Abstract
Background
Plants of the Myrcia genus have been widely used in folk medicine to treat various diseases, including cancer. Myrcia splendens species has a diverse chemical constitution, but the biological activities of its essential oil have not been well investigated. In this study to out the chemistry characterization of essential oil (EO) from the leaves of the species M. splendens from Brazil and evaluate cytotoxic effect in A549 lung cancer cells.
Methods
M. splendens EO was obtained by hydrodistillation and analyzed by Gas Chromatography-Mass Spectrometry (GC-MS). EO was isolated and evaluated for cellular viability in tumor cell lines by MTT assay. The evaluation of the formation of clones and the migratory capacity of the A549 cells treated with EO was done by the clonogenic assay and the wound healing assay. Morphological changes were observed in A549 cells by fluorescence using Phalloidin/FITC and DAPI.
Results
22 compounds were identified in the chemical analysis of EO, corresponding to 88% of the sample. Major compounds were the sesquiterpenic hydrocarbons bicyclogermacrene (15.4%), germacrene D (8.9%) and E-caryophyllene (10.1%). The biological analysis of the EO showed high cytotoxic activity with an IC50 below 20 µg/ml in the THP-1, A549 and B16-F10 tumor cells. The treatment with EO reduced colony formation and inhibited the migratory capacity of A549 cells. Furthermore, apoptotic morphological changes in the nucleus and cytoplasm of A549 cells was observed after of treatment with EO.
Conclusion
The findings of this study suggest that the M. splendens EO has cytotoxic compounds for the A549 lung cancer cells. Treatment with the EO decreased the colony formation and reduced the ability of lung cancer cells to migrate. Future studies may be used to isolate compounds from the EO for the study of lung cancer.
Similar content being viewed by others
Background
Cancer is a growing health problem in the world and there are still limitations in the treatment with chemotherapy, which in general cause several toxic effects [1]. The total number of cancer cases is expected to increase from 19.3 million in 2020 to 30.2 million in 2040, with nearly 10 million deaths worldwide accounted for in 2020 alone [2]. These data highlight the importance of using effective alternative and complementary treatments.
Recently there was growth in the studies of biological properties of natural products due its potential to treat several diseases, as cancer [3, 4]. Among these products, essential oils stand out, secondary metabolites that constitute a source of several bioactive compounds with anticancer potential, with their antitumor effects reported in in vitro and in vivo studies [5, 6]. In this regard, it is known that tropical plants have several essential oils with biological properties, as is the case with plants of the Myrtaceae family [7].
Myrcia splendens (SW.) DC. (Myrtaceae) is a tree plant with a wide distribution from Mexico to the South of Brazil [8]. In Brazil it is also known as “guamirim-da-folha-miúda” and in traditional medicine it is used to treat diarrhea, diabetes and hypertension [9, 10]. Some studies have demonstrated the biological properties of M. splendens EO including antibacterial [11], antifungal [12] and cytotoxic effects in tumor cells [9]. However, little is known about the antitumor activities of M. splendens EO. We hypothesize that EO has high in vitro antitumor activity. Thus, the present study aims to explore the antitumor effect of M. splendens EO on human tumor cell lines in vitro.
Methods
Plant material
Fresh leaves of M. splendens were collected in Ibura Flora, N. S. do Socorro, Sergipe, Brazil, and identified by Dr. Adauto de Souza Ribeiro, Federal University of Sergipe. The use of the leaves has been authorized by the Agency for Research in Federal Conservation Units (SISBIO) of the Chico Mendes Institute for Biodiversity Conservation (ICMBio) (collection authorization No. 68163/2019 – SISBIO – ICMBio). A voucher specimen (ASE 33399) is deposited at the Herbarium of the Department of Biology, Federal University of Sergipe, Brazil.
Essential oil isolation
Plant material was coarsely divided submitted to hydrodistillation, during 3 h, using a modified Clevenger apparatus and the procedure described in the European Pharmacopoeia [12]. The essential oil was obtained with a yield of 0.3% (w/w), dried over anhydrous sodium sulfate and kept in the dark at 4ºC prior to use.
