Abstract
Background
Cancer represents one of the biggest healthcare issues confronting humans and one of the big challenges for scientists in trials to dig into our nature for new remedies or to develop old ones with fewer side effects. Halophytes are widely distributed worldwide in areas of harsh conditions in dunes, and inland deserts, where, to cope with those conditions they synthesize important secondary metabolites highly valued in the medical field. Several Tamarix species are halophytic including T.nilotica which is native to Egypt, with a long history in its tradition, found in its papyri and in folk medicine to treat various ailments.
Methods
LC–LTQ–MS–MS analysis and 1H-NMR were used to identify the main phytoconstituents in the n- butanol fraction of T.nilotica flowers. The extract was tested in vitro for its cytotoxic effect against breast (MCF-7) and liver cell carcinoma (Huh-7) using SRB assay.
Results
T.nilotica n-butanol fraction of the flowers was found to be rich in phenolic content, where, LC–LTQ–MS–MS allowed the tentative identification of thirty-nine metabolites, based on the exact mass, the observed spectra fragmentation patterns, and the literature data, varying between tannins, phenolic acids, and flavonoids. 1H-NMR confirmed the classes tentatively identified. The in-vitro evaluation of the n-butanol fraction showed lower activity on MCF-7 cell lines with IC50 > 100 µg/mL, while the higher promising effect was against Huh-7 cell lines with an IC50= 37 µg/mL.
Conclusion
Our study suggested that T.nilotica flowers' n-butanol fraction is representing a promising cytotoxic candidate against liver cell carcinoma having potential phytoconstituents with variable targets and signaling pathways.
Similar content being viewed by others
Introduction
All over the world, cancer ranks as a primary cause of mortality and a significant roadblock to raising life expectancy [1, 2]. According to World Health Organization (WHO) estimations for 2022, globally cancer represented the cause of death for 16% before the age of 70 [3]. Hepatocellular carcinoma is the predominant primary cancer in most countries and the fourth most prevalent cancer across the globe [4, 5] besides being the third most lethal cancer-associated mortality in the world [6].
Additionally, breast cancer represents the first-leading cause of death for women, almost 2.3 million women received a breast cancer diagnosis in the world in 2020, and 685,000 of them passed away. Somewhere in the globe, a woman receives a breast cancer diagnosis every 14 s [6, 7]. The main regimen of treatment of various forms of cancer is to stop unregulated cell growth which can be achieved by using cytotoxic drug medications. The effect of these drugs can be estimated by using cell-based in vitro assays to measure the degree of tissue-level cell damage [8].
However, the use of conventional chemotherapeutic agents has been associated with a wide range of side effects and toxicities; therefore, new approaches for the prevention and cure of cancer represent a great challenge for researchers [9]. One of the most crucial methods for treating particular types of cancer is the discovery of natural anti-cancer medications, which requires constant monitoring of various sources such as marine animals, terrestrial plants, and seaweeds [10].
There are more than 60 species of halophyte plants in the genus Tamarix belonging to the Tamaricaceae family, which are cultivated in almost every region of the world under the common names “Tamarisk” and “salt cedar” [11, 12]. It has a variety of therapeutic uses in conventional medicine [11]. Due to the plant’s astringent and cleaning properties on internal organs, which were attributed to its bitter taste, it was known to have a chilly and dry nature [11]. Certain Tamarix species are recommended as mild laxatives, anti-tussive, antipyretics, and tonics for the liver and spleen [11, 13]. Some species are used to treat leucorrhea and uterine bleeding because they have anti-inflammatory and wound-healing characteristics [14]. It can be applied topically to treat skin conditions like eczema and anal fissure [13]. Biological studies have demonstrated that some Tamarix species can be used as anti-Alzheimer [15], anti-diabetic [16], anti-hyperlipidemic [17], anti-inflammatory [18, 19], antimicrobial [20, 21], antinociceptive [22], antioxidant [23], anti-coagulation [24], anti-rheumatoid [25], cytotoxicity [26], hepatoprotective [27] and wound healing [28] activities. Tamarix is represented in Egypt with two indigenous species which are T. aphylla (L.) H. Karst and T. nilotica (Ehrenb.) Bunge. T. nilotica is a rich source of polyphenolics including hydrolyzable tannins, sulfated and non-sulfated flavonoids, and phenylpropanoids [29, 30]. T. nilotica extracts have demonstrated antioxidant, antiangiogenic, cytotoxic, hepatoprotective, antifibrotic, antidiabetic, and antimicrobial activities in relation to their phenolic contents [29,30,31]. Although both species are indigenous in Egypt, many studies targeted T. aphylla which was mentioned for comparison to T. nilotica [16, 20, 22, 28, 32,33,34,35]. Besides, T. nilotica was the one easily available for us to carry on with this study.
In the previous published studies, T. nilotica received much attention in studying its cytotoxic activity. Various studies reported the effect of leaves, methanolic flower extracts on different cell lines including lung (A-549), liver (Huh-7), colon (HCT-116), and breast (MCF-7) cancer cell lines [36,37,38]. T. nilotica flower extract reported to exhibit hepatoprotective and antioxidant activities [38]. However, there are no studies concerning the cytotoxic activities of the n-butanol fraction of T. nilotica flower.
The present work aimed to investigate the possible cytotoxic activity of the n-butanol extract of T. nilotica flowers against liver (Huh-7) and breast (MCF-7) cell carcinoma while performing an in-depth phytochemical analysis of the same extract n-butanol extract using LC-MS/MS analysis to relate the activity to the extract’s metabolites.
Methods
Statement
All experiments and methods including the collection of the plant were performed following the relevant national, and international guidelines and legislation of the Faculty of Pharmacy, University of Sadat City, Sadat City, Egypt.
Extraction and Isolation
The air-dried flowers of T. nilotica (Ehrenb.) Bunge (1 kg) was exhaustively extracted with 80% methanol; excess solvent was removed using a rotary evaporator. The crude aqueous methanolic extract was further fractionated using solvents of different polarity viz., n-hexane, dichloromethane, n-butanol, and water. The fractions were dried under vacuum to give their corresponding weights of 30 gm, 25 gm 15 gm, and 45 gm, respectively. All fractions were stored at -20 °C till further analysis [39].
