluted in TBS at 37˚C for 1 h and washed thrice with TBST. The membranes were then incubated with rabbit polyclonal USP48 (dilution 1:500, ab237765, Abcam, San Diego, CA, USA) and rabbit monoclonal antibodies against GAPDH (dilution 1:10,000, AF7021, Affinity) at 4˚C for 12 h. The membranes were washed thrice with TBST. Secondary antibodies were detected using Goat Anti-Rabbit IgG H&L (HRP) (dilution, 1:10,000, ab205718, Abcam) and visualized using an enhanced chemiluminescent reagent (PerkinElmer Life Sciences, MA, USA).
Statistical analyses
The GEO database (https://www.ncbi.nlm.nih.gov/geo/) was used to analyze the differences in mRNA expression in the ovarian GCs of patients with PCOS. The search was conducted using the keywords [(granulosa cell) AND (polycystic ovary syndrome)] AND “Homo sapiens” in “GEO DataSets.” Further filtration was performed to identify datasets amenable to use “Analyze with GEO2R.” GSE106724, GSE137684, and GSE80432 were chosen to analyze differentially expressed genes in ovarian GCs of patients with PCOS according to GEO2R analysis. All data are presented as means ± standard deviation (SD) and were evaluated using SPSS analysis (SPSS Inc., U.S.A.). The significance of differences between control, H2O2, H2O2 + 0 µmol/L BAI, and H2O2 + 20 µmol/L BAI groups was assessed using one-way ANOVA with post hoc Dunnett’s multiple comparisons test used for comparison between two groups. Differences between the blank, siNC, and siUSP48 groups were assessed using one-way ANOVA. Statistical significance was set at p < 0.05.
Results
BAI treatment enhances the proliferation of H2O2-stimulated KGN cells
To identify the therapeutic potential of BAI against oxidative stress, KGN cells were treated with H2O2 for 24 h to mimic characteristics of oxidative stress and then treated with BAI. The molecular structure of BAI is shown in Fig. 1A. H2O2 inhibited KGN cell proliferation in a concentration-dependent manner (Fig. 1B). For BAI concentrations below 60 µmol/L, the treatment exhibited no notable impact on the proliferation of KGN cells when contrasted with the 0 µmol/L BAI treatment. However, 80 µmol/L BAI treatment could inhibit the proliferation of KGN cells (Fig. 1C). Next, 100 µmol/L H2O2 (≈IC30)-treated KGN cells were used as an in vitro model. At concentrations < 40 µmol/L, BAI promoted H2O2-induced KGN cell proliferation in a concentration-dependent manner (Fig. 1D). Proliferation in the 80 µmol/L treatment group was lower than that in the 40 µmol/L treatment group, indicating that BAI concentrations > 40 µmol/L inhibited proliferation (Fig. 1D). No significant difference in cell proliferation was observed between 20 and 40 µmol/L BAI treatment groups, whereas both the 20 and 40 µmol/L BAI treatment groups exhibited higher cell proliferation than the 10 µmol/L BAI treatment group (Fig. 1D). We selected the minimum concentration of BAI that demonstrated the most significant promotion of cell proliferation for subsequent experiments. Based on these results, we selected 20 µmol/L BAI for our subsequent studies. The findings also demonstrated that 100 µmol/L H2O2 promoted KGN cell apoptosis, whereas 20 µmol/L BAI treatment inhibited the apoptosis of H2O2-stimulated KGN cells (Fig. 1E).
