Abstract
Background
Nigella sativa (NS) oil has been found to have advantageous benefits in the management of inflammation and obesity. This study investigated the effect of NS supplementation on blood mRNA expressions and serum levels of IL-1β, IL-6, leptin, and insulin concentrations in overweight/obese women.
Methods
In a crossover design, participants were randomized to receive either NS supplements(2000 mg/day) or placebo for 2 durations(8 weeks). With between-subject and within-subject components and interactions, a repeated-measure ANOVA model was used considering the treatment, time, and the carryover effects. Cohen’s d(d) was used to measure the magnitude of the effects.
Results
Forty-six eligible participants were included. NS supplementation significantly reduced the mRNA expressions(d=-0.68, P = 0.03) and serum levels of IL-1β with medium-high effect sizes(d=-1.6, P < 0.001). Significant reductions with large effect sizes were observed in the gene expression and serum levels of IL-6(d=-1.8, d=-0.78, respectively; P < 0.01) and Leptin(d=-1.9, d=-0.89, respectively; P < 0.01, serum leptin P carryover < 0.001). Despite the meaningful carryover effect for serum leptin, results remained significant following the first intervention period analysis(P < 0.001). A significant but low effect size decrease in serum insulin was observed(d=-0.3, P = 0.02).
Conclusions
The clinical significance of present findings regarding improvements in obesity-related pro-inflammatory markers must be interpreted with caution due to some observed medium-low effect sizes.
Trial registration
IRCT20180430039475N1 (Date:25/6/2018).
Similar content being viewed by others
Background
Overweight and obesity are major global public health problems [1]. Nearly 40% of the world’s population is overweight or obese, which is predicted to rise steadily by 2030 [2]. Pro-inflammatory adipokine production increases as adipose tissue expands in obesity, contributing to metabolic disorders associated with obesity [3]. Obesity is linked to chronic inflammation, as well as alterations in the associated gene expression and lifestyle factors [4].
Pro-inflammatory interleukin-β (IL-1β) is a significant cytokine mainly produced by macrophages that plays a role in the development of obesity-associated insulin resistance [5]. Interleukin-6 (IL-6) is another cytokine generally referred to as an obesity-related inflammatory marker [6]. The elevated level of circulating IL-6 is correlated with adiposity and insulin resistance in humans [6]. Obese individuals are reported to have higher levels of serum IL-1β and IL-6 [7]. Adipose tissues also produce leptin, a hormone directly associated with the amount of fat stored [8]. Although it has a role in regulating food intake and body weight, leptin resistance is reported in obese individuals due to hyperinsulinemia, leading to reduced satiety and overeating [9]. Literature also suggests that leptin has pro-inflammatory properties by up-regulating cytokines such as IL-6 [10]. IL-1β may also increase the expression of leptin mRNA in adipose tissue, adding to the underlying causes of obesity development [11]. The up-regulation of these pro-inflammatory factors creates a vicious circle, reducing the efficacy of obesity prevention and control efforts [11].
Dietary constituents and herbal extracts rich in phytochemicals have been recommended as affordable, effective, and safe supplemental therapies to help regulate inflammatory factors [12]. Phytochemicals are natural antioxidants with anti-inflammatory properties. The beneficial effects of phytochemicals and their derivatives on down-regulating the gene expression of pro-inflammatory and inflammatory markers have been well studied [13, 14]. Nigella sativa (NS), commonly known as black seed, is an herb from the Ranunculaceae family with a broad spectrum of pharmacological properties. Literature suggests that NS and its main active component, thymoquinone(TQ), have anti-hypertensive, immune-stimulatory, and weight-regulatory properties [15]. In historical medical practices, Nigella sativa (NS) oil found extensive application in addressing gastrointestinal issues like indigestion, bloating, loose stools, and inflammatory bowel conditions, alongside its utility for various other health concerns such as respiratory challenges like asthma, bronchial spasms, and congested chests. Furthermore, NS oil has been recognized for its potential as a liver tonic, diuretic, enhancer of appetite, pain-reliever, and digestive aid. It has also exhibited potential in impacting lipid metabolism, potentially assisting in the control of cholesterol levels [16].
Previous animal and experimental studies have also reported the beneficial effects of NS oil or TQ supplementation on pro-inflammatory and inflammatory markers reporting a reduction in leptin and IL-6 levels in rats with metabolic disorders [17, 18].