LC–LTQ–MS–MS analysis
The n- butanol extract was analyzed and processed using LC–MS–MS. A Shimadzu LC-10 HPLC with a Grace Vydac Everest Narrowbore C-18 column (100 mm × 2.1 mm i.d., 5 μm, 300 Å). An LC–MS, connected to an LTQ Linear Ion Trap MS (Thermo Finnigan, San Jose, CA) was utilized with a mass range of 100–2000 m/z. A 2 µL sample was injected using an autosampler. A 35 min method was used as follows: 5 min isocratic run using 5% acetonitrile (Acn) and 0.05% formic acid (FA), then a gradient was run for 25 min until 95% AcN 0.05% FA. Finally, there was 5 min of conditioning the column with 5% AcN and 0.05% FA. The data were processed and analyzed using foundation 3.1_Xcalibur_3.1.6610 as well as MZmine3. Furthermore, the raw data files were converted to mzXML format using MSConvert from the ProteoWizard suite [40]. The molecular network was created using the Global Natural Products Social Molecular Networking (GNPS) online workflow. The spectra in the network were then searched against the GNPS spectral libraries and published data [41, 42].
Using the GNPS dataset, the raw MS file was analyzed. By analyzing the similarity between the fragmentation pattern from the raw mass spectrum and the GNPS library, GNPS assists in the identification and discovery of metabolites. Other installed programs, including MSConvert (https://proteowizard.sourceforge.io/), File Zilla (https://filezilla-project.org/), and Cytoscape version 3.5.1(https://cytoscape.org/), were used to operate with GNPS at the following link (https://gnps.ucsd.edu/) [43, 44].
1H-NMR analysis
1H-NMR spectra were recorded at 298 K on a Bruker 600 MHz (TCI CRPHe TR-1H and 19F/13C/15N 5 mm-EZ CryoProbe) spectrometer. Chemical shifts were referenced to the solvent peak for CH3OD at δH 3.3100 ppm [44, 45].
Cytotoxic evaluation of the n-butanol fraction of T. nilotica flowers
Cell cultures
Breast adenocarcinoma cell lines (MCF-7) and hepatocyte-derived cellular carcinoma cell lines, human liver (Huh-7) was obtained from Nawah Scientific Inc., (Mokatam, Cairo, Egypt). Cells were maintained in DMEM media supplemented with 100 mg/mL of streptomycin, 100 units/mL of penicillin, and 10% of heat-inactivated fetal bovine serum in humidified, 5% (v/v) CO2 atmosphere at 37 °C [46].
Cell cytotoxicity
Cell viability was assessed by sulforhodamine B (SRB) assay on two cancer cell lines [47, 48], the human liver cancer cell line (Huh-7) and the breast cancer cell line (MCF-7). Aliquots of 100 µL cell suspension (5 × 103^ cells) were in 96-well plates and incubated in complete media for 24 h. Cells were treated with another aliquot of 100 µL media containing the n-butanol T. nilotica flower extract at two different concentrations (10 and 100 µg/ml). After 72 h, cells were fixed by replacing media with 150 µL of 10% TCA and incubated at 4 °C for 1 h. The TCA solution was removed, and the cells were washed 5 times with distilled water. Aliquots of 70 µL SRB solution (0.4% w/v) were added and incubated in a dark place at room temperature for 10 min. Plates were washed 3 times with 1% acetic acid and allowed to air-dry overnight. Then, 150 µL of TRIS (10 mM) was added to dissolve the protein-bound SRB stain; the absorbance was measured at 540 nm using a BMG LABTECH- FLUOstar Omega microplate reader (Ortenberg, Germany) [49]. The cell morphological analysis was carried out according to M. Roy et al. 2017 [50].
Statistical analysis
Statistical analysis of the data was performed using one-way ANOVA, followed by Tukey’s multiple range tests for post hoc comparisons (GraphPad Prism, version 8.4.2). All the data are presented as the means of 3 determinations ± SE [51].
Results
Metabolic profiling of the n-butanol fraction of T. nilotica flowers using LC–LTQ–MS–MS analysis in positive mode
Based on the exact mass, the observed spectra fragmentation patterns, and literature data, the structural characterizations of chemical composition in the n-butanol fraction of the T. nilotica flowers were accomplished. Using MS/MS fragmentation pattern, 39 compounds from various classes of secondary metabolites were identified. The detected compounds’ structures were presented in (Fig. 1). Molecular ion, retention time, and MS/MS data of identified compounds were provided in (Table 1).
Chemical structures of the tentatively identified compounds in the n-butanol fraction of T. nilotica flowers numbered according to compounds listed in Table 1
LC–LTQ–MS–MS analysis of the n-butanol fraction of T. nilotica flowers using GNPS-Aided annotation
Metabolite profiling of the n-butanol fraction of T. nilotica flowers via GNPS based on tandem mass spectrometry data as well as a dictionary of natural products yielded the annotation of 35 metabolites (N1—N35); mainly flavonoids, phenolics, and fatty acids; respectively (Figs. 1 and 2; Table 2). Flavonoids were annotated by observing the common fragments of retro dials-alder reaction indicated at m/z 153, 152, 135 depending on structure as in N11, 15, 16, 17, 18, etc. Additionally, common fragments such as [M-18 Da] denoting loss of H2O molecule, [M-28 Da] denoting the loss of CO, [M + H-42]+ corresponding to C2H2O loss, besides [M + H-46]+, as in quercetin, kaempferol, and myricetin derivatives. A common fragment in O-methylated flavonoids is [M + H-15]+ formed by loss of methyl radical, as shown in N10 (Kaempferide-O-hexoside), N21 (Kaempferide-O-hexoside derivative), N28 (kaempferide), N20 (tamarixetin), N32 (kaempferol 4’,7-dimethyl ether), N30 (quercetin- dimethyl ether) and N18 (herbacetin-trimethyl ether). Flavanones were annotated in the form of dihydro derivatives of flavonols as presented in N26 (m/z 305) tentatively identified as dihydro-quercetin, N31 (m/z 321) identified as dihydromyricetin. Phenolic acids i.e., N5, N12, N13, and N24 were previously reported in Tamarix species. GNPS databases also aided in identifying N7, N9, N14, N25, and N34, besides kaempferol derivatives as well (Fig. 3).
Molecular network (showing clusters of metabolites of interest) based on tandem mass spectrometry data in the positive ionization mode of the n-butanol fraction of T. nilotica flowers. Twenty metabolites have been identified as labeled in Fig. 3, green color indicating the number of compounds in Table 2, light blue nodes are compounds identified using GNPS databases, while the identified compounds using fragmentation matching have the pink color
Nuclear magnetic resonance (NMR) analysis
To provide a broader scope of the n-butanol fraction T. nilotica flowers metabolome, 1H-NMR was used to provide insights into both secondary and primary metabolites that were not detected by LTQ-LC-MS-MS. 1H-NMR can also be used for structural elucidation and determining major metabolites. Sugars, flavonoids, phenolics, and coumarins were among the major metabolites classes detected in the n-butanol fraction of T. nilotica flowers using 1H-NMR as detailed in (Table 3).
