Abstract
Background
Drug resistance exists in almost all antimalarial drugs currently in use, leading to an urgent need to identify new antimalarial drugs. Medicinal plant use is an alternative approach to antimalarial chemotherapy. This study aimed to explore potent medicinal plants from Prabchompoothaweep remedy for antimalarial drug development.
Methods
Forty-eight crude extracts from Prabchompoothaweep remedy and its 23 plants ingredients were investigated in vitro for antimalarial properties using Plasmodium lactate dehydrogenase (pLDH) enzyme against Plasmodium falciparum K1 strain and toxicity effects were evaluated in Vero cells. The plant with promising antimalarial activity was further investigated using gas chromatography-mass spectrometry (GC-MS) to identify phytochemicals. Antimalarial activity in mice was evaluated using a four-day suppressive test against Plasmodium berghei ANKA at dose of 200, 400, and 600 mg/kg body weight, and acute toxicity was analyzed.
Results
Of the 48 crude extracts, 13 (27.08%) showed high antimalarial activity against the K1 strain of P. falciparum (IC50 < 10 μg/ml) and 9 extracts (18.75%) were moderately active (IC50 = 11–50 μg/ml). Additionally, the ethanolic extract of Prabchompoothaweep remedy showed moderate antimalarial activity against the K1 strain of P. falciparum (IC50 = 14.13 μg/ml). Based on in vitro antimalarial and toxicity results, antimalarial activity of the aqueous fruit extract of Terminalia arjuna (IC50 = 4.05 μg/ml and CC50 = 219.6 μg/ml) was further studied in mice. GC-MS analysis of T. arjuna extract identified 22 compounds. The most abundant compounds were pyrogallol, gallic acid, shikimic acid, oleamide, 5-hydroxymethylfurfural, 1,1-diethoxy-ethane, quinic acid, and furfural. Analysis of the four-day suppressive test indicated that T. arjuna extract at dose of 200, 400, and 600 mg/kg body weight significantly suppressed the Plasmodium parasites by 28.33, 45.77, and 67.95%, respectively. In the acute toxicity study, T. arjuna extract was non-toxic at 2000 mg/kg body weight.
Conclusions
The aqueous fruit extract of T. arjuna exerts antimalarial activity against Plasmodium parasites found in humans (P. falciparum K1) and mice (P. berghei ANKA). Acute toxicity studies showed that T. arjuna extract did not show any lethality or adverse effects up to a dose of 2000 mg/kg.
Similar content being viewed by others
Background
Malaria is an infectious disease transmitted to humans through the bites of infected female Anopheles mosquitoes. Five species of Plasmodium parasites, Plasmodium falciparum, Plasmodium malariae, Plasmodium vivax, Plasmodium ovale, and Plasmodium knowlesi cause malaria in humans [1]. The World Health Organization reported an estimated 241 million malaria cases worldwide, with an estimated 627,000 malaria deaths in 2020. The number of malaria cases in 2020 increased by 14 million compared with those in 2019 with accounted for 227 million cases [2]. Young children (under five years old), pregnant women, and non-immune people are the most vulnerable groups affected by malaria [2]. Antimalarial drug resistance has emerged as an essential problem requiring control. The first-line treatment, artemisinin-based combination therapy (ACTs) is now emergence and spread of resistance in the Greater Mekong subregion (GMS), which consists of Cambodia, Thailand, Vietnam, Myanmar and Laos. Resistance to the partner drugs piperaquine and mefloquine is also now common in the GMS, led to high failure rates of ACTs treatment [3, 4]. Therefore, there is an urgent need to develop novel therapeutic agents for malaria treatment.
Prabchompoothaweep remedy has been used in traditional Thai medicine for many years to treat allergic rhinitis and upper respiratory tract diseases [5, 6]. This remedy consists of 23 medicinal plants documented in the National List of Essential Medicine of Thailand. Among the medicinal plants, Terminalia arjuna Wight and Arn, commonly known as “arjuna” or “Sa-mor-tes” in Thai, has traditionally been used to treat several human diseases, including cardiovascular disorders (ischemia, cardiomyopathy, atherosclerosis, and myocardial necrosis) and blood diseases (anemia, and venereal and viral diseases) [7,8,9]. T. arjuna possesses several medicinal properties, including hypocholesterolemic, antibacterial, antimicrobial, antitumoral, antioxidant, anti-allergic, antifeedant, antifertility, and anti-human immunodeficiency virus activities [10,11,12].