BAI promotes cellular proliferation and inhibits apoptosis of H2O2-stimulated KGN cells. A The molecular structure of BAI. B After H2O2 treatment for 24 h, the effect of different H2O2 concentrations on KGN proliferation was assessed using CCK8. *p < 0.05 vs. 0 µmol/L H2O2. C After BAI treatment at 24 h, the effect of different BAI concentrations on KGN proliferation was assessed using CCK8. *p < 0.05 vs. 0 µmol/L BAI. D After co-treatment with H2O2 and BAI for 24 h, the effects of different BAI concentrations on H2O2-stimulated KGN cell proliferation were analyzed using CCK8. #p < 0.05 H2O2 vs. Control group; *p < 0.05 vs. H2O2 group. E After co-treatment with H2O2 and BAI at 24 h, the effect of BAI on apoptosis of H2O2-stimulated KGN was analyzed using an apoptosis kit. *p < 0.05, #p < 0.05
BAI ameliorated oxidative stress and inflammation in H2O2-stimulated KGN cells
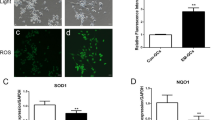
To determine whether BAI mitigates oxidative stress and inflammation in H2O2-stimulated KGN cells, these cells were treated with 20 µmol/L BAI for 24 h. Compared to the control group, 100 µmol/L of H2O2 increased ROS and MDA levels while reducing GSH-PX, CAT, and SOD levels (Fig. 2A and B). Treatment with 20 µmol/L BAI reduced ROS and MDA levels while enhancing GSH-PX, CAT, and SOD levels in H2O2-stimulated KGN cells (Fig. 2A and B). Compared to the control group, 100 µmol/L H2O2 enhanced IL-1β, IL-6, IL-8, and TNF-α levels in KGN cells, whereas 20 µmol/L BAI reduced IL-1β, IL-6, and TNF-αlevels in H2O2-stimulated KGN cells (Fig. 2C).
BAI treatment reduced oxidative stress and inflammation in H2O2-stimulated KGN cells. A ROS levels were measured by flow cytometry after BAI treatment for 24 h in H2O2-stimulated KGN cells. B MDA, GSH-PX, CAT, and SOD levels were measured using specific kits after BAI treatment for 24 h in H2O2-stimulated KGN cells. C IL-1β, IL-6, IL-8, and TNF-α levels were measured by ELISA after BAI treatment for 24 h in H2O2-stimulated KGN cells. #p < 0.05 and *p < 0.05
Screening for potential target genes of BAI in H2O2-stimulated KGN cells
According to GEO analysis, SNX29, MDM2, and USP48 were found at the intersection of GSE106724, GSE137684, and GSE80432 (Fig. 3A). Compared with the control group, 100 µmol/L H2O2 enhanced the mRNA expression of SNX29, MDM2, and USP48 in KGN cells. Furthermore, mRNA expression of only USP48 was significantly enhanced after treatment with 20 µmol/L BAI for 24 h (Fig. 3B). Next, USP48 protein was significantly inhibited in H2O2-treated KGN cells. This inhibition was reversed significantly after treatment with 20 µmol/L BAI (Fig. 3C).
USP48 is a potential target gene of BAI in H2O2-stimulated KGN cells. A The intersection of GSE106724, GSE137684, and GSE80432 included SNX29, MDM2, and USP48 genes. B The mRNA levels of SNX29, MDM2, and USP48 were measured by qRT-PCR after BAI treatment for 24 h in H2O2-stimulated KGN cells. C USP48 protein levels were measured by western blot after BAI treatment for 24 h in H2O2-stimulated KGN cells. #p < 0.05 and *p < 0.05
USP48 knockdown reversed the enhancement effect of BAI on the proliferation of H2O2-induced KGN cells
To examine whether USP48 influenced the inhibitory effect of BAI on the proliferation of H2O2-induced KGN cells, siUSP48 was transfected into KGN cells. USP48 mRNA expression was reduced in KGN cells after transfection (Fig. 4A). Then, the transfected KGN cells were treated with 100 µmol/L H2O2 and 20 µmol/L BAI for 24 h. The mRNA and protein levels of USP48 were reduced in transfected KGN cells (Fig. 4B and C). Compared to the H2O2 + BAI + siNC group, proliferation was inhibited in the H2O2 + BAI + siUSP48-1 and H2O2 + BAI + siUSP48-2 groups (Fig. 4D). In addition, apoptosis was enhanced in the H2O2 + BAI + siUSP48-1 group compared to that in the H2O2 + BAI + siNC group (Fig. 4E).
USP48 knockdown inhibited the proliferation of H2O2-stimulated and BAI-treated KGN cells. A USP48 mRNA levels in KGN cells were measured by qRT-PCR after siUSP48 transfection for 24 h without H2O2 and BAI treatment. B and C The mRNA and protein levels of USP48 in H2O2-stimulated KGN cells in response to BAI treatment were measured by qRT-PCR and western blotting after transfection with siUSP48-1 and siUSP48-2 at 24 h. D The effect of USP48 knockdown on BAI-treated H2O2-induced KGN cell proliferation was analyzed using CCK8 after transfection at 24 h. E The effect of USP48 knockdown on apoptosis in BAI-treated H2O2-stimulated KGN cells was analyzed using an apoptosis kit after siUSP48-1 transfection at 24 h. *p < 0.05
USP48 knockdown reversed the inhibitory effects of BAI on oxidative stress and inflammation in H2O2-stimulated KGN cells
The transfected KGN cells were treated with 100 µmol/L H2O2 and 20 µmol/L BAI for 24 h. Compared with the H2O2 + BAI + siNC group, the levels of ROS and MDA were enhanced, whereas those of GSH-PX, CAT, and SOD were reduced in the H2O2 + BAI + siUSP48-1 group (Fig. 5A and B). The levels of IL-1β, IL-6, IL-8, and TNF-α were also increased in the H2O2 + BAI + siUSP48-1 group (Fig. 5C).