Fatty acids were discriminated against by the presence of terminal (CH3 ) at δH 0.9 ppm, long chain methylene groups at δH 1.2 ppm, and olefinic (CH) showed at δH 5.3 ppm, as shown in (Fig. 4, M1).
Sugars, the second intense metabolites, were recognized by the presence of anomeric proton annotated as, α, β glucose, and sucrose, which exhibited anomeric protons at δH 5.18 (d, J = 3.8 Hz) for (Fig. 4, M2), δH 4.58 (d, J = 7.8 Hz) (Fig. 4, M3), and δH 5.40 (d, J = 3.8 Hz), δH 4.17 (d, J = 8.5 Hz) (Fig. 4, M4), respectively. Moreover, CHs attached to hydroxyl groups exhibited overlapped peaks at a range of δH 3.2—4.02 ppm as shown in (Fig. 4, M2-M4) [91]. A sharp singlet peak at δH 2.56 (s) indicated the presence of a common organic acid elucidated as succinic acid (Fig. 4, M5) [91]. Finally, flavonoids and coumarins were found in a region of aromaticity, which was recognized by the presence of δH 6.35, 7.60 (d, J = 15.8 Hz) corresponding to α, β unsaturated ketone in coumarins. Concerning flavonoids overlapped peaks at the region of δH 6.0—8.33 ppm, which was elucidated with the help of LTQ-LC-MS-MS data (Fig. 5).
1H-NMR spectrum exhibiting the identified metabolites in the n-butanol fraction of T. nilotica flowers; primary metabolites i.e., fatty acids and sugars (M1-M4) as well as organic acid (M5) at the aliphatic region δH 0.5—5.5 ppm as mentioned in Table 3
Cytotoxic evaluation of the n-butanol fraction of T. nilotica flowers
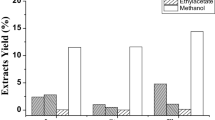
The cytotoxic effect of the n-butanol fraction T. nilotica flowers was investigated as a cytotoxicity SRB quick screening against MCF-7 and Huh-7 cells. The n-butanol fraction inhibited cancer cells in a dose-dependent manner since the activity increased with increasing the dose. For instance, at a concentration of 100 µg/ml, the viability percentage was 54.27% compared to 100% with 10 µg/mL on MCF-7 with an IC50 ˃100 µg/mL. However, the best effect was observed with Huh-7 where the percentage viability decreased from 51.89% at 10 µg/mL to 7.22% at 100 µg/mL with an IC50 = 37 µg/mL (Table 4).
Cell viability was assessed at five different concentrations (0.01, 0.1, 1, 10, and 100 µg/mL) using the SRB assay revealed that T. nilotica flowers n-butanol fraction possesses a dose-dependent cytotoxic effect with an IC50 of 37 µg/mL with Huh-7 cell lines while it showed IC50 > 100 µg/mL with MCF-7 cell lines (Fig. 6).
Discussion
One of the leading causes of death on the globe is cancer. Given their significant toxicity to cancer cells, natural products, and their secondary metabolites are highly significant for research into potential anticancer treatments. Previous research found that several Tamarix species have displayed varying cytotoxic activities. Breast adenocarcinoma cells (MCF-7) were suppressed by the methanolic extract of T. aphylla in a concentration-dependent manner [33]. Different extracts of T. senegalensis demonstrated anti-cancer effects in human liver (Huh-7) and lung (A-549) carcinoma cells [31]. T. gallica shoots, flowers, and leaves methanolic extracts were able to inhibit the proliferation of colon cancer (Caco-2) cells at concentrations of 50 and 100 g/mL [82]. Furthermore, T. articulata methanolic extract demonstrated promising antiproliferative activity against hepatocellular carcinoma [92], as well as against prostate cancer (LnCaP) cells’ motility and invasiveness in a dose-dependent manner [93]. In this study, the n-butanol fraction of T. nilotica flowers showed cytotoxic activity against MCF-7 and Huh-7 cells (Fig. 6) in a dose-dependent manner with a more promising effect against liver cancer cell Huh-7 (IC50 = 37 µg/mL). The optical microscope-stained images were recorded as shown in Fig. 7 comparing the cytotoxic effect of n-butanol fraction of T. nilotica flowers at a concentration of 10 and 100 µg/mL with comparison to (-ve control). Images clearly show the cytotoxic effect of the extract against MCF-7 and Huh-7 cell lines (Fig. 7C, E & F) where no morphological changes were observed on MCF-7 at conc. 10 µg/mL (Fig. 7B) as well as the negative control of both cell lines (Fig. 7A & D) while more potent effect was observed against Huh-7 (Fig. 7E & F). This confirms that the n-butanol fraction of T. nilotica flowers possess cytotoxic effects which are clearer and more potent on Huh-7 cells over MCF-7 cells.
T.nilotica has been previously reported for promising cytotoxic activity against human colon (HCT-116) and breast (MCF-7) cancer cells [94], whereas ethyl acetate was active against lung cancer cell line with increased expression levels of p-53 and Bax whereas that of Bcl-2 was decreased [36, 37], while flowers were effective and selective against liver cell carcinoma (Huh-7) [38].
The chemical investigation of various Tamarix species was reported. Gallic acid, flavones, and flavonols were among the polyphenols found in this study that were recognized as compounds that had previously been found in other species of Tamarix [34, 95]. For example, a study on the alcohol-soluble fraction of an aqueous extract of T. nilotica aerial parts collected from Egypt and Saudi Arabia was discussed by Sekkien A. et al. 2018 [30]. The study reported that the major compounds in the Egyptian species extract were (iso)ferulic acid-3-sulphate, methyl ferulate sulfate, and coniferyl alcohol sulfate derivative. Moreover, this species exhibited the presence of kaempferide, gallic acid, nilocitin, kaempferol dimethyl ether sulfate, tamarixetin, kaempferol, quercetin, methyl gallate methyl ether, kaempferol 3-O-β-glucuronide and 4ʹ-O-methyl quercetin 3-O-β-hexoside which was following the identified compounds in our study [30]. Also, the tannin-identified compounds in our study as hirtellin B, gemin D, nilotinin D1, and tamarixinin A were following those reported in T. nilotica, T. pakistanica, T. tetrandra, and T. senegalensis by [56, 64, 68, 96]. These several identified polyphenolic compounds in this genus explain its widespread biological activity as stated in [11].