In humans, a few randomized controlled clinical trial (RCT) studies investigated the effect of NS oil supplements on pro-inflammatory markers and reported controversial results [19,20,21]. A recent study showed that a 3-month supplementation with black seed oil significantly reduced serum IL-6 and inflammatory markers in patients with chronic pulmonary disease [19]. However, no significant effects were observed on serum IL-6 levels after NS oil supplementation combined with a low-calorie diet in overweight and obese women [20]. NS oil supplementation in patients with type-2 diabetes mellitus also resulted in a significant drop in blood leptin concentration [21]. Literature on the effects of NS oil or TQ supplementation on IL-1β is scarce. Also, there is no clinical trial investigating the mechanisms of the effects of NS or TQ on pro-inflammatory cytokines in individuals with overweight or obesity. According to the evidence, the development or progression of obesity would also be independently shown by changes in peripheral blood mononuclear cell (PBMC) gene expression profiles. Therefore, this study aimed to investigate the effect of NS oil supplementation on serum concentrations and gene expression of IL-1β, IL-6, and leptin in PBMCs as well as insulin levels in a sample of overweight and obese women. The findings of this study may guide the development of more effective therapies and interventions to prevent and manage overweight and obesity in adult populations.
Materials
Study design and participants
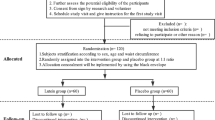
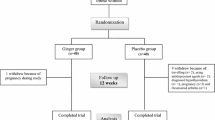
This study was a crossover, double-blind, placebo-controlled, randomized clinical trial with two intervention periods of 8 weeks each and a 4-week washout period. Findings regarding the effects of Nigella sativa on major adipogenesis-related parameters have been published previously [22]. The trial was designed following the Consolidated Standards of Reporting Trials (CONSORT) guidelines, revised for randomized crossover trials [23]. Individuals were recruited from the obesity clinic at Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Otherwise healthy women aged 25 to 55 with a body mass index (BMI) between 25 and 35 kg/m2 were eligible to participate. Participants were not included if they had comorbidities conditions such as type-2 diabetes mellitus, cardiovascular diseases, hyperlipidemia, pancreatic or hepatic disorders, thyroid or renal diseases, were pregnant or breastfeeding, or smoked or consumed alcohol. Those with a history of surgery, allergy, or cancer, as well as those taking obesity medications, nutritional supplements, or participating in a specific weight-loss program for at least six months prior to the study’s start date, were also excluded.
An independent researcher blinded to the study randomized participants into two groups (1:1) of NS and placebo using block randomization and computer-generated random numbers stratified based on age. Concealment was performed using sealed envelopes. Literature suggests at least 1000 mg/day of NS oil supplementation is required to observe significant changes in leptin concentration [21], and that a daily intake of 2000 mg of NS is well-tolerated with no adverse reactions [24]. Participants were given either 2000 mg/day of NS oil supplement (two capsules of 1000 mg/day oil each) or two capsules/day of a paraffin oil placebo (2000 mg in each capsule). Nigella sativa and placebo capsules were provided by Barij Essence Pharmaceutical Company (Kashan, Iran). The NS oil was extracted from the Ranunculaceae family grown in Iran and processed using the cold-press method. TQ was the most active component in each 1000 mg of black seed oil capsule, accounting for 1.1% of the total. The intervention and placebo capsules looked and tasted the same, and the bottles were labelled with unique codes. Additional file 1 presents the results of the gas chromatography-mass spectrometry (GC/MS) test on each 50 g of NS. Participants were instructed to consume one capsule before lunch and another before dinner. They were not allowed to alter their usual physical activities. Regular drugs, such as lipid-lowering agents or blood pressure meds, were permitted as long as the dosages were maintained during the trial. A four-week washout period was selected based on the literature to cover at least five times the half-life of the treatment intervention to eliminate the carryover effect [25]. The elimination half-life of oral administration of TQ, as the main constituent of NS, is reported to be around 275 min [26]. The washout time was extended to four weeks in this study to factor in the maximum elimination of the carryover effects. All participants and researchers were blinded to the study until the final analysis.
Individuals were also provided with an individualized diet plan without any calorie deficit to control for energy and macronutrient intakes during the study. The diet regimen was designed by a certified nutritionist based on every individual’s body weight at the start of each intervention phase. The required energy was first estimated, and then a dietary plan with portions from each food group was designed to meet the macronutrient distribution, which was 55% carbohydrates, 30% fat, and 1.2 g of protein per kg of body weight. During each intervention session, a certified dietitian monitored participant compliance with dietary intakes in both groups (at weeks 4, and 8). Participants were required to return the empty bottles or any remaining supplements at the end of each intervention period. Compliance with the intervention was also monitored three times per week through phone and face-to-face interviews, and any adverse reactions were recorded.
Sample size was calculated using the formula suggested for crossover studies [27]; after considering a non-inferiority margin of 0.05, a significance level of 0.05, a power of 80%, and anticipating a 15% dropout rate, a total of 40 participants were required for this crossover study.