USP48 knockdown reversed the inhibitory effect of BAI on oxidative stress and inflammation in H2O2-stimulated KGN cells. KGN cells in three groups were treated with 100 µmol/L H2O2 and 20 µmol/L BAI for 24 h. A ROS levels were measured by flow cytometry after siUSP48-1 transfection at 24 h. B The levels of MDA, GSH-PX, CAT, and SOD were measured after siUSP48-1 transfection at 24 h. C The levels of IL-1β, IL-6, IL-8, and TNF-α were measured by ELISA after siUSP48-1 transfection at 24 h. *p < 0.05
Discussion
Oxidative stress involves a disturbance in the balance between prooxidants and antioxidants, which tends to favor an oxidized state. SOD, CAT, and GSH-Px are antioxidants that eliminate excess ROS and inhibit oxidative stress. Conversely, ROS, which are prooxidants, can generate free radicals and hydrogen peroxide when synthesized in excess, thereby promoting oxidative stress and induce cell apoptosis [25]. GCs are sensitive to oxidative stress, which contributes to impaired steroid synthesis and inflammation and consequently to the dysfunction and apoptosis of GCs [26, 27]. GCs support the energy metabolism of oocytes by providing substances that oocytes cannot produce, thereby supporting oocyte development and controlling meiotic division. Conversely, oocytes actively regulate the development and function of GCs by secreting various growth factors, promoting their proliferation and preventing apoptosis [28]. In addition, oocytes work in conjunction with estrogen to regulate the development and functionality of granulosa cells by promoting the expression of FOXL2 [29]. Progesterone also inhibits apoptosis in granulosa cells [30]. Hence, while oocytes, estrogen, and progesterone regulate the proliferation of GCs, GCs support the normal development of oocytes and secretion of estrogen and progesterone. Therefore, oxidative stress-induced apoptosis of GCs can affect oocyte development. Conversely, abnormally developed oocytes further inhibit the proliferation of GCs. Abnormal oxidative stress and inflammation in GCs are closely associated with variations in chemical toxins (MC-LR), PM2.5, air pollution, and heavy metal (lead) [31,32,33]. Previous studies have indicated the activation of the PI3K-AKT, MAPK, FOXO, Nrf2, NF-κB, and AMPK pathways during the process of oxidative stress within GCs [7, 34]. H2O2 is an intermediate product of oxidative stress and is further degraded in water by CAT and GSH-PX. Elevated levels of H2O2 within the cell may lead to the generation of more toxic hydroxyl radicals, resulting in breaks and damage to the DNA strand, ultimately leading to apoptosis [25]. In the ovary, there is a transient increase in ROS levels after a surge in gonadotropins before ovulation; this elevation serves as a necessary signal for ovulation. It appears that primordial and small follicles are more resistant to H2O2 than other ovarian cell types, and H2O2 does not induce small follicular atresia in cultured neonatal ovaries nor does it result in apoptosis of primordial follicles [35]. However, H2O2 treatment enhances oxidative stress and inflammation, promoting GC apoptosis via the Nrf2/HO-1, ER stress, mTOR, and JNK/FOXO1 pathways [12, 17, 36, 37]. GC apoptosis is associated with a reduction in primordial follicular activity, PCOS, and other diseases associated with reduced ovarian functional reserves [28,29,30]. This study also showed that 100 µmol/L H2O2 treatment inhibited cell proliferation and promoted apoptosis, accompanied by increased MDA, IL-1β, IL-6, IL-8, and TNF-α levels and reduced SOD, CAT, and GSH-PX activities in KGN cells. These results suggest that H2O2 treatment enhanced oxidative stress and inflammation and promoted GC apoptosis.