However, the antimalarial properties and toxicity of Prabchompoothaweep remedy have not yet been reported. Therefore, this study aimed to investigate the in vitro antimalarial activity and toxicity of this remedy and its 23 medicinal plant ingredients. Additionally, a good candidate plant for antimalarial drug development was selected for further in vivo study of antimalarial activity and acute toxicity in mice.
Methods
Plant materials
Twenty-three plant ingredients from the Prabchompoothaweep remedy were purchased from a traditional Thai drug store in the Nakhon Si Thammarat Province, Thailand (Table 1). The use of plant materials complied with the relevant guidelines and regulations of the Plant Varieties Protection, Department of Agriculture, Ministry of Agriculture and Cooperatives, Thailand. The plant species were identified by Assoc. Prof. Tanomjit Supavita, School of Pharmacy at Walailak University. Voucher herbarium specimens were deposited in the School of Medicine, Walailak University (Table 1).
Plant extraction
All plant samples were cleaned with distilled water to remove dirt and dried at 60 °C in a hot air oven for 72 h. The plant samples were then cut into small pieces and weighed into portions of 60 g. Each plant was extracted using ethanol and distilled water. Ethanol was selected as a solvent due to it can dissolve most slightly non-polar and slightly polar molecules. Distilled water was used as the solvent to be related to the usage almost plants as Thai folk medicines. For ethanolic extraction, the plant samples (60 g) were macerated in 600 ml of 80% ethanol at 25–30 °C for 72 h and this procedure was repeated three times. The aqueous extract was obtained using the decoction method and 60 g of each plant was extracted three times by mixing with 600 ml of distilled water and boiled at 90–100 °C for 30 min. The resulting extract in each method was filtered through Whatman No. 1 filter paper, evaporated in a rotary evaporator (N-1200B, EYELA Co., Ltd., Shanghai, China) at 60 °C, and lyophilized to dryness using a freeze-dryer (Gamma 2–16 LSCplus, Martin Christ, Osterode am Harz, Germany). The crude extracts were collected and stored at 4 °C until use.
Phytochemical analysis
All the extracts were subjected to standard phytochemical analyses to determine the presence of flavonoids, terpenoids, alkaloids, tannins, anthraquinone, cardiac glycosides, saponins, and coumarins, as previously described with some modifications [13, 14].
In vitro cultivation of Plasmodium falciparum
To investigate in vitro antimalarial activity, P. falciparum K1 strain was cultured as previously described with minor modifications [15]. The Plasmodium parasite was cultured in uninfected O+ red blood cells (RBCs) as host cells and maintained in complete medium (RPMI-1640) containing 2 mg/ml sodium bicarbonate, 10 μg/ml hypoxanthine (Sigma-Aldrich, New Delhi, India), 4.8 mg/ml HEPES (HiMedia, Mumbai, India), 0.5% Albumax II (Gibco, Waltham, MA, USA), and 2.5 μg/ml gentamicin (Sigma-Aldrich). The culture flasks were incubated at 37 °C and 5% CO2. The percentage of parasitemia was monitored daily using a light microscope.
In vitro antimalarial activity assay
Prabchompoothaweep remedy and its plant ingredient extracts were tested for their antimalarial activity using an in vitro Plasmodium lactate dehydrogenase (pLDH) assay [16]. In this assay plates containing 2% parasite cultures were incubated with crude extract at final concentrations between 0.3–2500 mg/ml for 72 h at 37 °C in a CO2 incubator. Artesunate (0.3–2500 mg/ml) (Sigma-Aldrich) and dimethyl sulfoxide (DMSO; Merck, Darmstadt, Germany) were added to each well as positive and negative controls, respectively. Non-infected RBCs were used as blank controls. After 72 h of incubation, the plates were frozen at − 20 °C and thawed at 37 °C three times. The supernatant from each well was transferred to a new microplate containing the Malstat reagent (Sigma-Aldrich). Nitroblue tetrazolium/phenazine ethosulfate solution (Sigma-Aldrich) was added to the plate and cultured in the dark for 60 min. Next, 5% acetic acid (Merck) was added to each well to stop the reaction. The absorbance at 650 nm was measured using a microplate reader. Each sample was tested in triplicates. Finally, a log dose-response curve was generated and used to determine the percent inhibition and half-maximal inhibitory concentration (IC50).