Anthropometric, dietary intake, and physical activity assessments
Weight (to the nearest 0.1 kg precision), height (to the nearest 0.1 cm precision), and body composition of individuals in light clothing and an overnight fasted state were measured using a body analyzer (In-body, 770, USA) machine. BMI was computed by dividing weight (kg) by height (m2). Every participant’s dietary intake was measured twice during the study using 3-day food diaries (once at the start of each intervention period) and then converted to g/day using home measurements [28]. Physical activity level was assessed using a validated questionnaire based on metabolic equivalent [29]. The questionnaire comprises nine different types of physical activity, each with different levels of intensity. The sum of the average hours spent on one day for each activity depending on the quantity of MET equivalent was used to compute the overall number of MET hours per day (h/day).
Biochemical assessment
After a 12-h overnight fast, a venous blood sample (12 ml) was collected from participants at the beginning and end of each intervention period. Blood samples were then centrifuged for 10 min (3,000 g, at room temperature; Eppendorf AG, Hamburg), and serum samples were separated and stored at − 70°C. The enzyme-linked immunoassay (ELISA) method was used to assess serum levels of IL-1β, IL-6, leptin and insulin (Thermo Fisher Co., USA; inter and intra-assay coefficients of variations were < 10%).
RNA extraction and real-time polymerase chain reaction (RT-PCR)
Literature suggests that PBMCs reflect the effects of dietary modifications at the gene expression level [30]. As reported in the GETx portal (https://gtexportal.org), IL-1β, IL-6 and leptin genes have been identified in blood by RNA sequencing (Additional file 2). The standard Ficoll method was performed to isolate PBMCs from whole blood. Total RNA was extracted immediately using the Qiagen RNA purification kit (Qiagen Co., Germany, Cat No. 74,104). The spectrophotometer (NanoDrop, Thermo Scientific) was used for assessing the quality and purity (260/280 nm ratio between 1.8 and 2.2) of the extracted RNA. Then, total mRNA was reverse-transcribed into cDNA using a cDNA synthesis kit (Thermo Fisher Co., USA, Cat No. RR037).
The expressions of IL-1β, IL-6 and Leptin genes were quantified using real-time polymerase chain reaction (RT-PCR) along with the SYBR Green method (Takara Bio, Inc., Japan). To verify the product’s specificity, melting curve analyses were performed at the end of every run. Primers for RT-PCR were designed using Primer Blast, Oligocalc, and Gene-runner 5.0.99 (Table 1). All quantifications were normalized to glyceraldehyde phosphate dehydrogenase (GAPDH) as the housekeeping gene. The efficacy of PCR was examined using LinRegPCR software [31]. Finally, cycle threshold (Ct) values were obtained, and fold changes (FD) were calculated through ∆∆Ct method [32].
Statistical analysis
Means and confidence intervals (CI) were reported for continuous variables. Differences in baseline characteristics and outcomes of interest between groups were evaluated using independent samples t-test. Skewness and Kurtosis tests were performed to assess the normality of data. If any baseline variables did not have a normal distribution (P Skewness < 0.05), the BoxCox transformation was used before the final analysis. The final analysis was based on the intention-to-treat (ITT) method. All randomized individuals for whom relative data were available for at least the first period of intervention were considered as the ITT population. Specific statistical operations are required for the cross-over design. With between-subject and within-subject components and interactions, a repeated-measure ANOVA model was used considering the effect of treatment, time, and interaction between them, which was called as the carryover effect. If the carryover effect was found to be significant (residual P < 0.05), the results of the first intervention period were analyzed using ANCOVA test. Additionally, The magnitude of the treatment effects was then assessed using a one-sample t-test (two-sided, alpha level of 0.05), estimating Cohen’s d, for all participants with complete data, evaluating differences in outcome while on the NS oil period vs. changes in outcome when on the placebo period. The effect size was defined as small (Cohen’s d = 0.2), medium (Cohen’s d = 0.5), and large (Cohen’s d = 0.8). The Cohen’s d value of zero indicates that there are no differences between the means of the two comparative groups, and 50% of the observations in the control group are located below the mean of the experimental group. The Cohen’s d values of 0.2, 0.5, and 0.8 are located at the 58th, 69th, and 79th percentiles of the distribution of the control group, respectively [33]. A P < 0.05 was considered statistically significant. All analyses were performed using SPSS version 21.
Ethical approval
The protocol of the study was registered at the Iranian Registry of Clinical Trials (registration no. IRCT20180430039475N1, date:25/6/2018) [34]. Ethical approval was obtained from the Medical Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (approval no. IR.SSU.SPH. REC.1397.006) and the Committee of Ethics of Shahid Beheshti University of Medical Sciences (approval no. IR.SBMU.ENDOCRINE.REC.1400.084). All methods were performed in accordance with the Declaration of Helsinki. Participants were recruited in April 2019. They were provided with an information sheet and asked to complete an informed consent form prior to the study. Informed consent was obtained from all participants.