BAI can inhibit cell skeleton rearrangement caused by loss of actomyosin stress fibers, regulate nuclear translocation of proteins, and suppress protein translation in ribosomes, thereby playing a beneficial role in disease treatment [38,39,40]. Accumulating evidence indicates that BAI reduces oxidative stress and inflammation. BAI treatment can reduce ROS levels, enhance cellular antioxidant capacity, and reduce intestinal epithelial cell apoptosis and skin aging under conditions involving H2O2 treatment [41, 42]. BAI treatment reduces oxidative stress and inflammation and ameliorates myocardial, nerve, and acute liver injuries [43,44,45]. In addition, 50 µM BAI treatment can reverse H2O2-induced GC cell apoptosis, upregulate the expression of P450arom and stAR, and increase the secretion of estradiol and progesterone [12]. Our results also showed that BAI exhibited antioxidant and anti-inflammatory effects in H2O2-stimulated KGN cells and reduced KGN apoptosis. Periplaneta americana, polydatin, and caffeic acid inhibit inflammation and oxidative stress, improve GC apoptosis, and enhance ovarian function [17, 32, 37]. The pharmacological mechanisms of these drugs are similar to those of BAI, in that they aim to improve oxidative stress and inflammation to exert their therapeutic effects. In addition, BAI treatment reduces testosterone concentrations and hormone secretion and promotes follicular development in PCOS models [13,14,15]. This evidence indirectly proves that BAI improves female reproductive system diseases, such as PCOS and premature ovarian failure. This may be related to improvements in oxidative stress-induced GC damage, further validating the therapeutic value of BAI. In addition, previous studies have indicated that BAI concentrations below 50 µM do not affect KGN survival, whereas concentrations above 80 µM can inhibit the survival of KGN [12, 15]. This study also demonstrates that under normal culture conditions, 80 µM BAI could inhibit KGN survival, whereas concentrations of BAI below 60 µM did not affect KGN survival. Under conditions involving H2O2 treatment, 10–40 µM BAI can improve KGN proliferation, with 20 µM and 40 µM being the most effective concentrations. Hence, the findings of this study propose that BAI concentrations ranging from 20 to 40 µM are advantageous for mitigating oxidative stress in KGN cells. Importantly, this concentration range aligns with the typical tolerable levels for KGN.
USP48 is a deubiquitination enzyme. Primarily localized in the nucleus, USP48 plays a crucial regulatory role in inherited retinal dystrophy (IRD) and ciliopathies by removing the ubiquitination of ARL3 and stabilizing UNC119a [22]. Similarly, USP48, primarily localized to the nucleus, impedes metabolic reprogramming to inhibit hepatocellular carcinoma development by removing K48-linked ubiquitination at the K33 and K128 sites of SIRT6 to stabilize SIRT6 [23]. USP48 promotes pyroptosis in cancer cells by removing K48-linked ubiquitination at positions K120 and K189 of the GSDME [21]. These studies indicate that USP48 can reverse disease progression via deubiquitination of targeted genes. In the present study, USP48 expression was reduced by H2O2 treatment and restored by BAI treatment. USP48 knockdown promoted inflammation, oxidative stress, and apoptosis in BAI-treated H2O2-stimulated KGN cells, indicating that USP48 knockdown abrogated the protective effects of BAI. These results show that BAI inhibited inflammation and oxidative stress by enhancing USP48 protein expression. In addition, these results showed that the functionality of USP48 is associated with inflammation and oxidative stress in GCs. Notably, USP48 inhibits NF-κB activity by regulating RelA deubiquitination to reduce cell inflammation and apoptosis [46, 47]. The activation of the PI3K/AKT/NF-κB signaling pathway in GCs of patients with PCOS contributes to an increase in the levels of ROS, IL-1β, IL-6, IL-8, and TNF-α [48]. Exposure of GCs to lead inhibits the expression of NRF2, activates NF-κB, increases levels of ROS and TNF-a, and decreases the expression of the antioxidant factors SOD and CAT [31]. Hence, NF-κB signaling pathway can enhance inflammation and oxidative stress in GCs. It may act as a key signaling pathway that links USP48 to inflammation and oxidative stress. However, the regulatory role of USP48 in the NF-κB pathway requires further research.
This study has some limitations. Firstly, the expression of USP48 in the ovarian tissue of patients with PCOS is not yet fully understood, precluding a definitive determination of its precise role in the pathogenesis of PCOS. Second, it remains unclear whether BAI directly binds to USP48 or regulates USP48 through other pathways, necessitating further research to elucidate their regulatory mechanisms. Additionally, the target genes and signaling pathways regulated by USP48, such as NF-KB, require further investigation to reveal their detailed mechanisms.