GC/MS analysis
Composition of the oil was accessed by gas chromatography (GC) and gas chromatography-mass spectroscopy (GC/MS). Analytical GC was carried out in a Hewlett-Packard 6890 (Agilent Technologies, Palo Alto, CA, USA) gas chromatograph with HP GC Chem-Station Rev. A.05.04 data handling system, equipped with a single injector and two flame ionization detection (FID) systems. A graphpack divider (Agilent Technologies, part no. 5021–7148) was used for simultaneous sampling to two Supelco (Supelco, Bellefonte, PA, USA) fused silica capillary columns with different stationary phases: SPB-1 (polydimethylsiloxane 30 m x 0.20 mm i.d., film thickness 0.20 μm) and Supelcowax-10 (polyethylene glycol 30 m × 0.20 mm i.d., film thickness 0.20 μm). Oven temperature program is 70–220 °C (3 °C.min− 1), 220 °C (15 min), with injector temperature: 250 °C, carrier gas: helium, adjusted to a linear velocity of 30 cm.s− 1; splitting ratio 1:40; detectors temperature: 250 °C. GC-MS was carried out in a Hewlett-Packard 6890 gas chromatograph fitted with an HP1-fused silica column (polydimethylsiloxane 30 m × 0.25 mm i.d., 0.25 μm film thickness) interfaced with a Hewlett-Packard 5973 mass selective detector (Agilent Technologies) operated by HP Enhanced ChemStation software, version A.03.00. GC parameters are described above, with interface temperature: 250 °C, MS source temperature: 230 °C, MS quadrupole temperature: 150 °C, ionization energy: 70 eV, ionization current: 60 mA, scan range: 35–350 units, and scans.s− 1: 4.51. Essential oil components were identified by their retention indices on both SPB-1 and Supelcowax-10 columns and from their mass spectra. Retention indices, calculated by linear interpolation to retention times of C8–C23 n-alkanes, were compared with those of reference samples included in the Faculty of Pharmacy, University of Coimbra database. Acquired mass spectra were compared with reference spectra from laboratory database, Wiley/NIST library and literature data [13]. Relative amounts of individual components were calculated based on GC raw data areas without FID response factor correction.
Cells
The cytotoxicity of the EO was tested against lung adenocarcinoma (A549), melanoma (B16-F10) and acute monocytic leukemia (THP-1) cancer cell lines, all obtained from American Type Culture Collection (ATCC). Cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium or in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with heat-inactivated Fetal Bovine Serum (FBS, 10%) and 100 U/mL penicillin with 100 µg/mL streptomycin, and incubated at 37 °C with a 5% CO2 atmosphere.
MTT assay
The cytotoxicity activity was quantified using the MTT (3 - [4, 5-dimethylthiazole-2-yl]-2, 5-diphenyl-tetrazolium bromide) assay, as previously described by [14]. For all experiments, cells were seeded in 96-well plates (2 × 104 cells/mL in 200 µL of medium) in incubated overnight to permit attachment. After 24 h, the EO (2,5–100 µg/mL), dissolved in Dimethylsulfoxide (DMSO) or Doxorubicin (25 µg/mL) was added to each well and incubated for 24 h. Subsequently, 200 µL of MTT (5 mg/mL in PBS) was added to each well and further incubated for 3 h later, the formazan product was dissolved in 150 µL of DMSO, and the absorbance was read in a microplate reader (Synergy H1, Biotek, VT, EUA) at 570 nm. Cytotoxicity was expressed as the concentration of oil inhibiting cell viability by 50% (IC50). All measurements were performed in triplicate and the means and standard errors were calculated.
Clonogenic assay
The procedure of Franken et al. (2006) was employed with some modifications [15]. A549 cells were seeded in 6-well plate (300 cells/well) in RPMI medium containing 10% FBS and 1% antibiotic (penicillin 10,000 U/ml; streptomycin 10,000 mg/ml). After 24 h of incubation conditions in an oven with an atmosphere of 5% CO2 at 37 °C, the cells were treated with EO at concentrations of 10, 20 and 40 µg/mL, which correspond to the values of 0.5xIC50, 1xIC50 and 2xIC50, respectively. DMSO 0.1% and Doxorubicin 25 µg/mL were used as a negative control and a positive death control, respectively. After the treatment time, the media were removed and complete DMEM medium was added to the wells, and the cells were incubated in an oven with a 5% CO2 atmosphere at 37 ºC for 10 days. After aqueous solution with acid + water (3:5 min) stained with crystal violet, 5% in 30 min. At the end of the experiment, the growth pattern in number of colonies of cells was observed and counted, with the aid of Image J software.