In vitro cytotoxicity assay
Vero cells (1 × 105/well) were plated in 200 μl of complete medium per well in 96-well plates. After cell attachment, the plant extracts were added and incubated at 37 °C for 24 h. Concentrations of plant extract varied from 0 to 80 μg/ml. The culture medium was then replaced with 100 μl of fresh medium/well containing 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) per well and incubated at 37 °C for 3 h. DMSO was then added to each well and incubated for another 20 min at room temperature in the dark. Lastly, the absorbance at 560 and 670 nm was measured using a microplate reader. All experiments were repeated thrice. The 50% cytotoxic concentration (CC50) of the extracts was determined by dose-response curve analysis [17].
Selectivity index
A selectivity index (SI), which is the ratio between cytotoxic and antimalarial activities [18], was calculated for each extract according to the following formula:
GC-MS analysis
The relative quantities of the phytochemicals present in the extracts were determined using gas chromatography with a 7000C Triple Quadrupole GC/MS (Agilent Technologies, Santa Clara, CA, USA) equipped with an HP-5MS column (30 m × 0.25 mm; 0.25 μm). Spectroscopic detection by GC-MS involves an electron ionization system that utilizes high energy electrons of 70 eV, ion source temperature of 250 °C, and mass scanning range of 33–600 amu in full scan. Pure helium gas (99.99%) was used as the carrier gas at a constant flow rate of 1 ml/min. The injector temperature was maintained at a constant of 250 °C, and the oven temperature was programmed as follows: 60 °C for 2 min, 150 °C at an increasing rate of 10 °C/min, and finally, 300 °C at an increasing rate of 5 °C/min. The sample (1 μl) in ethanol was injected in the split mode at a split ratio of 20:1, respectively. The compounds in the test samples were identified by comparing their retention times and mass spectra with those in the spectral database of the National Institute of Standards and Technology (NIST2011) structural library. Only peaks with 80% similarity and above with the NIST libraries were selected and identified.
Animals
Male Institute of Cancer Research (ICR) mice aged 6–8 weeks old and weighing 25–30 g in body weight were purchased from Nomura Siam International Co., Ltd. (Pathumwan, Bangkok, Thailand). The mice housing temperature was maintained at a room temperature of approximately 22 °C (± 3 °C) and relative humidity of 50–60%. The lighting environment was set to a 12:12 h light/dark cycle. Mice were allowed free access to food pellets and clean drinking water.
Four-day suppressive test (Peter’s test)
The four-day suppressive test was used to measure the schizonticidal activity of the aqueous extract of T. arjuna against P. berghei ANKA-infected ICR mice. The method was performed as previously described with minor modifications [19, 20]. Briefly, male ICR mice were randomly divided into five groups of five animals. Twenty-five mice were injected with 1 × 107 RBCs infected with P. berghei ANKA via intraperitoneal injection [20]. The treatment started 4 h following inoculation. In the extract treatment groups, the animals received daily oral doses of 200, 400, or 600 mg/kg body weight aqueous extract of T. arjuna in 200 μl of 7% Tween 80 solution. The dosage was selected with increasing as low, moderate and high doses of crude extract with 200, 400, and 600 mg/kg body weight according to previous studies [20,21,22]. The negative control group received 200 μl of 7% Tween 80 solution, while the positive control group was administered 6 mg artesunate/kg body weight orally per day. The mice were administered each substance daily for 4 days (at 4, 24, 48, and 72 h after inoculation). On the fifth day, the percentage of parasitemia was determined using Giemsa staining. Percent inhibition was calculated using the following formula [23]:
Determination of mean survival time (MST)
MST was determined as described by Chaniad et al. [20]. Twenty-five mice were used in the four-day suppressive test and fed ad libitum. Mouse mortality was monitored daily until day 30 after parasite inoculation. Any deaths in the treatment and control groups that occurred during the follow-up period were recorded. The MST for each group was calculated using the following formula [23]:
Acute toxicity test
The crude aqueous extract of T. arjuna was assessed for toxicity in non-infected ICR mice aged 6–8 weeks old and weighing 25–30 g according to the standard guidelines of the Organization for Economic Cooperation and Development [24]. Fifteen mice were randomly divided into three groups of five mice each: mice treated with 2000 mg/kg T. arjuna aqueous extract, negative control, and untreated. The aqueous extract of T. arjuna was dissolved in 7% Tween 80 to a dose of 2000 mg/kg body weight. Mice in the experimental group received a single dose of 2000 mg/kg T. arjuna extract orally, while mice in the control group were administered 200 μl of 7% Tween 80 solution. Blood samples were collected into heparinized tubes using a cardiac puncture technique. The plasma samples were used for biochemical analysis of liver function (alanine aminotransferase [ALT], aspartate aminotransferase [AST], and alkaline phosphatase [ALP]) and kidney function (blood urea nitrogen [BUN] and creatinine [Cr]) using an AU480 chemistry analyzer (Beckman Coulter, Brea, CA, USA). Furthermore, liver and kidney tissues were removed and fixed in formalin for histopathological examination.