Results
Forty-six eligible women were included. A total of seven participants were lost to follow-up due to pregnancy (n = 1), a new diagnosis of disease (n = 4), and personal reasons (n = 2) (Fig. 1).
The baseline characteristics of participants are summarized in Table 2. The mean ± SD age, height, and BMI of participants were 36 ± 10 years, 159 ± 6.4 cm, and 32 ± 5 kg/m2, respectively. Baseline characteristics of physical activity status, dietary intake, medication, and serum biomarkers of IL-1β, IL-6, and leptin were not significantly different between the NS and placebo groups. Therefore, pooled baseline measures were used for the final analyses. No severe adverse reactions were reported. There was a significant decrease in BMI following the NS supplementation [mean change (SD)=-0.34 (0.62) kg/m2; the mean (SD) BMI was 31.02 (5.17) kg/m2 for the intervention period and 31.36 (5.30) kg/m2 for the placebo period; P effect = 0.004; P carryover effect = 0.09].
BMI, body mass index; MET, metabolic equivalent; PUFA, poly-unsaturated fatty acids; MUFA, mono-unsaturated fatty acids; SFA, saturated fatty acids; IL-1β, interleukin-1 beta; IL-6, interleukin-6.
Effects of Nigella sativa (black seed) oil supplement on mRNA gene expressions of IL-1β, IL-6, and Leptin
Figure 2 presents the changes in mRNA expression levels of IL-1β, IL-6 and leptin following NS or placebo supplementation. After accounting for the treatment, period, and carryover effects, NS supplementation led to a 0.21 fold change (FC) decrease in IL-1β gene expression with a medium effect (P carryover effect = 0.16 and P effect = 0.03, Cohen’s d=-0.68), a 0.17-FC decrease in the gene expression levels of IL-6 with a large effect size (P carryover effect = 0.11; P effect = 0.003, Cohen’s d=-1.8), and a 0.57-FC decrease in leptin mRNA gene expression with a relatively large effect size (P carryover effect = 0.67; P effect < 0.001, Cohen’s d=-1.9) compared to the placebo.
Fold changes (means ± SDs) in mRNA expression levels of IL-1β, IL-6 and Leptin in PBMCs during NS intervention period versus placebo period among women with overweight and obesity (n = 46)
A repeated-measure ANOVA model was used considering the effects of treatment, time, and interaction (carryover effect)
* P value < 0.05; ** P value < 0.001
Abbreviations: NS, Nigella Sativa; IL-1β, interleukin-1 beta; IL-6, interleukin-6
Changes in serum concentrations of IL-1β, IL-6, leptin and insulin
Consistent with the findings from RT-PCR, NS supplementation resulted in a significant reduction in serum concentrations of IL-1β with a large effect size (P effect < 0.001, P carryover effect = 0.31, Cohen’s d=-1.6), IL-6 with a medium-large effect size (P carryover effect = 0.08; P effect = 0.002 Cohen’s d=-0.78), and serum leptin concentration with a large effect size (P carryover effect < 0.001; P effect = 0.008, Cohen’s d=-0.89) compared to placebo (Table 3). Although a significant carryover effect was seen in the analysis of leptin concentration, a significantly lower level of leptin was still observed in the NS group compared to the placebo group at the end of the first treatment period (28.34 ± 6.5 ng/ml vs. 39.65 ± 7.2 pg/ml, respectively, P < 0.001). Results also showed a significant reduction but with a low effect size in serum fasting insulin levels following NS supplementation (P effect = 0.02, P carryover effect = 0.81, Cohen’s d=-0.3).
Discussion
This study meticulously investigated the effect of 2000 mg/day NS oil supplementation on blood mRNA gene expressions and serum concentrations of major obesity-related pro-inflammatory factors in overweight and obese women. Our findings suggested a significant down-regulation in the mRNA expression of IL-1β, IL-6, and Leptin. Similarly, significant reductions in the serum levels of IL-1β, IL-6, leptin, and insulin were observed following NS supplementation. These findings align with existing literature that suggests the beneficial effect of NS oil supplementation on leptin levels in patients with type-2 diabetes [21] and patients with non-alcoholic fatty liver disease [35]. Notably, the substantial reduction in serum leptin levels following NS supplementation holds clinical significance. A previous prospective RCT demonstrated that a reduction of 3.3 ng/mL in leptin levels over one month is a strong predictor of long-term weight loss in adults with overweight and obesity [36]. Our study recorded a mean decrease of 3.1 ng/mL after NS supplementation.