Wound healing assay
A549 cells were seeded in 12-well plate at a density of 3 × 105 cells per well. After 24 h, the cell monolayer was scratched with a tip of p200 pipette creating a straight-line wound, the debris were removed by washing with PBS and the cells were treated with 10, 20 and 40 µg/mL the EO for 24 h. DMSO 0.1% was used as vehicle control and Doxorubicin 10 µM was used as positive control. The images were acquired 0, 24 and 48 h of the scratch using a microscopy Olympus. The percentage of wound closure was calculated for each treatment and controls comparing the time points 24 and 48 h with the time point zero, using the equation proposed by Yarrow et al. (2004) [16]. The images were analyzed using ImageJ 1.46 software.
Cell morphology assay
A549 cells were seeded at a concentration of 1 × 104 in 48-well plates, in RPMI medium containing 10% FBS and 1% antibiotic (penicillin 10,000 U/mL; streptomycin 10,000 mg/mL). After 24 h of incubation, the cells were exposed to concentrations of 10, 20 and 40 µg/mL the EO and incubated for 24 h in an oven. DMSO 0.1% and Doxorubicin 25 µg/mL were used as negative control and positive control, respectively. Culture medium from all wells of the plate was removed and the cells washed three times with 1X PBS. After washing, cells were fixed with 4% formaldehyde at room temperature for 15 min and then washed again with 1X PBS. Cells were permeabilized using 0.2% Triton X-100 solution diluted in PBS for 15 min and then 1% Bovine Serum Albumin (BSA) also diluted in PBS was used for 30 min. To visualize the cytoskeleton, cells were stained with Phalloidin/FITC (25 µg/mL) for 30 min, in the dark, followed by 2 washes with PBS. The cell nucleus was counterstained with DAPI (1 µg/mL) for 10 min, in the absence of light. Images were captured at 200x and 400x magnification under a fluorescence microscope (Olympus, USA).
Statistical analysis
For all experiments, a 95% confidence interval was used and p < 0.05 values considered statistically significant. Analyzes and graphs as well as IC50 were obtained using the GraphPad Prism 8. Shapiro-Wilk normality test was applied to assess the normal distribution of the data. For comparison between groups, ANOVA was used, followed by Dunnett post-test.
Results
Chemical composition of the M. splendens essential oil
The analysis of the essential oil from the leaves of M. splendens resulted in the identification of 22 constituents that represent 88% of the total composition (Table 1). The oil is composed of hydrocarbon sesquiterpenes (48.5%) and oxygen containing sesquiterpenes (39.6%), being bicyclogermacrene (15.4%), E-caryophyllene (10.1%) and germacrene D (8.9%) the major constituents.
M. splendens essential oil reduce the viability of cancer cell lines
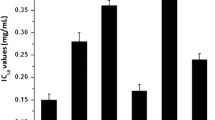
In order to assess the effects of EO on the viability of cancer cell lines, after the treatment of M. splendens EO at varied concentrations for 24 h, the cytotoxic activities were evaluated in three tumor cell lines: A549 (lung cancer), THP-1 (acute monocytic leukemia) and B16-F10 (melanoma), using the MTT assay. IC50 values and confidence interval are shown in Table 2. EO was cytotoxic in all tested tumor lines, presenting IC50 values of 5.37 µg/mL (2.17–13.30) in THP-1 cells, of 17.76 µg/mL (9.48–33 0.26) in B16-F10 cells and 20.14 µg/mL (16.46–24.64) in the A549 cells. Doxorubicin was used as a positive control and showed an IC50 value of 25.51 µg/mL in A549 cells. Considering the high rates of incidence and mortality of lung cancer, the A549 strain was chosen to continue the experiments.
M. splendens essential oil reduces the ability of A549 lung cancer cells to form colonies
To assess whether EO has the ability to inhibit the formation of colonies of A549 cells, a clonogenic assay was performed. Cells were treated for 24 h with EO at concentrations of 10, 20 and 40 µg/mL, which correspond to the values of 0.5xIC50, 1xIC50 and 2xIC50, respectively. After the treatment, the cells were kept growing for 10 days, and then the analyzes were performed. The results obtained are shown in Fig. 1. A significant reduction in the number of colonies (p < 0.0001) was observed in the treatment with EO at all concentrations tested, compared to the negative control. Treatment with Doxorubicin (25 µg/mL) also caused a significant reduction (p < 0.0001) compared to untreated cells. The data are represented in Fig. 1B.