Histopathology
Histopathological examination of the liver and kidney tissues was performed according to previously described histological procedures [25, 26]. All tissue were fixed in 10% buffered formalin, then dehydrated using a gradient series of ethanol solutions, rinsed three times with xylene, and placed in a mold containing paraffin. The paraffin blocks were then serially sectioned at 5 μm thickness, transferred to glass slides, and stained with hematoxylin and eosin solution. To evaluate histopathological changes, the stained slides were observed using a light microscope by two independent researchers blinded to the experimental groups.
Statistical analysis
The results are presented as mean ± standard error of the mean (SEM). IBM SPSS Statistics version 23.0 software was used for the statistical analysis. The Kolmogorov-Smirnov goodness-of-fit test was used to test the normal distribution. The statistical significance of parasitemia inhibition was analyzed using one-way analysis of variance, followed by Tukey’s multiple comparison test. Statistical significance was set at p-value less than 0.05 (p ≤ 0.05).
Results
Extraction of plant materials
The percentage of crude extract yield (%yield) as shown in Table 2. Extraction of the roots of A. lancea with water produced the highest crude extract, 31.60 g of dark brown solid were afforded after freeze drying representing 52.67% yield with respect to the plant material used. Ethanolic extract was produced with brown sticky mixed with yellow liquid with 13.26 g accounting for a 22.11% yield. Extraction of the fruits of T. arjuna with ethanol, 8.85 g of a caramel sticky solid were obtained representing 14.76% yield and aqueous extract was afforded as 27.87 g of caramel sticky solid translating to 46.44% yield. Preparation of T. chebula extracts in ethanol produced dark brown semi-solid with the 25.58% yield and aqueous extract also produced dark brown semi-solid with 36.15% yield.
Phytochemical screening
Phytochemical analysis of each plant component in Prabchompoothaweep remedy revealed the presence of flavonoids, terpenoids, alkaloids, tannins, saponins, and coumarins, whereas anthraquinones and cardiac glycosides were not detected in any of the extracts (Table 3). Moreover, the ethanolic extract of the remedy contained terpenoids, alkaloids, tannins, and coumarins, whereas the aqueous extract of the remedy contained flavonoids, terpenoids, alkaloids, tannins, saponins, and coumarins (Table 3).
In vitro antimalarial activity
The in vitro antimalarial activity of Prabchompoothaweep remedy and its ingredients is shown in Table 4. The activity of the extracts was considered high if IC50 < 10 μg/ml, moderately active if IC50 ranged between 11 and 50 μg/ml, mildly active if IC50 ranged between 51 and 100 μg/ml, and inactive if IC50 > 100 μg/ml [27]. According to these criteria, 13 extracts (27.08%) of 10 plants showed high antimalarial activity against the K1 strain of P. falciparum with IC50 values lower than 10 μg/ml. Nine extracts (18.75%) were moderately active and five extracts (10.42%) possessed mild activity. Of the total tested plant extracts, the aqueous flower extract of S. aromaticum was the most active against P. falciparum, with the lowest IC50 value (1.96 μg/ml), followed by the ethanolic flower extract of P. chaba, ethanolic rhizome extract of Z. officinale, aqueous fruit extract of T. arjuna, ethanolic fruit extract of P. nigrum, and ethanolic fruit extract of T. arjuna (IC50 = 2.06, 3.42, 4.05, 4.38, and 4.72 μg/ml, respectively). The ethanolic extract of Prabchompoothaweep showed moderate antimalarial activity against the K1 strain of P. falciparum (IC50 = 14.13 μg/ml). Artesunate, the positive control, exhibited antimalarial activity at an IC50 of 1.25 ng/ml.