The phytochemical analysis of the n-butanol extract of T. nilotica flowers using LC-MS/MS analysis reveals the identification of various phenolic compounds such as gallic acid, caffeic acid, ferulic acid, luteolin, kaempferol, quercetin, kaempferol-3-O-glucuronide, tamarixetin, besides various galloyl and gallate moieties. Fragments at m/z [M-H-152]− and [M-H-170] – denoted the losses of galloyl and gallate moieties respectively, eliminated by gallotannins or galloylated esters [60]. Tannins were previously isolated and identified in T. nilotica and have shown potent cytotoxic effects with high tumor specificity [68]. The promising cytotoxic effect against liver carcinoma can be well correlated with the tentatively identified phenolic compounds where caffeic and gallic acid was reported to reduce the growth of MCF-7 breast cancer cells and altered the expression of apoptotic genes [97], ferulic acid also promotes apoptosis in cancer cell lines MCF-7 and HepG-2 and activated the caspase-8 and − 9 pathways, has cytotoxic action and [98]. while nilocitin showed a G2/M and S cell cycle arrest as a consequence of the G1 phase [99], furthermore, the flavonoid hispidulin (4’,5,7-trihydroxy-6-methoxyflavone) causes ERS-mediated apoptosis in hepatocellular carcinoma cells by stimulating the AMPK/mTOR pathway, [100]. HepG-2 cells were more vulnerable to hispidulin-mediated cell death than immortalized L929 fibroblasts, indicating that this substance has a distinct level of toxicity in tumor-related cell lines than normal cell lines [101]. When kaempferol was administered to the human breast cancer cell line MCF-7, it suppressed the expression of PLK-1, a protein-like kinase that has been shown to control mitotic development and to be elevated in several human cancers. Kaempferol’s anticancer activity is mediated via inhibition of the EGFR-related Src, ERK1/2, and AKT pathways, and it may be a powerful inhibitor of pancreatic cancer cells [102]. Luteolin is a very significant flavonoid that is present in many foods. It has several health benefits, including its ability to prevent cancer, induce cell cycle arrest and apoptosis in some human cancer cells, and enhance the antitumor effects of 5-FU on Bel7402 and HepG-2 cells. These effects may be connected to apoptosis and the control of 5-FU metabolism [103,104,105]. The dietary flavonoid quercetin, which is found in berries, demonstrated high cytotoxicity it prevented HepG-2 cancer cells from proliferating and surviving while inducing apoptosis by increasing the expression of p53 and BAX [106, 107].
Our findings imply that the T. nilotica flower’s n-butanol fraction has the potential to be a promising cytotoxic candidate against Huh-7 cancer cells.
Conclusion
This study documents a detailed metabolites profiling for the unexplored n-butanol fraction of Tamarix nilotica flowers. A total of 39 constituents including tannins, flavonoids, and phenolic acids, were tentatively identified. The in vitro cytotoxicity study revealed significant cytotoxic action towards the hepatocyte-derived cellular carcinoma cell lines, human liver (Huh-7). However, further studies are necessary to correlate this activity to the identified compounds to demonstrate T.nilotica as a prospective drug candidate that inhibits cancer.
Availability of data and materials
The datasets generated and analyzed during the current study are all mentioned in the presented manuscript.
References
Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–30.
El-Seedi HR, Yosri N, Khalifa SAM, Guo Z, Musharraf SG, Xiao J, Saeed A, Du M, Khatib A, Abdel-Daim MM, et al. Exploring natural products-based cancer therapeutics derived from egyptian flora. J Ethnopharmacol. 2021;269:113626.
Reports W. World health statistics 2022: monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization. In.; 2022.
Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Chapter One - Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. In: Advances in Cancer Research. Volume 149, edn. Edited by Sarkar D, Fisher PB: Academic Press; 2021: 1–61.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Breast Cancer Statistics And Resources. In: bcrforg/breasti>-cancer-statistics-and-resources/. Breast Cancer Research Foundation 2023.
Swetha GMS, Keerthana M, Rayginia CK, Anto TP. RJ: Cancer Chemoprevention: A Strategic Approach Using Phytochemicals. Front Pharmacol. 2022;12.
Abosedera DA, Emara SA, Tamam OAS, Badr OM, Khalifa SAM, El-Seedi HR, Refaey MS. Metabolomic Profile and In vitro Evaluation of the Cytotoxic Activity of Asphodelus microcarpus against Human Malignant Melanoma Cells A375. Arab J Chem. 2022:104174.
Abosedera DA, Emara SA, Tamam OAS, Badr OM, Khalifa SAM, El-Seedi HR, Refaey MS. Metabolomic profile and in vitro evaluation of the cytotoxic activity of Asphodelus microcarpus against human malignant melanoma cells A375. Arab J Chem. 2022;15(10):104174.
Bahramsoltani R, Kalkhorani M, Abbas Zaidi SM, Farzaei MH, Rahimi R. The genus Tamarix: traditional uses, phytochemistry, and pharmacology. J Ethnopharmacol. 2020;246:112245.
El-Seedi HR, Khalifa SA, Yosri N, Khatib A, Chen L, Saeed A, Efferth T, Verpoorte R. Plants mentioned in the islamic scriptures (Holy Qur’ân and ahadith): traditional uses and medicinal importance in contemporary times. J Ethnopharmacol. 2019;243:112007.
Quattrocchi U. CRC World Dictionary of Medicinal and poisonous plants: common names, scientific names, Eponyms, Synonyms, and etymology. Volume 5, 1st ed. CRC Press; 2012.
Mughal M: Kitab Al-Shifa Bi Ta'Rif Huquq Al-Mustafa (Sallallahu 'Alayhi Wasallam) کِتَابُ الشِّفَاءِ بِتَعۡرِیۡفِ حُقُوۡقِ الۡمُصۡطَفیٰ(صَلَّی للهُ عَلیۡہِ وَآلِہٖ وَسَلَّم) By Qadi ‘Iyad Maliki Andulusi قاضی عیاض مالکی اندلسی(رحمۃ لله علی) Translated into English by Justice ® Dr. Munir Ahmad Mughal [Chapter – 3 (Pages 1 to 1022)]. 2011.
Salissou MTM, Mahaman YAR, Zhu F, Huang F, Wang Y, Xu Z, Ke D, Wang Q, Liu R, Wang JZ, et al. Methanolic extract of Tamarix Gallica attenuates hyperhomocysteinemia induced AD-like pathology and cognitive impairments in rats. Aging. 2018;10(11):3229–48.