Inhibitory effect of the M. splendens EO on the formation of A549 lung cancer cells colonies. (A) Colonies formed after treatment with increasing concentrations (10, 20 and 40 µg/mL) for 24 h of M. splendens EO and 10 days of growth. (B) Percentage of colony formation after treatment compared to control cells. Doxorubicin (25 µg/mL) was used as a positive control. Data show are mean ± SD obtained from three independent experiments. Statistical differences, compared to untreated control cells, were assessed by a one-way ANOVA with Dunnett post-test (****) p < 0.0001
M. splendens essential oil inhibits the migratory and invasive ability of A549 cells
The migration assay, using the Wound Healing technique, was performed to verify the effects of EO treatment on the migratory capacity of A549 cells. EO concentrations of 10, 20 and 40 µg/mL were used, and the analyzes were performed at 0, 24 and 48 h. Figure 2 shows the wound closure process in the cell monolayer, with representative images of the wells. According to the data obtained, it was possible to observe a significant effect on the migratory capacity (Fig. 2) of A549 cells at a concentration of 40 µg/mL after 24 h of treatment.
Inhibitory effect of the M. splendens EO on the migratory and invasive ability of A549 cells. (A) Representative photograph of the Wound Healing assay after treatment with increasing concentrations (10, 20 and 40 µg/mL) for 0, 24 and 48 h of M. splendens EO. (B) Percentage wound closure after treatment compared to control cells. Doxorubicin (25 µg/mL) was used as a positive control. Data show are mean ± SD obtained from three independent experiments. Statistical differences, compared to untreated control cells, were assessed by a Two-way ANOVA with Dunnett post-test (**) p < 0.05
M. splendens essential oil induces apoptosis in A549 cells
The apoptotic morphological changes in the nucleus and cytoplasm of A549 cells after 24 h of treatment with EO were evaluated using DAPI and Phalloidin/FITC dyes. Figure 3 shows the morphological changes caused by increasing concentrations of EO. According to the data presented, a reduction in cytoplasmic volume was observed in A549 cells at a concentration of 10 µg/mL of EO. At a concentration of 20 µg/mL, in addition to the reduction in cytoplasmic volume, cells with greater accumulation of DAPI in the nucleus were also observed, indicating DNA fragmentation and chromatin condensation. In cells treated at a concentration of 40 µg/mL, rounding and cytoplasmic and nuclear shrinkage were observed, in addition to chromatin condensation and DNA fragmentation. Treatment with Doxorubicin caused important cellular changes such as reduced cytoplasmic volume, chromatin condensation and DNA fragmentation. Overall, the most observed changes after treatment with EO concentrations were: decrease in cell number, cell rounding and shrinkage, and reduction of cytoplasmic volume, characteristics of programmed cell death induction. For the same treatment time, the observed changes were more intense as the EO concentration was increased.
Morphological changes in the cytoskeleton and nucleus of A549 cells, observed with DAPI and Phalloidin/FITC staining, after treatment for 24 h with M. splendens EO at concentrations of 10, 20 and 40 µg/mL. Doxorubicin (25 µg/mL) was used as a positive control. Filled arrows represent chromatin condensation and DNA fragmentation; hollow arrows represent reduction in cytoplasmic volume and thin arrows, rounding and cell shrinkage. Bar: 20 μm
Discussion
In this study, we demonstrated the cytotoxicity of essential oil from M. splendens leaves against the human lung adenocarcinoma cell line (A549). A previous study investigated the chemical characterization and biological activities, including cytotoxicity, of the EO from the leaves of M. splendens collected in Amazonian Ecuador. The authors found trans-nerolidol and α-bisabolol as the major EO compounds [9]. Interestingly, in the present study we observed a different chemical composition of the EO, in which the major compounds found were bicyclogermacrene, E-caryophyllene and germacrene D. This difference in the chemical composition of the M. splendens EOs may have occurred mainly due to the variation of the place of collection of the leaves, since the samples used in the present study were collected in the northeast of Brazil. Previous studies have reported that factors such as high genetic diversity can influence the variability of essential oils from species of the Myrtaceae family [17]. Despite the difference between the major M. splendens EO compounds found in our study and that of Scalvenzi et al. (2017), the chemical composition of the EO evaluated in both studies is compatible with regard to the predominance of sesquiterpenes.