In vitro cytotoxicity
The evaluation of in vitro toxicity in Vero cells is shown in Table 4. A non-toxic effect is defined as a CC50 value greater than 50 μg/ml [28]. Therefore, all extracts were non-toxic to Vero cells with CC50 values greater than 50 μg/ml, except five ethanolic extracts that showed toxic effects: L. sibiricus leaf, A. lancea root, F. vulgare fruit, M. fragrans aril, and A. graveolens fruit (CC50 = 20.51, 29.54, 31.50, 38.30, and 49.51 μg/ml, respectively).
Of the total 48 extracts, the aqueous extract of T. arjuna was found in the top five extracts with an antimalarial effect and was non-toxic to Vero cells. This extract showed promising antimalarial activity (IC50 = 4.05 μg/ml) against the K1 strain of P. falciparum and no cytotoxic effect against Vero cells (CC50 > 200 μg/ml). Based on the high antimalarial activity and SI values obtained for the aqueous fruit extract of T. arjuna and no previous report of its antimalarial activity, the in vivo antimalarial activity and acute toxicity of this extract was further evaluated in mice.
GC-MS analysis of ethanolic aqueous fruit extract of T. arjuna
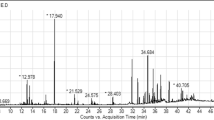
The GC-MS chromatograms of the fruit extract of T. arjuna are shown in Fig. 1. The mass spectra of the phytochemical compounds were compared with those in the spectral database of known compounds in the NIST library. Twenty-two compounds were identified and characterized (Table 5). The most abundant compound was pyrogallol with a retention time of 11.690 min (40.69%), followed by gallic acid (9.87%), shikimic acid (7.19%), oleamide (6.11%), and 5-hydroxymethylfurfural (5.72%), 1,1-diethoxy-ethane (3.11%), quinic acid (2.44%) and furfural (1.08%). Other compounds were present at concentrations below 1%. Chemical structure of eight compounds with the peak area greater than 1% was illustrated in Fig.2. Particularly, 5-hydroxymethylfurfural, compounds 8 with a retention time of 9.631 and maltol, compound 9 with a retention time of 9.944 have the same formula as C6H6O3 but the spectrum patterns are different (Fig. 3). Interestingly, the identified compounds, i.e., benzenetriol (pyrogallol), trihydroxybenzoic acid (gallic acid), shikimic acid, and cinnamic acid, are interrelated via the biosynthesis pathway (Fig.4).
Biosynthesis pathay of shikimic cid, cinnamic acid, gallic acid and pyrogallol. DAHPS: 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase; SK II: shikimate kinase II; CM: chorismate mutase; PAL: phenylalanine ammonia lyase; 3-DHD: 3-dehydroshikimate dehydratase; 3-DHG: 3-dehydroshikimate dehydrogenase; AR: aromatase; DC: decarboxylase [29,30,31].
Four-day suppressive test
The four-day suppressive test showed that mice treated with aqueous fruit extract of T. arjuna at concentrations of 200, 400, and 600 mg/kg presented significantly (p < 0.001) lower percentages of parasitemia (24.58, 18.60, and 10.99%, respectively) compared with that in the negative control group (34.30%). Mice treated with aqueous fruit extracts of T. arjuna exhibited parasite suppression rates in a dose-dependent manner, with a maximum activity of 67.95%, followed by 45.77 and 28.33% at doses of 600, 400 and 200 mg/ml, respectively (Table 6). The survival time of all mice was also assessed over a 30-d period, as shown in Table 6. The MST of the extract-treated groups was dose-dependent. Extract doses of 200, 400, and 600 mg/kg body weight significantly (p < 0.05) prolonged the survival time by 13.00, 16.00, and 17.40 d, respectively, compared with that in the negative control mice (8.60 d). Additionally, compared with the 200 mg/kg extract-treated group, the mean survival durations of the 400 and 600 mg/kg extract-treated groups were significantly extended (Table 6).
In vivo acute toxicity biochemical tests
All mice treated with 2000 mg/kg aqueous fruit extract of T. arjuna revealed no gross physical or behavioral changes, including lacrimation, altered feeding activities, vomiting, diarrhea, abnormal secretion, abnormal sleep, excitement, and hair erection for 24 h, and no mortality occurred during the 14-d follow-up period. Therefore, the lethal dose of the extract was greater than 2000 mg/kg body weight. To determine the effects of the aqueous fruit extract of T. arjuna on the liver and kidney, plasma biomarkers of liver and kidney functions were examined. The findings demonstrated that the mean levels of ALT, ALP, BUN, and Cr in the mice treated with 2000 mg/kg T. arjuna extract did not significantly differ from those in the 7% Tween 80 and untreated control groups (Table 7). However, the mean levels of AST in mice treated with 2000 mg/kg T. arjuna extract were significantly higher than those in the 7% Tween 80 group (p < 0.05).