Ullah R, Tariq SA, Khan N, Sharif N, Ud Din Z, Mansoor K. Antihyperglycemic effect of methanol extract of Tamarix aphylla L. Karst (Saltcedar) in streptozocin–nicotinamide induced diabetic rats. Asian Pac J Trop Biomed. 2017;7(7):619–23.
Hebi M, Eddouks M. Hypolipidemic activity of Tamarix articulata Vahl. in diabetic rats. J Integr Med. 2017;15(6):476–82.
Bose A, Mondal S, Gupta JK, Ghosh T, Dash GK, Si S. Analgesic, anti-inflammatory and antipyretic activities of the ethanolic extract and its fractions of Cleome rutidosperma. Fitoterapia. 2007;78(7–8):515–20.
Chen R, Yang Y, Xu J, Pan Y, Zhang W, Xing Y, Ni H, Sun Y, Hou Y, Li N. Tamarix hohenackeri Bunge exerts anti-inflammatory effects on lipopolysaccharide-activated microglia in vitro. Phytomedicine: Int J Phytotherapy Phytopharmacology. 2018;40:10–9.
Khalid M, Hassani D, Bilal M, Butt ZA, Hamayun M, Ahmad A, Huang D, Hussain A. Identification of oral cavity biofilm forming bacteria and determination of their growth inhibition by Acacia arabica, Tamarix aphylla L. and Melia azedarach L. medicinal plants. Arch Oral Biol. 2017;81:175–85.
Benmerache A, Benteldjoune M, Alabdul Magid A, Abedini A, Berrehal D, Kabouche A, Gangloff SC, Voutquenne-Nazabadioko L, Kabouche Z. Chemical composition, antioxidant and antibacterial activities of Tamarix balansae J. Gay aerial parts. Nat Prod Res. 2017;31(24):2828–35.
Qadir M, Abbas K, Hamayun R, Ali M. Analgesic, anti-inflammatory and anti-pyretic activities of aqueous ethanolic extract of Tamarix aphylla L. (Saltcedar) in mice. Pak J Pharm Sci. 2014;27 6:1985–8.
Bettaib J, Talarmin H, Droguet M, Magné C, Boulaaba M, Giroux-Metges MA, Ksouri R. Tamarix gallica phenolics protect IEC-6 cells against H(2)O(2) induced stress by restricting oxidative injuries and MAPKs signaling pathways. Biomed Pharmacother. 2017;89:490–8.
Xing Y, Liao J, Tang Y, Zhang P, Tan C, Ni H, Wu X, Li N, Jia X. ACE and platelet aggregation inhibitors from Tamarix hohenackeri Bunge (host plant of Herba Cistanches) growing in Xinjiang. Pharmacognosy Magazine. 2014;10(38):111–7.
Yao Y, Jiang CS, Sun N, Li WQ, Niu Y, Han HQ, Miao ZH, Zhao XX, Zhao J, Li J. Tamaractam, a New Bioactive Lactam from Tamarix ramosissima, Induces Apoptosis in Rheumatoid Arthritis Fibroblast-Like Synoviocytes. Molecules. 2017;22(1).
Abaza MS, Al-Attiyah R, Bhardwaj R, Abbadi G, Koyippally M, Afzal M. Syringic acid from Tamarix aucheriana possesses antimitogenic and chemo-sensitizing activities in human colorectal cancer cells. Pharm Biol. 2013;51(9):1110–24.
Komal S, Malik A, Akhtar N, Kazmi S, Anjum F, Rida A. Tamarix dioica (Ghaz) Protective Potential in the Carbon Tetrachloride-Induced Hepatotoxicity Animal Model. 2021;35:37–43.
Ali SAS, Ahmad S, Ali M, Ahsan W, Siddiqui MR, Ansari S, Shamim S, Ali MD. Wound Healing Activity of Alcoholic Extract of Tamarix Aphylla L. on Animal Models. Biomed Pharmacol J. 2019;12(1).
Abdelgawad A. Tamarix nilotica (Ehrenb) Bunge: A Review of Phytochemistry and Pharmacology. J Microb Biochem Technol. 2017;09.
Sekkien A, Swilam N, Ebada SS, Esmat A, El-Khatib AH, Linscheid MW, Singab AN. Polyphenols from Tamarix nilotica: LC-ESI-MS(n) Profiling and In Vivo Antifibrotic Activity. Molecules. 2018;23(6).
Riham OB, Mohamed AE-AER, Rehab SA. Phenolic content, radical scavenging activity and cytotoxicity of Tamarix nilotica (Ehrenb.) bunge growing in Egypt. J Pharmacognosy Phytotherapy. 2013;5(3):47–52.
Ali M, Alhazmi H, Ansari S, Hussain A, Ahmad S, Alam M, Ali M, El-Sharkawy K, Hakeem K. Tamarix aphylla (L.) Karst. Phytochemical and Bioactive Profile Compilations of Less Discussed but Effective Naturally Growing Saudi Plant: Pharmacology and Therapeutic Uses. In., edn.; 2019: 343–52.
Sobeai S, ANTICANCER, CYTOTOXIC EFFECT OF TAMARIX APHYLLA, AND ANTIBACTERIAL SCREENING EFFICIENCY AGAINST MULTIDRUG-RESISTANT HUMAN PATHOGENS. Asian J Pharm Clin Res. 2018;11:241.
Mahfoudhi A, Prencipe FP, Mighri Z, Pellati F. Metabolite profiling of polyphenols in the tunisian plant Tamarix aphylla (L.) Karst. J Pharm Biomed Anal. 2014;99:97–105.
Akhlaq M, Mohammed A. New phenolic acids from the galls of Tamarix aphylla (L.) Karst. Int Res J Pharm. 2011;4:222–5.
Abdel-Tawab FM, Shebl SA, Al-Senosy NK, Abdou HS. Assessment of anticancerogenic effect of Tamarix nilotica on human lung cancer cell line. Egypt J Genet Cytol 2019;48.
Bakr RO, Raey MAE, Ashour RMS. Phenolic content, radical scavenging activity and cytotoxicity of Tamarix nilotica (Ehrenb.) bunge growing in Egypt. J Pharmacognosy Phytotherapy. 2013;5:47–52.
Abouzid S, Sleem A. Hepatoprotective and antioxidant activities of Tamarix nilotica flowers. Pharm Biol. 2011;49(4):392–5.
Bakr RO, Fayed MAA, Fayez AM, Gabr SK, El-Fishawy AM, Taha SE-A. Hepatoprotective activity of Erythrina × neillii leaf extract and characterization of its phytoconstituents. Phytomedicine: Int J Phytother Phytopharmacol. 2019;53:9–17.