Previous studies suggest that for an EO to be considered promising for cancer drug development, its IC50 values need to be less than 30 µg/mL [18]. In the present study, we observed that EO caused cytotoxicity in THP-1 (acute monocytic leukemia), B16-F10 (melanoma) and A549 (human lung adenocarcinoma) cell lines, with IC50 values less than 21 µg/mL. A study by Mohamed et al. (2018) reported cytotoxic activity in A549 cells treated with essential oil of Pistacia lentiscus L., which contained sesquiterpene germacrene D as one of the main compounds [19]. Another study reported that germacrene D and bicyclogermacrene, in synergism, were responsible for the high cytotoxic activity of essential oil from Porcelia macrocarpa leaves against leukemic cells (HL-60) [20]. In research carried out by The et al. (2021), the cytotoxic potential of the essential oil of the leaves of Polyalthia suberosa was evaluated, whose major constituents were bicyclogermacrene, E-caryophyllene and β-pinene. The results obtained showed cytotoxic activity against tumor cell lines of hepatocellular carcinoma (HepG2), breast cancer (MCF-7) and human lung adenocarcinoma (A549) [21]. These studies demonstrate that the sesquiterpenes bicyclogermacrene, E-caryophyllene and germacrene D are compounds that have antitumor action. The fact that these are the major constituents found in EO suggests that they may be the main components responsible for the antitumor action of M. splendens EO. However, studies are needed to investigate the action of these isolated compounds, for a definition of their antitumor activity.
In addition, EO was shown to significantly reduce (p < 0.05) the percentage of A549 cell colonies at all concentrations evaluated. Our results were similar to those obtained in the study by Dahham et al. (2015), who evaluated the anticlonogenic activity of sesquiterpene β-caryophyllene in colon carcinoma cells (HCT 116) [22]. A study by Toyang et al. (2013) reported the anticlonogenic activity of two sesquiterpenes isolated from Vernonia guineensis leaves, Vernopicrin and Vernomelitensin, which reduced the number of colonies in the prostate adenocarcinoma cell line (PC-3), during the nine-day exposure period, in a period similar to that used in the present study [23].
Among the mechanisms involved in the development and establishment of cancer is the process of tumor cell migration. During this process, tumor cells migrate from the primary site to a secondary organ, being a critical process of invasion, which allows the adaptation of primary tumors to metastasis [24, 25]. Our results indicate that EO causes a significant reduction in the migration capacity of A549 cells at the highest concentration (40 µg/mL), after 24 h of treatment. Studies show that essential oils have the ability to reduce tumor cell migration [26, 27]. The study by Chen et al. (2018), the essential oil of Eupatorium adenophorum Spreng., consisting mainly of sesquiterpenes, reduced the migration of hepatocellular carcinoma (HepG2) cells [28].
Furthermore, M. splendens EO was able to induce apoptosis in A549 cells, a cell death mechanism observed through changes in the nucleus and cytoplasm of A549 cells [29]. All concentrations promoted a reduction in cytoplasmic volume, while DNA fragmentation and chromatin condensation, evidenced by the accumulation of DAPI present in the cell nucleus, were only observed from a concentration of 20 µg/mL. This apoptotic effect was also observed in the study by Pereira et al. (2017), when evaluating the essential oil of Baccharis milleflora (Less.) DC against non-Hodgkin’s lymphoma (Raji) cells. The authors found bicyclogermacrene, germacrene D, E-caryophyllene and α-humulene as major compounds in the essential oil of Baccharis milleflora (Less.) DC [30].
Conclusion
This study showed that M. splendens EO has a cytotoxic effect on A549 lung cancer cells. The EO was able to reduce colony formation and cell migration, in addition to inducing death by apoptosis. Based on GC/MS, the study demonstrated that the EO is rich in sesquiterpenes. Considering the biological activities of sesquiterpenes, including antitumor activity, the cytotoxic effect of EO on A549 cells could have been attributed to these compounds. Thus, additional research is needed to investigate the biochemical mechanisms of M. splendens EO and its isolated compounds. Such findings may be useful to guide searches for potential new antitumor agents.
Data Availability
The datasets generated and/or analyzed in this study are available from the corresponding author on reasonable request.