Histopathological changes
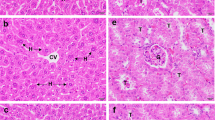
Histopathological examination revealed that the mice treated with 2000 mg/kg T. arjuna extract exhibited normal histopathological features in both liver and kidney tissues compared with those in the negative control group (Fig. 5). Therefore, the aqueous fruit extract of T. arjuna at a dose of 2000 mg/kg body weight did not have acute hepatotoxic or nephrotoxic effects.
Discussion
Prabchompoothaweep remedy has long been used in Thai traditional medicine to relieve the common cold, hay fever, allergic rhinitis, and upper respiratory tract disease [5, 6]. The aqueous and ethanolic extracts of all plant ingredients from the Prabchompoothaweep remedy were investigated for the presence of phytochemical constituents and then for antimalarial properties against P. falciparum K1 strain and cytotoxicity in Vero cells.
In our in vitro study, the extracts of Prabchompoothaweep remedy and its plant ingredients were tested using enzymatic detection of the pLDH enzyme. The toxicity of the extract was next examined in Vero cells. According to the cell cytotoxicity classification, the CC50 value was used to define the potency of cytotoxicity. A non-toxic effect is classified as a CC50 value greater than 50 μg/ml [28]. The extracts exhibited varying degrees of antimalarial activity. Among the 48 crude extracts tested in the present study, the aqueous flower extract of S. aromaticum showed the highest antimalarial activity with the lowest IC50 value of 1.96 μg/ml and CC50 value of 134.70 μg/ml, followed by the ethanolic flower extract of P. chaba with IC50 value of 2.06 and CC50 value of 198.60 μg/ml followed by ethanolic rhizome extract of Z. officinale, aqueous fruit extract of T. arjuna, ethanolic fruit extract of P. nigrum with IC50 values of 3.42, 4.05 and 4.38 μg/ml and CC50 values of 30.73, > 54.22 and 44.36 μg/ml, respectively. Regarding S. aromaticum, it also known as clove. It has been reported that methanolic extract of this plant possesses slightly antimalarial effect in mice infected with P. berghei [32]. For P. chaba, piperine which is the major isolated constituent of this plant has been reported to exhibit the antimalarial effect against both chloroquine-sensitive and chloroquine-resistant P. falciparum clones [33]. Among the plant extracts that exhibited high antimalarial activity, the aqueous fruit extract of T. arjuna exhibited promising antimalarial properties, with an IC50 of 4.05 μg/ml. This extract possessed potent effect approximately 14.2–23.8 times compared to mildly active plants (IC50 ranged between 51 and 100 μg/ml). The in vitro result revealed that the CC50 value of the aqueous extract of T. arjuna was greater than 200 μg/ml, indicating that no toxic effects were present. This result is consistent with previous reports of the methanolic extract of T. arjuna bark exhibiting a non-cytotoxic effect on human peripheral blood mononuclear cells [34]. Therefore, the aqueous extract of T. arjuna was selected for further in vivo experiments.
The SI value is a crucial parameter for determining whether further work on an extract is warranted [35]. When the SI results are greater than 10, the extract is considered potentially safe in terms of cytotoxicity parameters [36]. Therefore, the aqueous T. arjuna extract with an SI value greater than 54.22 suppressed P. falciparum infection without acute toxic effects in mammalian cells. To confirm the in vitro antiplasmodial results, the antimalarial properties and toxic effects of this plant extract were further tested in an animal model.