The observed significant reductions in blood insulin levels among overweight and obese women echo findings from a study by Mahdavi et al., where NS oil supplementation in combination with a low-calorie diet led to similar outcomes [20]. However, this prior study did not report significant changes in IL-6 measures. Discrepancies in results could arise from variations in study design, sample size, population characteristics, and intervention specifics such as types and doses of NS supplementation. It’s worth mentioning that follow-up studies have associated a median reduction of 0.16 pg/ml in IL-6 levels with a reduced hazard ratio of 1.44 in cardiovascular diseases [37] and lower adiposity measurements [38]. In line with this, our research showcased a considerable decrease in IL-6 levels. Furthermore, the observed significant reductions with comparatively large effect sizes in PBMC gene expressions of IL-6, IL-1β, and leptin suggest the potential for controlled inflammation in obesity after NS oil supplementation. These results resonate with evidence indicating that alterations in PBMC gene expression profiles are independently related to obesity’s onset or progression [4]. Consequently, the significant reductions in PBMC gene expressions of IL-6, IL-1β, and leptin, marked by their comparatively large effect sizes, collectively point toward a potential state of controlled inflammation in the realm of obesity subsequent to NS oil supplementation.
A clear connection exists between cholesterol levels and pro-inflammatory cytokines, such as IL-1β and IL-6, particularly among participants dealing with overweight and obesity [39]. Excess body fat, especially around organs, triggers ongoing inflammation, with adipose tissue releasing these cytokines. This inflammatory state disrupts metabolism, leading to lipid imbalances like elevated low-density lipoprotein cholesterol (LDL-C) and triglycerides, alongside lowered high-density lipoprotein cholesterol (HDL-C). Raised levels of these proinflammatory cytokines can worsen inflammation, accelerating the progression of atherosclerosis and potentially heightening the risk of cardiovascular events [39, 40]. In light of this, our study demonstrated reduced cytokine levels following NS interventions. This suggests that such interventions might have the potential to modify cytokine levels, ease inflammation, and ultimately lower the risk of future cardiovascular diseases, alongside promoting other aspects of a healthy lifestyle.
The plausible mechanism behind the anti-inflammatory effects of NS supplements lies in its long-chain unsaturated fatty acids, bioactive substances, and the regulation of IL-1, IL-6, and leptin gene expressions as pro-inflammatory markers [15]. This aligns with the inhibition of NF-κB, TNF-α, and IL-1 receptor-associated kinase (IRAK-1) by NS or its active component TQ, leading to the suppression of the mitogen-activated protein kinase (MAPK) pathway and down-regulation of gene expressions of pro-inflammatory markers [41]. These insights could also offer an explanation for the observed beneficial changes in BMI post-NS supplementation. Furthermore, the interplay between weight gain, inflammation, and NS’s potential role in expediting weight loss underscores the multifaceted approach NS might offer in obesity management [3]. Nonetheless, it’s noteworthy that the observed final mean changes in insulin levels among overweight and obese women in our study exhibited a relatively modest effect size. Therefore, it is crucial to approach the interpretation of these results, especially concerning the insulin status and the potential beneficial role of NS in managing insulin resistance in obesity, with careful consideration.
We recognize the importance of including obesity-related stress markers in our analysis to provide a more holistic view of our results. Our study concentrated on some of the most vital obesity stress markers, such as leptin and IL-6, which represent crucial facets of the intricate obesity-associated stress network. Leptin’s role beyond appetite regulation involves signaling potential resistance patterns in obesity. Similarly, IL-6, a key cytokine, has implications in inflammation and metabolic disturbances [42]. However, it is important to acknowledge that these markers are just a subset of the complex physiological responses engendered by obesity. Exploring stress markers like adrenaline and cortisol could yield insights into the broader stress adaptation mechanisms triggered by obesity.
This study has several strengths. To our knowledge, no human studies have assessed the effect of NS supplements on gene expression of pro-inflammatory cytokines, especially in overweight and obese women. Using a crossover design with a long carryover elimination period and effect size estimations improved the validity of the results observed [43]. Also, because the NS supplementation used in this study was purely NS oil extract, the effect observed can be attributed to NS alone, as compared to previous studies that investigated the effects of NS extracts combined with other substances such as honey [44] or herbal extracts [45]. To avoid the heterogeneity that could arise from including other groups (such as male populations), this study exclusively included overweight or obese women. Finally, due to the potential confounding effects of dietary consumption, all research participants were given a customized diet plan that did not include any calorie restrictions.
However, this study also has some limitations. While a significant reduction in leptin mRNA expression was observed following the two intervention periods of 8 weeks, the relative decrease in serum leptin concentration can only be attributed to the first treatment period, due to the significant carryover effect observed. This carryover effect could be due to the rapid short-term changes in serum leptin levels [46]. Moreover, despite the significant reduction in serum insulin, the effect size was estimated to be small which may limit the interpretation of results for the effect of NS on serum insulin levels or the status of insulin resistance. Further assessments may also be needed to accurately measure the half-life time of NS oil supplements. In this study, the blood or urine concentration of TQ, metabolites of NS, or other pro-inflammatory markers were not measured. This could help providing a better understanding of the anti-inflammatory effects of NS oil supplementation. In addition, limiting the study participants to women to reduce the gender bias limits the generalization of the findings as females may react differently to NS bioactive compounds than males [47].