Abbreviations
- A549:
-
Human Lung Carcinoma Epithelial Cell Line
- B16-F10:
-
Melanoma Cell Line
- CDK:
-
Cyclin Dependent Kinases
- DAPI:
-
6-Diamidine-2-phenylindole dihydrochloride
- DMSO:
-
Dimethylsulfoxide
- DOX:
-
Doxorubicin
- EDTA:
-
Ethylenediaminetetraacetic acid
- EO:
-
Essential Oil
- FBS:
-
Fetal Bovine Serum
- GC-MS:
-
Gas Chromatography Coupled with Mass Spectrometry
- IC50 :
-
Inhibitory concentration for 50% of cells
- INCA:
-
José Alencar da Silva National Cancer Institute
- MCF-7:
-
Adenocarcinoma Cell Line
- MSEO:
-
Myrcia splendens Essential Oil
- PHALLOIDIN/FITC:
-
Fluorescein Isothiocyanate
- PSB:
-
Phosphate Saline Buffer
- THP-1:
-
Leukemia Cell Line
- WHO:
-
World Health Organization
References
Regnard C, Kindlen M. Chemotherapy: side effects. Support Palliat Care Cancer. 2019;:39–41.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111–29.
Munari CC, De Oliveira PF, Campos JCL, Martins SDPL, Da Costa JC, Bastos JK, et al. Antiproliferative activity of Solanum lycocarpum alkaloidic extract and their constituents, solamargine and solasonine, in tumor cell lines. J Nat Med. 2014;68:236–41.
Kubatka P, Uramova S, Kello M, Kajo K, Samec M, Jasek K et al. Anticancer activities of thymus vulgaris L. In experimental breast carcinoma in vivo and in vitro. Int J Mol Sci. 2019;20.
Jamali T, Kavoosi G, Ardestani SK. In-vitro and in-vivo anti-breast cancer activity of OEO (Oliveria decumbens vent essential oil) through promoting the apoptosis and immunomodulatory effects. J Ethnopharmacol. 2020;248:112313.
Ferreira OO, Cruz JN, de Moraes ÂAB, Franco C, de Lima JP, Dos Anjos RR. Essential oil of the plants growing in the brazilian Amazon: Chemical Composition, Antioxidants, and Biological Applications. Molecules. 2022;27:1–29.
Brand MM, Vieira A. 957 Estrutura Genética em Microescala Espacial De. Rev Árvore. Viçosa-MG. 2011;5:957–64.
Scalvenzi L, Grandini A, Spagnoletti A, Tacchini M, Neill D, Ballesteros JL, et al. Myrcia splendens (sw.) DC. (syn. M. fallax (Rich.) DC.) (myrtaceae) essential oil from amazonian ecuador: a chemical characterization and bioactivity profile. Molecules. 2017;22:1–12.
Botânica S, Atlântica M, Iheringia. Govaerts 2014. 2016;71:261–8.
Alarcon L, Pena A, Gonzales N, Quintero A, Meza M, Usubillaga A, et al. Composition and antibacterial activity of the essential oil of Myrcia fallax (Rich.) DC. From Venezuela. Rev la Soc Química del Perú. 2009;75:221–8.
Pontes FC, Abdalla VCP, Imatomi M, Fuentes LFG, Gualtieri SCJ. Antifungal and antioxidant activities of mature leaves of Myrcia splendens (sw.) DC. Brazilian J Biol. 2019;79:127–32.
Rodrigues DF, Arenas Velásquez AM, Cavaleiro C, Salgueiro L, Martins GZ, Magalhães NO, Koenig et al. (Zingiberaceae). ISRN Infect Dis. 2013;2013:1–6.
Mitra I, Mukherjee S, Reddy Venkata PB, Dasgupta S, Jagadeesh Bose CK, Mukherjee S, et al. Benzimidazole based Pt(II) complexes with better normal cell viability than cisplatin: synthesis, substitution behavior, cytotoxicity, DNA binding and DFT study. RSC Adv. 2016;6:76600–13.
Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9.
Yarrow JC, Perlman ZE, Westwood NJ, Mitchison TJ. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004;4:1–9.
De Cerqueira MD, Souza-Neta LC, Passos MDGVM, Lima EDO, Roque NF, Martins D, et al. Seasonal variation and antimicrobial activity of Myrcia myrtifolia essential oils. J Braz Chem Soc. 2007;18:998–1003.
Suffness M, Pezzuto JM. Assays related to cancer drug discovery,” in methods in Plant Biochemistry: assays for Bioactivity. K Hostettmann Academic Press. 1990;6:71–133.