Conclusion
In summary, 20 µmol/L BAI treatment improved cell proliferation and reduced oxidative stress and inflammation by enhancing USP48 protein expression in 100 µmol/L H2O2-stimulated KGN cells. Thus, BAI could be used as a clinical drug to ameliorate oxidative stress-induced damage in GCs.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- BAI:
-
Baicalin
- CAT:
-
Catalase
- CCK-8:
-
Cell Counting Kit-8
- GCs:
-
Granulosa cells
- GSH-Px:
-
Glutathione peroxidase
- IL-1β:
-
Interleukin 1β
- MDA:
-
Malondialdehyde
- PCOS:
-
Polycystic ovarian syndrome
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor-α
References
Baldani DP, Skrgatic L, Ougouag R. Polycystic ovary syndrome: important underrecognised cardiometabolic risk factor in reproductive-age women. Int J Endocrinol. 2015;2015:786362.
Fu J, Liu Y, Wang C, Zhang H, Yu B, Wang Y, Zhu H. Persistent follicular granulosa cell senescence and apoptosis induced by methotrexate leading to oocyte dysfunction and aberrant embryo development. Clin Transl Sci. 2021;14(5):2043–54.
Li H, Wang X, Mu H, Mei Q, Liu Y, Min Z, Zhang L, Su P, Xiang W. Mir-484 contributes to diminished ovarian reserve by regulating granulosa cell function via YAP1-mediated mitochondrial function and apoptosis. Int J Biol Sci. 2022;18(3):1008–21.
Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(3):881–7.
Alam F, Syed H, Amjad S, Baig M, Khan TA, Rehman R. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr Res Physiol. 2021;4:119–24.
Shi YQ, Zhu XT, Zhang SN, Ma YF, Han YH, Jiang Y, Zhang YH. Premature ovarian insufficiency: a review on the role of oxidative stress and the application of antioxidants. Front Endocrinol (Lausanne). 2023;14:1172481.
Liu S, Jia Y, Meng S, Luo Y, Yang Q, Pan Z. Mechanisms of and potential medications for oxidative stress in ovarian granulosa cells: a review. Int J Mol Sci. 2023;24(11): 9205.
Bao M, Ma Y, Liang M, Sun X, Ju X, Yong Y, Liu X. Research progress on pharmacological effects and new dosage forms of baicalin. Vet Med Sci. 2022;8(6):2773–84.
Sharawi ZW, Ibrahim IM, Abd-Alhameed EK, Althagafy HS, Jaber FA, Harakeh S, Hassanein EHM. Baicalin and lung diseases. Naunyn Schmiedebergs Arch Pharmacol. 2023:https://doi.org/10.1007/s00210-00023-02704-00211.
Hu Q, Hou S, Xiong B, Wen Y, Wang J, Zeng J, Ma X, Wang F. Therapeutic effects of Baicalin on diseases related to gut-brain axis dysfunctions. Molecules. 2023;28(18):6501.
Xin L, Gao J, Lin H, Qu Y, Shang C, Wang Y, Lu Y, Cui X. Regulatory mechanisms of baicalin in cardiovascular diseases: a review. Front Pharmacol. 2020;11:583200.
Fan H, He J, Bai Y, He Q, Zhang T, Zhang J, Yang G, Xu Z, Hu J, Yao G. Baicalin improves the functions of granulosa cells and the ovary in aged mice through the mTOR signaling pathway. J Ovarian Res. 2022;15(1):34.
Wang W, Zheng J, Cui N, Jiang L, Zhou H, Zhang D, Hao G. Baicalin ameliorates polycystic ovary syndrome through AMP-activated protein kinase. J Ovarian Res. 2019;12(1):109.
Yu J, Liu Y, Zhang D, Zhai D, Song L, Cai Z, Yu C. Baicalin inhibits recruitment of GATA1 to the HSD3B2 promoter and reverses hyperandrogenism of PCOS. J Endocrinol. 2019;240(3):497–507.
Xu X, Xu X, Wang X, Shen L. Baicalin suppress the development of polycystic ovary syndrome via regulating the miR-874-3p/FOXO3 and miR-144/FOXO1 axis. Pharm Biol. 2023;61(1):878–85.
Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142(1):437–45.
Chiang YF, Lin IC, Huang KC, Chen HY, Ali M, Huang YJ, Hsia SM. Caffeic acid’s role in mitigating polycystic ovary syndrome by countering apoptosis and ER stress triggered by oxidative stress. Biomed Pharmacother. 2023;166: 115327.
Yang Z, Hong W, Zheng K, Feng J, Hu C, Tan J, Zhong Z, Zheng Y. Chitosan oligosaccharides alleviate H(2)O(2)-stimulated granulosa cell damage via HIF-1α signaling pathway. Oxid Med Cell Longev. 2022;2022:4247042.
Lockhart PJ, Hulihan M, Lincoln S, Hussey J, Skipper L, Bisceglio G, Wilkes K, Farrer MJ. Identification of the human ubiquitin specific protease 31 (USP31) gene: structure, sequence and expression analysis. DNA Seq. 2004;15(1):9–14.
Yu J, Hou B, Huang Y, Wang X, Qian Y, Liang Y, Gu X, Ma Z, Sun Y. USP48 alleviates bone cancer pain and regulates MrgC stabilization in spinal cord neurons of male mice. Eur J Pain. 2023;27(6):723–34.
Ren Y, Feng M, Hao X, Liu X, Li J, Li P, Gao J, Qi Q, Du L, Wang C, et al. USP48 stabilizes gasdermin E to promote pyroptosis in cancer. Cancer Res. 2023;83(7):1074–93.
Sánchez-Bellver L, Férriz-Gordillo A, Carrillo-Pz M, Rabanal L, Garcia-Gonzalo FR, Marfany G. The deubiquitinating enzyme USP48 interacts with the retinal degeneration-associated proteins UNC119a and ARL3. Int J Mol Sci. 2022;23(20): 12527.
Du L, Li Y, Kang M, Feng M, Ren Y, Dai H, Wang Y, Wang Y, Tang B. USP48 is upregulated by Mettl14 to attenuate hepatocellular carcinoma via regulating SIRT6 stabilization. Cancer Res. 2021;81(14):3822–34.
Bian W, Xiao S, Yang L, Chen J, Deng S. Quercetin promotes bone marrow mesenchymal stem cell proliferation and osteogenic differentiation through the H19/miR-625-5p axis to activate the Wnt/β-catenin pathway. BMC Complement Med Ther. 2021;21(1):243.
Alkadi H. A review on free radicals and antioxidants. Infect Disord Drug Targets. 2020;20(1):16–26.
Wang J, Wu J, Zhang Y, Zhang J, Xu W, Wu C, Zhou P. Growth hormone protects against ovarian granulosa cell apoptosis: alleviation oxidative stress and enhancement mitochondrial function. Reprod Biol. 2021;21(2):100504.
Luderer U. Ovarian toxicity from reactive oxygen species. Vitam Horm. 2014;94:99–127.
Sugiura K, Maruyama N, Akimoto Y, Matsushita K, Endo T. Paracrine regulation of granulosa cell development in the antral follicles in mammals. Reprod Med Biol. 2023;22(1):e12538.
Emori C, Wigglesworth K, Fujii W, Naito K, Eppig JJ, Sugiura K. Cooperative effects of 17β-estradiol and oocyte-derived paracrine factors on the transcriptome of mouse cumulus cells. Endocrinology. 2013;154(12):4859–72.
Peluso JJ, Fernandez G, Pappalardo A, White BA. Characterization of a putative membrane receptor for progesterone in rat granulosa cells. Biol Reprod. 2001;65(1):94–101.
Aglan HS, Gebremedhn S, Salilew-Wondim D, Neuhof C, Tholen E, Holker M, Schellander K, Tesfaye D. Regulation of Nrf2 and NF-κB during lead toxicity in bovine granulosa cells. Cell Tissue Res. 2020;380(3):643–55.
Zhou S, Xi Y, Chen Y, Zhang Z, Wu C, Yan W, Luo A, Wu T, Zhang J, Wu M, et al. Ovarian dysfunction induced by chronic whole-body PM2.5 exposure. Small. 2020;16(33):e2000845.
Wu J, Yuan M, Song Y, Sun F, Han X. MC-LR exposure leads to subfertility of female mice and induces oxidative stress in granulosa cells. Toxins (Basel). 2015;7(12):5212–23.