Conclusions
In conclusion, our findings showed that daily supplementation of 2000 mg of NS oil after 2 periods of 8 weeks resulted in significant reductions in blood mRNA expression levels of obesity-related pro-inflammatory genes, including IL-6, IL-1β, and leptin. Serum levels of IL-6, IL-1β, leptin, and insulin were also significantly reduced but the changes in insulin levels are of minor clinical significance. As the changes in blood mRNA gene expressions of interested outcomes were with high effect sizes, these findings suggest that NS oil can be considered a beneficial supplemental therapy to help prevent and manage overweight and obesity, and reduce the burden of the disease in the general population. However, further interventions are required to confirm the carryover effects observed for leptin concentration and the small effect size estimated in serum insulin changes. Future studies investigating the effects of NS supplements on various pro-inflammatory markers in different genders and populations may help improve the generalizability of these findings. The use of the next-generation sequencing (NGS) method in future gene-diet interaction investigations is strongly recommended prior to assessing gene expression to have the most comprehensive genomic coverage and to more accurately detect variations of significance.
Data Availability
Data are available from the correspondence author upon reasonable request and with permission of Shahid Sadoughi Medical University.
Abbreviations
- BMI:
-
Body mass index
- cDNA:
-
Complementary DNA
- CI:
-
Confidence intervals
- CONSORT:
-
Consolidated Standards of Reporting Trials
- Ct:
-
Cycle threshold
- d:
-
Cohen’s d
- ELISA:
-
Enzyme-linked immunoassay
- FD:
-
Fold changes
- GAPDH:
-
Glyceraldehyde phosphate dehydrogenase
- GC/MS:
-
Gas chromatography-mass spectrometry
- IL-1β:
-
Interleukin 1 beta
- IL-6:
-
Interleukin six
- ITT:
-
The intention-to-treat
- MAPK:
-
Mitogen-activated protein kinase
- MET:
-
Metabolic equivalent
- NF-κB:
-
Nuclear factor kappa B
- NS:
-
Nigella sativa
- PBMC:
-
Peripheral blood mononuclear cells
- RCT:
-
Randomized controlled trial
- RNA:
-
Ribonucleic acid
- RT-PCR:
-
Real-time polymerase chain reaction
- TQ:
-
Thymoquinone
References
Gnacińska M, Małgorzewicz S, Guzek M, Łysiak-Szydłowska W, Sworczak K. Adipose tissue activity in relation to overweight or obesity. Endokrynologia Polska. 2010;61(2):160–8.
CDC. Adult Obesity Facts [https://www.cdc.gov/obesity/data/prevalence-maps.html]
Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016;5:47–56.
Jang K, Tong T, Lee J, Park T, Lee H. Altered gene expression profiles in Peripheral Blood mononuclear cells in obese subjects. Obes Facts. 2020;13(3):375–85.
Bing C. Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte. 2015;4(2):149–52.
Kern L, Mittenbühler MJ, Vesting AJ, Ostermann AL, Wunderlich CM, Wunderlich FT. Obesity-Induced TNFα and IL-6 signaling: the missing link between obesity and inflammation-driven liver and colorectal cancers. Cancers (Basel). 2018;11(1):24.
Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50.
Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, Gojobori T, Isenovic ER. Leptin and obesity: role and clinical implication. Front Endocrinol 2021, 12(563).
Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC. Leptin, obesity, and Leptin Resistance: where are we 25 years later? Nutrients 2019, 11(11):2704.
Rajendran K, Devarajan N, Ganesan M, Ragunathan M. Obesity, inflammation and Acute Myocardial Infarction - expression of leptin, IL-6 and high sensitivity-CRP in Chennai based population. Thromb J. 2012;10(1):13.
Iikuni N, Lam QLK, Lu L, Matarese G, La Cava A. Leptin and inflammation. Curr Immunol Rev. 2008;4(2):70–9.
Teng L, Lee EL, Zhang L, Barnes J. Herbal preparations for weight loss in adults. Cochrane Database Syst Rev. 2020;2020(4):CD013576.
Denzler KL, Waters R, Jacobs BL, Rochon Y, Langland JO. Regulation of inflammatory gene expression in PBMCs by Immunostimulatory Botanicals. PLoS ONE. 2010;5:e12561.
Abdollahi S, Salehi-Abargouei A, Toupchian O, Sheikhha MH, Fallahzadeh H, Rahmanian M, Tabatabaie M, Mozaffari-Khosravi H. The Effect of Resveratrol Supplementation on Cardio-metabolic risk factors in patients with type 2 Diabetes: a Randomized, double-blind controlled trial. Phytother Res. 2019;33(12):3153–62.