Mohamed K, Zine K, Fahima K, Abdelfattah E, Sharifudin SM, Duduku K. NiO nanoparticles induce cytotoxicity mediated through ROS generation and impairing the antioxidant defense in the human lung epithelial cells (A549): preventive effect of Pistacia lentiscus essential oil. Toxicol Rep. 2018;5:480–8.
Da Silva EBP, Matsuo AL, Figueiredo CR, Chaves MH, Sartorelli P, Lago JHG. Chemical constituents and cytotoxic evaluation of essential oils from leaves of Porcelia macrocarpa (Annonaceae). Nat Prod Commun. 2013;8:277–9.
The SN, Le Tuan A, Thu TDT, Dinh LN, Thi TT. Essential Oils of Polyalthia suberosa Leaf and Twig and Their Cytotoxic and Antimicrobial Activities. Chem Biodivers. 2021;18.
Dahham SS, Tabana YM, Iqbal MA, Ahamed MBK, Ezzat MO, Majid ASA, et al. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules. 2015;20:11808–29.
Toyang NJ, Wabo HK, Ateh EN, Davis H, Tane P, Sondengam LB, et al. Cytotoxic sesquiterpene lactones from the leaves of Vernonia guineensis Benth. (Asteraceae). J Ethnopharmacol. 2013;146:552–6.
Woo CC, Loo SY, Gee V, Yap CW, Sethi G, Kumar AP, et al. Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-γ pathway. Biochem Pharmacol. 2011;82:464–75.
Hendriks LEL, Smit EF, Vosse BAH, Mellema WW, Heideman DAM, Bootsma GP, et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer. 2014;84:86–91.
Cho SM, Lee EO, Kim SH, Lee HJ. Essential oil of Pinus koraiensis inhibits cell proliferation and migration via inhibition of p21-activated kinase 1 pathway in HCT116 colorectal cancer cells. BMC Complement Altern Med. 2014;14:1–9.
Bhagat M, Kumar A, Suravajhala R. Cedrus deodara (bark) essential oil induces apoptosis in human Colon Cancer cells by Inhibiting Nuclear factor kappa B. Curr Top Med Chem. 2020;20:1981–92.
Chen H, Zhou B, Yang J, Ma X, Deng S, Huang Y, et al. Essential oil derived from Eupatorium adenophorum Spreng. Mediates anticancer effect by inhibiting STAT3 and AKT activation to induce apoptosis in hepatocellular carcinoma. Front Pharmacol. 2018;9 MAY:1–17.
Zhang Y, Chen X, Gueydan C, Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28:9–21.
Pereira CB, Kanunfre CC, Farago PV, Borsato DM, de Budel JM et al. Noronha Sales Maia BHL,. Cytotoxic mechanism of Baccharis milleflora (Less.) DC. essential oil. Toxicol Vitr. 2017;42 May:214–21.
Acknowledgements
Declared none.
Funding
Research partially funded by Fundação para a Ciência e Tecnologia, Portugal, ref. UIDP/00102/2020.
Author information
Authors and Affiliations
Contributions
MMM carried out the research and wrote the first draft of the manuscript. FBF and EWPS assisted in the research work and revised the manuscript. JFS helped in the clonogenic assay and wound healing experiments. WLJ contributed to the research work. ASR collected and identified the leaves of Myrcia splendens. ASF and SMFM prepared Myrcia splendens essential oils. CC revised the manuscript. RS helped with data interpretation and revised the manuscript. CBC guided the research, reviewed and submitted the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Fresh leaves of Myrcia splendens were collected in Ibura Flora, N. S. do Socorro, Sergipe, Brazil. The use of the leaves of species Myrcia splendens has been authorized by the Agency for Research in Federal Conservation Units (SISBIO) of the Chico Mendes Institute for Biodiversity Conservation (ICMBio) (collection authorization No. 68163/2019 – SISBIO - ICMBio), and identified by Dr. Adauto de Souza Ribeiro, Federal University of Sergipe. A voucher specimen (ASE 33399) is deposited at the Herbarium of the Department of Biology, Federal University of Sergipe, Brazil. The experimental research and feld studies on plants, including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation.
Competing interests
The authors report no conflicts of interest of this work.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Montalvão, M.M., Felix, F.B., Propheta dos Santos, E.W. et al. Cytotoxic activity of essential oil from Leaves of Myrcia splendens against A549 Lung Cancer cells. BMC Complement Med Ther 23, 139 (2023). https://doi.org/10.1186/s12906-023-03969-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-03969-y