Amr A, Abd El-Wahed A, El-Seedi HR, Khalifa SAM, Augustyniak M, El-Samad LM, Abdel Karim AE, El Wakil A. UPLC-MS/MS Analysis of Naturally Derived Apis mellifera Products and Their Promising Effects against Cadmium-Induced Adverse Effects in Female Rats. Nutrients. 2022;15(1).
Azeem HHA, Osman GY, El-Seedi HR, Fallatah AM, Khalifa SAM, Gharib MM. Antifungal Activity of Soft Tissue Extract from the Garden Snail Helix aspersa (Gastropoda, Mollusca). Molecules. 2022;27(10).
Darwish AMG, Abd El-Wahed AA, Shehata MG, El-Seedi HR, Masry SHD, Khalifa SAM, Mahfouz HM, El-Sohaimy SA. Chemical Profiling and Nutritional Evaluation of Bee Pollen, Bee Bread, and Royal Jelly and Their Role in Functional Fermented Dairy Products. Molecules. 2022;28(1).
El-Din MIG, Fahmy NM, Wu F, Salem MM, Khattab OM, El-Seedi HR, Korinek M, Hwang T-L, Osman AK, El-Shazly M. Comparative LC–LTQ–MS–MS analysis of the Leaf extracts of Lantana camara and Lantana montevidensis growing in Egypt with Insights into their Antioxidant, anti-inflammatory, and cytotoxic activities. Plants. 2022;11(13):1699.
El-Garawani IM, Abd El-Gaber AS, Algamdi NA, Saeed A, Zhao C, Khattab OM, AlAjmi MF, Guo Z, Khalifa SAM, El-Seedi HR. In Vitro induction of apoptosis in isolated Acute myeloid leukemia cells: the role of Anastatica hierochuntica Methanolic Extract. Metabolites. 2022;12(9):878.
Dawod A, Fathalla SI, Elkhatam A, Osman N, Sheraiba N, Hammad MA, El-Seedi HR, Shehata AA, Anis A. UPLC-QToF nanospray MS and NMR analysis of Ficus sycomorus Stem Bark and its Effects on rabbit. Processes. 2021;9(7):1201.
Basiouni S, Fayed MAA, Tarabees R, El-Sayed M, Elkhatam A, Töllner KR, Hessel M, Geisberger T, Huber C, Eisenreich W, et al. Characterization of sunflower oil extracts from the lichen usnea barbata. Metabolites. 2020;10(9):1–16.
Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1(3):1112–6.
Kamiloglu S, Sari G, Ozdal T, Capanoglu E. Guidelines for cell viability assays. Food Front. 2020;1(3):332–49.
Bakr RO, El-Behairy MF, Elissawy AM, Elimam H, Fayed MAA. New adenosine derivatives from Aizoon canariense L.: In vitro anticholinesterase, antimicrobial, and cytotoxic evaluation of its extracts. Molecules. 2021;26(5).
Roy M, Chakraborty S, Mali K, Chatterjee S, Banerjee S, Mitra S, Naskar R, Bhattacharjee A. Cellular image processing using morphological analysis. In: 2017 IEEE 8th Annual Ubiquitous Computing, Electronics and Mobile Communication Conference (UEMCON): 19–21 Oct 2017 2017; 2017: 237–241.
Abdou EM, Fayed MAA, Helal D, Ahmed KA. Assessment of the hepatoprotective effect of developed lipid-polymer hybrid nanoparticles (LPHNPs) encapsulating naturally extracted β-Sitosterol against CCl4 induced hepatotoxicity in rats. Sci Rep. 2019;9(1):19779.
Chernonosov AA, Karpova EA, Lyakh EM. Identification of phenolic compounds in Myricaria bracteata leaves by high-performance liquid chromatography with a diode array detector and liquid chromatography with tandem mass spectrometry. Revista Brasileira De Farmacognosia-brazilian Journal of Pharmacognosy. 2017;27:576–9.
Iskhanov Y. Chemical compositional analysis of the Tamarix hispida aerial part extract obtained in ethanol solutions of different concentration. J Appl Eng Sci. 2018;16:233–41.
Nawwar MAM, El-Sissi HI, Barakat HH. Flavonoid constituents of Ephedra alata. Phytochemistry. 1984;23:2937–9.
Ren X, Wang W, Bao Y, Zhu Y, Zhang Y, Lu Y, Peng Z, Zhou G. Isorhamnetin and Hispidulin from Tamarix ramosissima Inhibit 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP) Formation by Trapping Phenylacetaldehyde as a Key Mechanism. Foods (Basel Switzerland). 2020;9(4).
Orabi MA, Taniguchi S, Hatano T. Monomeric and dimeric hydrolysable tannins of Tamarix nilotica. Phytochemistry. 2009;70(10):1286–93.
Lefahal M, Benahmed M, Louaar S, Zellagui A, Duddeck H, Kamel M, Akkal S. Antimicrobial activity of Tamarix gallica L. extracts and isolated flavonoids. Adv Nat Appl Sci. 2010;4:289–92.
Sharifi-Rad J, Song S, Ali A, Subbiah V, Taheri Y, Suleria HAR. LC-ESI-QTOF-MS/MS characterization of phenolic compounds from Pyracantha coccinea M.Roem. and their antioxidant capacity. Cell Mole Biol (Noisy-le-Grand, France). 2021;67(1):201–211.
Tomás-Barberán FA, Iniesta-Sanmartín E, Tomás-Lorente F, Rumbero Á. Antimicrobial phenolic compounds from three spanish Helichrysum species. Phytochemistry. 1990;29:1093–5.
Hooi Poay T, Sui Kiong L, Cheng Hock C. Characterisation of Galloylated Cyanogenic Glucosides and Hydrolysable Tannins from Leaves of Phyllagathis rotundifolia by LC-ESI-MS/MS. Phytochem Anal. 2011;22(6):516–25.
Orabi MA, Taniguchi S, Yoshimura M, Yoshida T, Hatanoa T. New monomeric and dimeric hydrolyzable tannins from Tamarix nilotica. Heterocycles. 2010;80(1):463–75.
AbouZid SF, Ali S, Choudhary MI. A new ferulic acid ester and other constituents from Tamarix nilotica leaves. Chem Pharm Bull. 2009;57 7:740–2.
Yoshida T, Ahmed A, Memon M, Okuda T. Tannins of tamaricaceous plants II. New Monomeric and Dimeric Hydrolyzable Tannins from Reaumuria hirtella and Tamarix pakistanica. Chem Pharm Bull. 1991;39:2849–54.