Ahmad MF, Ahmad FA, Ashraf SA, Saad HH, Wahab S, Khan MI, Ali M, Mohan S, Hakeem KR, Athar MT. An updated knowledge of black seed (Nigella sativa Linn.): review of phytochemical constituents and pharmacological properties. J Herb Med. 2021;25:100404–4.
Ahmad MF, Ahmad FA, Ashraf SA, Saad HH, Wahab S, Khan MI, Ali M, Mohan S, Hakeem KR, Athar MT. An updated knowledge of black seed (Nigella sativa Linn.): review of phytochemical constituents and pharmacological properties. J Herb Med. 2021;25:100404.
Fadishei M, Ghasemzadeh Rahbardar M. Effects of Nigella sativa oil and thymoquinone against bisphenol A-induced metabolic disorder in rats. Phytother Res. 2021;35(4):2005–24.
Harphoush S, Wu G, Qiuli G, Zaitoun M, Ghanem M, Shi Y, Le G. Thymoquinone ameliorates obesity-induced metabolic dysfunction, improves reproductive efficiency exhibiting a dose-organ relationship. Syst Biology Reproductive Med. 2019;65(5):367–82.
Al-Azzawi MA, AboZaid MMN, Ibrahem RAL, Sakr MA. Therapeutic effects of black seed oil supplementation on Chronic Obstructive Pulmonary Disease patients: a randomized controlled double blind clinical trial. Heliyon. 2020;6(8):e04711.
Mahdavi R, Namazi N, Alizadeh M, Farajnia S. Nigella sativa oil with a calorie-restricted diet can improve biomarkers of systemic inflammation in obese women: a randomized double-blind, placebo-controlled clinical trial. J Clin Lipidol. 2016;10(5):1203–11.
Hosseinpour-Niazi S, Hadi S, Mirmiran P. Effect of Nigella Sativa Oil Extract on Leptin and adiponectin in type 2 diabetic patients: a Randomized, double blind, Placebo controlled clinical trial. Research-in-Medicine. 2020;44(4):521–5.
Razmpoosh E, Safi S, Nadjarzadeh A, Salehi-Abargouei A, Mazaheri M, Mirmiran P, Meyre D. Effects of Nigella sativa supplementation on blood concentration and mRNA expression of TNF-α, PPAR-γ and adiponectin, as major adipogenesis-related markers, in obese and overweight women: a crossover, randomised-controlled trial. Br J Nutr 2022:1–10.
Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;366:l4378.
Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, Damanhouri ZA, Anwar F. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3(5):337–52.
Qidwai W, Hamza HB, Qureshi R, Gilani A. Effectiveness, Safety, and tolerability of Powdered Nigella sativa (Kalonji) seed in capsules on serum lipid levels, blood Sugar, blood pressure, and body weight in adults: results of a Randomized, double-blind controlled trial. J Altern Complement Med. 2009;15(6):639–44.
Ibrahim RM, Hamdan NS, Ismail M, Saini SM, Abd Rashid SN, Abd Latiff L, Mahmud R. Protective effects of Nigella sativa on metabolic syndrome in Menopausal Women. Adv Pharm Bull. 2014;4(1):29–33.
Sample Size Calculations in Clinical Research. [https://doi.org/10.1201/9781584889830]
Gibson AA, Hsu MSH, Rangan AM, Seimon RV, Lee CMY, Das A, Finch CH, Sainsbury A. Accuracy of hands v. household measures as portion size estimation Aids. J Nutritional Sci. 2016;5:e29.
Aadahl M, JØRgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc 2003, 35(7).
de Mello VD, Kolehmanien M, Schwab U, Pulkkinen L, Uusitupa M. Gene expression of peripheral blood mononuclear cells as a tool in dietary intervention studies: what do we know so far? Mol Nutr Food Res. 2012;56(7):1160–72.
Robledo D, Hernández-Urcera J, Cal RM, Pardo BG, Sánchez L, Martínez P, Viñas A. Analysis of qPCR reference gene stability determination methods and a practical approach for efficiency calculation on a turbot (Scophthalmus maximus) gonad dataset. BMC Genomics. 2014;15(1):648–8.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego Calif). 2001;25(4):402–8.
Kim H-Y. Statistical notes for clinical researchers: effect size. Restor Dent Endod. 2015;40(4):328–31.