Ji R, Jia FY, Chen X, Wang ZH, Jin WY, Yang J. Salidroside alleviates oxidative stress and apoptosis via AMPK/Nrf2 pathway in DHT-induced human granulosa cell line KGN. Arch Biochem Biophys. 2022;715:109094.
Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86(2):27.
Chen X, Song QL, Li ZH, Ji R, Wang JY, Cao ML, Mu XF, Zhang Y, Guo DY, Yang J. Pterostilbene ameliorates oxidative damage and ferroptosis in human ovarian granulosa cells by regulating the Nrf2/HO-1 pathway. Arch Biochem Biophys. 2023;738:109561.
Kong C, Su J, Wang Q, Liu K, Fu R, Sui S. Signaling pathways of Periplaneta Americana peptide resist H(2)O(2)-induced apoptosis in pig-ovary granulosa cells through FoxO1. Theriogenology. 2022;183:108–19.
Qin S, Huang X, Qu S. Baicalin induces a potent innate immune response to inhibit respiratory syncytial virus replication via regulating viral non-structural 1 and matrix RNA. Front Immunol. 2022;13: 907047.
Yang J, Hai Z, Hou L, Liu Y, Zhang D, Zhou X. Baicalin attenuates panton-valentine leukocidin (PVL)-induced cytoskeleton rearrangement via regulating the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β pathways in bovine mammary epithelial cells. Int J Mol Sci. 2023;24(19): 14520.
Zhang L, Yu G, Yu Q, Wang L, Wu L, Tao Z, Ding J, Lin D. Baicalin promotes random-pattern skin flap survival by inducing autophagy via AMPK-regulated TFEB nuclear transcription. Phytother Res. 2023;37(9):3926–38.
Liang J, Zhou Y, Cheng X, Chen J, Cao H, Guo X, Zhang C, Zhuang Y, Hu G. Baicalin attenuates H(2)O(2)-Induced oxidative stress by regulating the AMPK/Nrf2 signaling pathway in IPEC-J2 cells. Int J Mol Sci. 2023;24(11):9435.
Kim G, Han DW, Lee JH. The cytoprotective effects of Baicalein on H(2)O(2)-Induced ROS by maintaining mitochondrial homeostasis and cellular tight Junction in HaCaT keratinocytes. Antioxid (Basel). 2023;12(4):902.
Li J, Yang Y, Wang H, Ma D, Wang H, Chu L, Zhang Y, Gao Y. Baicalein ameliorates myocardial ischemia through reduction of oxidative stress, inflammation and apoptosis via TLR4/MyD88/MAPK(S)/NF-κB pathway and regulation of ca(2+) homeostasis by L-type ca(2+) channels. Front Pharmacol. 2022;13:842723.
Mao X, Cao Y, Li X, Yin J, Wang Z, Zhang Y, Mao C, Fan K, Zhou H, Cai J, et al. Baicalein ameliorates cognitive deficits in epilepsy-like tremor rat. Neurol Sci. 2014;35(8):1261–8.
Zhou HC, Wang H, Shi K, Li JM, Zong Y, Du R. Hepatoprotective effect of baicalein against acetaminophen-induced acute liver injury in mice. Molecules. 2018;24(1):131.
Jantaree P, Chaithongyot S, Sokolova O, Naumann M. USP48 and A20 synergistically promote cell survival in helicobacter pylori infection. Cell Mol Life Sci. 2022;79(8):461.
Mirra S, Sánchez-Bellver L, Casale C, Pescatore A, Marfany G. Ubiquitin specific protease USP48 destabilizes NF-κB/p65 in retinal pigment epithelium cells. Int J Mol Sci. 2022;23(17):9682.
Zhao Y, Zhang C, Huang Y, Yu Y, Li R, Li M, Liu N, Liu P, Qiao J. Up-regulated expression of WNT5a increases inflammation and oxidative stress via PI3K/AKT/NF-κB signaling in the granulosa cells of PCOS patients. J Clin Endocrinol Metab. 2015;100(1):201–11.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
WB designed the experiments. JC, CL, XH performed the experiments, contributed data collection and analysis. JC wrote the manuscript and WB critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, J., Lin, C., Huang, X. et al. Baicalin enhances proliferation and reduces inflammatory-oxidative stress effect in H2O2-induced granulosa cells apoptosis via USP48 protein regulation. BMC Complement Med Ther 24, 42 (2024). https://doi.org/10.1186/s12906-024-04346-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-024-04346-z