Orabi MAA, Taniguchi S, Sakagami H, Yoshimura M, Yoshida T, Hatano T. Hydrolyzable Tannins of Tamaricaceous plants. V. Structures of Monomeric–Trimeric Tannins and cytotoxicity of macrocyclic-type tannins isolated from Tamarix nilotica. J Nat Prod. 2013;76(5):947–56.
Sanz M, de Simón BF, Cadahía E, Esteruelas E, Muñoz AM, Hernández T, Estrella I, Pinto E. LC-DAD/ESI-MS/MS study of phenolic compounds in ash (Fraxinus excelsior L. and F. americana L.) heartwood. Effect of toasting intensity at cooperage. J mass spectrometry: JMS. 2012;47(7):905–18.
Orabi MAA, Zidan SAH, Attia GH, Alyami HS, Matsunami K, Hatano T. Ellagitannins and simple phenolics from the halophytic plant Tamarix nilotica. Nat Prod Res. 2022;36(1):177–85.
Yoshida T, Hatano T, Ahmed AF, Okonogi A, Okuda T. Structures of isorugosin E and hirtellin B, dimeric hydrolyzable tannins having a trisgalloyl group. Tetrahedron. 1991;47(22):3575–84.
Orabi MAA, Taniguchi S, Yoshimura M, Yoshida T, Kishino K, Sakagami H, Hatano T. Hydrolyzable Tannins of Tamaricaceous plants. III. Hellinoyl- and macrocyclic-type ellagitannins from Tamarix nilotica. J Nat Prod. 2010;73(5):870–9.
Thambugala KM, Daranagama DA, Phillips AJL, Bulgakov TS, Bhat DJ, Camporesi E, Bahkali AH, Eungwanichayapant PD, Liu Z-Y, Hyde KD. Microfungi on Tamarix. Fungal Divers. 2017;82(1):239–306.
Hatano T, Orabi M, Taniguchi S, Yoshida T, Yoshimura M. New Monomeric and Dimeric Hydrolyzable Tannins from Tamarix nilotica. Heterocycles 2010, 80.
Francescato LN, Debenedetti SL, Schwanz TG, Bassani VL, Henriques AT. Identification of phenolic compounds in Equisetum giganteum by LC-ESI-MS/MS and a new approach to total flavonoid quantification. Talanta. 2013;105:192–203.
Nawwar MAM, Souleman AMA, Buddrus J, Linscheid M. Flavonoids of the flowers of Tamarix nilotica. Phytochemistry. 1984;23(10):2347–9.
Bakr RO, Amer RI, Fayed MAA, Ragab TIM. A Completely Polyherbal Conditioning and Antioxidant Shampoo: A Phytochemical Study and Pharmaceutical Evaluation. J Pharm Bioallied Sci. 2019;11(2).
Abdul Khaliq H, Ortiz S, Alhouayek M, Muccioli GG, Quetin-Leclercq J. Dereplication and Quantification of Major Compounds of Convolvulus arvensis L. Extracts and Assessment of Their Effect on LPS-Activated J774 Macrophages. Molecules. 2022;27(3).
el-Mousallami A, Hussein S, Nawwar M. Polyphenolic metabolites of the flowers of Tamarix tetragyna. Nat Prod Sci. 2000;6:193–8.
Jenkinson SF, Cowley A, Kato A, Kato N, Hollinshead J, Nash RJ, Booth KV, Fleet GWJ. (2R, 3S)-3-Hydroxy-N, N-dimethylproline monohydrate. Acta Crystallogr Sect E: Struct Rep Online. 2007;63(9):o3717–7.
Nawwar MAM, Souleman AMA. 3, 4, 8, 9, 10-Pentahydroxy-dibenzo [b, d] pyran-6-one from Tamarix nilotica. Phytochemistry. 1984;23(12):2966–7.
Abouzid SF, Ali SA, Choudhary MI. A new ferulic acid ester and other constituents from Tamarix nilotica leaves. Chem Pharm Bull. 2009;57(7):740–2.
Mahrous FSM, Mohammed H, Sabour R. LC-ESI-QTOF-MS/MS of Holoptelea integrifolia (Roxb.) Planch. Leaves and in silico study of phenolic compounds’ antiviral activity against the HSV1 virus. Azhar Int J Pharm Med Sci. 2021;1(3):91–101.
Sekkien A, Swilam N, Ebada SS, Esmat A, El-Khatib AH, Linscheid MW, Singab AN. Polyphenols from Tamarix nilotica: LC–ESI-MSn profiling and in vivo antifibrotic activity. Molecules. 2018;23(6):1411.
Dang Y, Lin G, Xie Y, Duan J, Ma P, Li G, Ji G. Quantitative determination of myricetin in rat plasma by ultra performance liquid chromatography tandem mass spectrometry and its absolute bioavailability. Drug Res. 2014;64(10):516–22.
Boulaaba M, Tsolmon S, Ksouri R, Han J, Kawada K, Smaoui A, Abdelly C, Isoda H. Anticancer effect of Tamarix gallica extracts on human colon cancer cells involves Erk1/2 and p38 action on G2/M cell cycle arrest. Cytotechnology. 2013;65(6):927–36.
Stevens JF, Wollenweber E, Ivancic M, Hsu VL, Sundberg S, Deinzer ML. Leaf surface flavonoids of Chrysothamnus. Phytochemistry. 1999;51(6):771–80.
Parmalr VS, Rathore JS, Singh S, Jain AK, Gupta SR. Troupin, a 4-methylcoumarin from Tamarix troupii. Phytochemistry. 1985;24(4):871–2.
Riham O, Bakr AT, Heba A, El-Gizawy N, Tawfik UR, Abdelmohsen, Miada F, Abdelwahab WA, Alshareef SM, Fayez, Shereen MS, El-Mancy AM, El-Fishawy MA. Abdelkawy and Marwa A. A. Fayed: The metabolomic analysis of five Mentha species: cytotoxicity, anti-Helicobacter assessment, and the development of polymeric micelles for enhancing the anti-Helicobacter activity. 2021.
Karker M, De Tommasi N, Smaoui A, Abdelly C, Ksouri R, Braca A. New sulphated flavonoids from Tamarix africana and biological activities of its polar extract. Planta Med. 2016;82(15):1374–80.
Abad-García B, Garmón‐Lobato S, Berrueta LA, Gallo B, Vicente F. A fragmentation study of dihydroquercetin using triple quadrupole mass spectrometry and its application for identification of dihydroflavonols in Citrus juices. Rapid Commun Mass Spectrometry: Int J Devoted Rapid Dissemination Up‐to‐the‐Minute Res Mass Spectrom. 2009;23(17):2785–92.