IRCT clinical trial registeration. Effect of Nigella Sativa supplement on the expression of genes involved in insulin resistance, glycemic status and anthropometric indices among obese women. REGISTERATION CODE: IRCT20180430039475N1 [https://www.irct.ir/trial/30955]
Rashidmayvan M, Mohammadshahi M, Seyedian SS, Haghighizadeh MH. The effect of Nigella sativa oil on serum levels of inflammatory markers, liver enzymes, lipid profile, insulin and fasting blood sugar in patients with non-alcoholic fatty liver. J Diabetes Metab Disord. 2019;18(2):453–9.
Kempf K, Röhling M, Banzer W, Braumann KM, Halle M, Schaller N, McCarthy D, Predel HG, Schenkenberger I, Tan S et al. Early and strong leptin reduction is predictive for long-term weight loss during High-Protein, low-glycaemic meal Replacement-A subanalysis of the randomised-controlled ACOORH Trial. Nutrients 2022, 14(12).
Liu Y, Berthier-Schaad Y, Fallin MD, Fink NE, Tracy RP, Klag MJ, Smith MW, Coresh J. IL-6 haplotypes, inflammation, and risk for Cardiovascular Disease in a multiethnic dialysis cohort. J Am Soc Nephrology: JASN. 2006;17(3):863–70.
Santos FSD, Oliveira IO, Mintem GC, Horta BL, Gigante DP. Epidemiology of interleukin-6: the 30-year follow-up of the 1982 Pelotas (Brazil) birth cohort study. Ann Hum Biol. 2021;48(6):525–33.
Ridker PM. From C-Reactive protein to Interleukin-6 to Interleukin-1: moving Upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145–56.
Ridker PM, MacFadyen JG, Glynn RJ, Bradwin G, Hasan AA, Rifai N. Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: secondary analyses from the Cardiovascular inflammation reduction trial. Eur Heart J. 2020;41(31):2952–61.
Hossen MJ, Yang WS, Kim D, Aravinthan A, Kim J-H, Cho JY. Thymoquinone: an IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci Rep. 2017;7(1):42995.
Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the Complications. Arch Med Sci. 2017;13(4):851–63.
Verma N, Mudge JD, Kasole M, Chen RC, Blanz SL, Trevathan JK, Williams JC, Ludwig KA. Auricular vagus neuromodulation – A systematic review on quality of evidence and clinical effects. medRxiv 2021:2020.2011.2026.20239509.
Dastani Z, Hivert M-F, Timpson N, Perry JRB, Yuan X, Scott RA, Henneman P, Heid IM, Kizer JR, Lyytikäinen L-P, et al. Novel loci for adiponectin levels and their influence on type 2 Diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8(3):e1002607.
Iwase M, Yamamoto T, Nishimura K, Takahashi H, Mohri S, Li Y, Jheng HF, Nomura W, Takahashi N, Kim CS, et al. Suksdorfin promotes adipocyte differentiation and improves abnormalities in glucose metabolism via PPARγ activation. Lipids. 2017;52(7):657–64.
Shemirani F, Golzarand M, Salari-Moghaddam A, Mahmoudi M. Effect of low-carbohydrate diet on adiponectin level in adults: a systematic review and dose-response meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr 2021:1–10.
Parhizkar S, Latiff LA, Rahman SA, Dollah MA, Parichehr H. Assessing estrogenic activity of Nigella sativa in ovariectomized rats using vaginal cornification assay. Afr J Pharm Pharmacol 2011.
Acknowledgements
We would like to thank all the participants and members who were involved in this study, and the obesity clinic at Shahid Sadoughi University of Medical Sciences. We also thank Barij Essence Pharmaceutical Co. for their executive supports. We would also like to express our gratitude to Shahid Sadoughi University of Medical Sciences for their financial and executive support and Shahid Beheshti University of Medical Sciences for their in-kind support.
Funding
This work was supported by Shahid Sadoughi University of Medical Sciences (Grant No. 961020).
Author information
Authors and Affiliations
Contributions
Each author has made substantial contributions to the conception of the work; ER wrote the main manuscript text and MN prepared the figures and genetics analyses. ER and SS contributed to the acquisition, analysis, and interpretation of data; SK has drafted the work. MM and AN contributed to the design of the work and substantively revised it. All authors have approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Medical Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (approval no. IR.SSU.SPH. REC.1397.006) and the Committee of Ethics of Shahid Beheshti University of Medical Sciences (approval no. IR.SBMU.ENDOCRINE.REC.1400.084). All methods were carried out in accordance with the Declaration of Helsinki. Participants were recruited in April 2019. They were provided with an information sheet and asked to complete a consent form prior to the study; informed consent was obtained from all study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Razmpoosh, E., Safi, S., Mazaheri, M. et al. A crossover randomized controlled trial examining the effects of black seed (Nigella sativa) supplementation on IL-1β, IL-6 and leptin, and insulin parameters in overweight and obese women. BMC Complement Med Ther 24, 22 (2024). https://doi.org/10.1186/s12906-023-04226-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-04226-y