Dalia I, Hamdan MAAF, Adel R. Echinops taeckholmiana Amin: optimization of a tissue culture protocol, Biological evaluation, and Chemical Profiling using GC and LC-MS. ACS Omega. 2021;6(20):13105–15.
Xiang D, Fan L, Hou Xl, Xiong W, Shi, Cy. Wang Wq, Fang Jg: uptake and transport mechanism of Dihydromyricetin Across Human intestinal Caco-2 cells. J Food Sci. 2018;83(7):1941–7.
Fayed MAA, Abouelela ME, Refaey MS. Heliotropium ramosissimum metabolic profiling, in silico and in vitro evaluation with potent selective cytotoxicity against colorectal carcinoma. Sci Rep. 2022;12:12539.
Kim HK, Choi YH, Verpoorte R. NMR-based plant metabolomics: where do we stand, where do we go? Trends Biotechnol. 2011;29(6):267–75.
Alnuqaydan AM, Rah B. Tamarix articulata inhibits cell proliferation, promotes cell death mechanisms and triggers G(0)/G(1) cell cycle arrest in Hepatocellular Carcinoma cells. Food Technol Biotechnol. 2021;59(2):162–73.
Abdullah MA, Abdulmajeed GA, Ohoud YA, Hanan Ali H, Abdullah MA, Abdullah A, Abdullah A, Mohd Younis R, Bilal R. Evaluation of the cytotoxic activity of Tamarix articulata and its anticancer potential in prostate cancer cells. J Appl Pharm Sci. 2022.
Hassan LEA, Ahamed MBK, Majid ASA, Baharetha HM, Muslim NS, Nassar ZD, Majid AMSA. Correlation of antiangiogenic, antioxidant and cytotoxic activities of some sudanese medicinal plants with phenolic and flavonoid contents. BMC Complement Altern Med. 2014;14(1):406.
Umbetova AK, Choudhary MI, Sultanova NA, Burasheva GS, Abilov ZA. Flavonoids of plants from the Genus Tamarix. Chem Nat Compd. 2004;41:728–9.
Orabi MA, Taniguchi S, Terabayashi S, Hatano T. Hydrolyzable tannins of tamaricaceous plants. IV: Micropropagation and ellagitannin production in shoot cultures of Tamarix tetrandra. Phytochemistry. 2011;72(16):1978–89.
Rezaei-Seresht H, Cheshomi H, Falanji F, Movahedi-Motlagh F, Hashemian M, Mireskandari E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: an in silico and in vitro study. Avicenna J Phytomedicine. 2019;9(6):574–86.
El Khazendar M, Chalak J, El-Huneidi W, Vinod A, Abdel-Rahman W, Abu-Gharbieh E. Antiproliferative and proapoptotic activities of ferulic acid in breast and liver cancer cell lines. 2019.
Nawwar MA, Youb NA, El-Raey MA, Zaghloul SS, Hashem AM, Mostafa ES, Eldahshan O, Werner V, Becker A, Haertel B, et al. Polyphenols in Ammania auriculata: structures, antioxidative activity and cytotoxicity. Die Pharmazie Int J Pharmaceut Sci. 2014;69(11):860–4.
Han M, Gao H, Xie J, Yuan YP, Yuan Q, Gao MQ, Liu KL, Chen XH, Han YT, Han ZW. Hispidulin induces ER stress-mediated apoptosis in human hepatocellular carcinoma cells in vitro and in vivo by activating AMPK signaling pathway. Acta Pharmacol Sin. 2019;40(5):666–76.
Lv L, Zhang W, Li T, Jiang L, Lu X, Lin J. Hispidulin exhibits potent anticancer activity in vitro and in vivo through activating ER stress in non–small–cell lung cancer cells. Oncol Rep. 2020;43(6):1995–2003.
Bhasker S, Madhav H, Chinnamma M. Molecular evidence of insulinomimetic property exhibited by steviol and stevioside in diabetes induced L6 and 3T3L1 cells. Phytomedicine Int J Phytother Phytopharmacol. 2015;22(11):1037–44.
Xu H, Yang T, Liu X, Tian Y, Chen X, Yuan R, Su S, Lin X, Du G. Luteolin synergizes the antitumor effects of 5-fluorouracil against human hepatocellular carcinoma cells through apoptosis induction and metabolism. Life Sci. 2016;144:138–47.
Han K, Meng W, Zhang JJ, Zhou Y, Wang YL, Su Y, Lin SC, Gan ZH, Sun YN, Min DL. Luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301. OncoTargets Therapy. 2016;9:3085–94.
Sato Y, Sasaki N, Saito M, Endo N, Kugawa F, Ueno A. Luteolin attenuates doxorubicin-induced cytotoxicity to MCF-7 human breast cancer cells. Biol Pharm Bull. 2015;38(5):703–9.
Li SZ, Li K, Zhang JH, Dong Z. The effect of quercetin on doxorubicin cytotoxicity in human breast cancer cells. Anti-cancer Agents Med Chem. 2013;13(2):352–5.
Maurya AK, Vinayak M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol Biol Rep. 2015;42(9):1419–29.
Acknowledgements
The authors acknowledge the Pharmacognosy Department, Faculty of Pharmacy, University of Sadat City, Sadat City 32897, Egypt, for supporting the run of this work in their labs.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that this study was self-funded.
Author information
Authors and Affiliations
Contributions
M.A.F., R. O. B.: Conceptualization; Methodology; Data curation; Resources; Supervision; Validation; Visualization; N. Y., S. A.M. K., H. R.E.: LC-MS & GNPS analysis; Software; D. I. H. and M. S. R.: Identification of LC-MS compounds; All authors shared writing – original draft; Writing – review & editing and approved the final submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The flowers of Tamarix nilotica (Ehrenb.) Bunge and Family Tamaricaceae were collected from Al-Wahat road, Egypt, in April 2019 with license approval from the Faculty of Pharmacy, University of Sadat City, Sadat City, Egypt according to relevant guidelines and regulations. The plant material was kindly identified by Prof. Dr. A. A. Fayed, Professor of Plant Taxonomy, Faculty of Science, Assiut University, Assiut, Egypt. We deposited a voucher sample (alphabetically ordered under the letter “T” for the genus “Tamarix”) in the Herbarium of the Faculty of Science, Assiut University, Assiut, Egypt.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fayed, M.A.A., Bakr, R.O., Yosri, N. et al. Chemical profiling and cytotoxic potential of the n-butanol fraction of Tamarix nilotica flowers. BMC Complement Med Ther 23, 169 (2023). https://doi.org/10.1186/s12906-023-03989-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-03989-8