Malaria-infected mice treated with the aqueous T. arjuna extract showed a significant dose-dependent decrease in the number of Plasmodium parasites. Furthermore, MST is an important parameter for evaluating the antimalarial activity of plant extracts. T. arjuna extract prolonged the survival of P. berghei-infected mice in a dose-dependent manner. This may be because secondary metabolites that exhibit anti-inflammatory and antioxidant functions were present and prevented the overall pathologic effect of the parasite in the infected mice [37, 38]. Since, malaria is a highly inflammatory and oxidative disease. During the blood stage of malaria infection, in response to the presence of the parasite, the host’s immune system produces proinflammatory cytokines, including IL-6, IL-8, IFN-γ, and TNF which play a pivotal role in controlling the growth of the parasite and its elimination [39]. In addition, during the blood stage of infection, the level of oxidative stress in plasma is increased, since it contributes to the elimination of invading pathogens, but also causes molecular damage in the host [40]. The potential of T. arjuna extract that exerts anti-inflammatory and antioxidant effects was supported by a previous report [41]. It inhibited the lipid peroxidation, maintained endogenous antioxidant enzyme activities and decreasing cytokine levels leading to decelerate the disease progression. Therefore, the antimalarial effect of the T. arjuna extract may be possessed by anti-inflammatory and antioxidant properties.
To confirm the safety of the extract, mice received a single dose of 2000 mg/kg aqueous T. arjuna extract. There were no visible signs or symptoms of toxicity or mortality in the mice. This indicated that the lethal dose of 50% was greater than 2000 mg/kg. Our study is in accordance with previous studies in which oral administration of methanolic extract of T. arjuna bark at various concentrations of 250–2000 mg/kg body weight did not show any adverse signs of toxicity or mortality in acute toxicity study in mice [34].
Biochemical analysis of liver and kidney functions plays an important role in evaluating the toxicological effects of xenobiotics [42, 43]. The plasma levels of ALT and ALP in mice treated with aqueous T. arjuna extracts were not significantly different compared with the untreated control and 7% Tween 80 groups. Regarding the kidney function test, BUN and Cr levels were not significantly different between the groups. Histopathological analysis of the liver and kidneys revealed normal features compared with those in healthy mice.
Phytochemical analysis of Prabchompoothaweep remedy showed a diversity of phytochemical constituents, including flavonoids, terpenoids, alkaloids, tannins, saponins, and coumarins. These secondary metabolites prevent the generation of free radicals and block protein synthesis in the Plasmodium parasite [5, 44,45,46,47]. Saponins may also modulate the immune system of infected mice [22]. Moreover, saponins are amphiphilic nature and can complex with cholesterol in biomembranes with their lipophilic moiety and bind to surface glycoproteins and glycolipids. Most terpenoids are lipophilic in nature and readily interact with the lipophilic inner core of membrane bilayers [37]. Flavonoids inhibit the influx of L-glutamine and myoinositol into P. falciparum-infected erythrocytes [48]. These phytochemical constituents may inhibit parasite growth and multiplication, resulting in a reduction in parasitemia and body temperature.
We found that the aqueous fruit extract of T. arjuna presented a group of flavonoids, terpenoids, alkaloids, tannins, and saponins. Our results are consistent with those of previous reports of the chemical constituents of T. arjuna [49, 50]. Secondary metabolites, particularly flavonoids, alkaloids, tannins, and saponins, are protective against Plasmodium parasites [44,45,46,47]. The most abundant compounds in the fruit extract of T. arjuna were pyrogallol, gallic acid, shikimic acid, oleamide, 5-hydroxymethylfurfural,1,1-diethoxy-ethane, quinic acid, and furfural. The antimalarial activity of this extract may be attributed to the synergistic effects of these compounds. Interestingly, the identified compounds from the fruit extract of T. arjuna including pyrogallol, gallic acid, shikimic acid, cinnamic acid, and quinic acid are interrelated via biosynthesis pathway [29,30,31]. Since, most non-volatiles will decompose at between 400 and 1000 °C [51]. Therefore, the identified compounds which non-volatiles cannot be vaporized and decomposed easily. In addition, in this study, the injector temperature of GC-MS was set at a constant of 250 °C, and the maximum temperature of the oven was set at 300 °C. So, this thermal condition inapplicable for decomposition process.
The bioactivities of the two major compounds, shikimic acid and 5-hydroxymethylfurfural, further explains why the T. arjuna extracts significantly enhanced the survival in mice. For instance, shikimic acid a key intermediate in the biosynthesis of aromatic compounds, exerts antibacterial, anti-inflammatory, analgesic, antioxidant, antithrombotic, and antibacterial activities [52]; 5-hydroxymethylfurfural is a furan-containing aldehyde present in sacchariferous foods (fruit juices and dried fruits), Codonopsis pilosula and garlic exerts antioxidant, anti-inflammatory, anti-proliferative, and cardioprotective effects [53, 54]. For, gallic acid or 3, 4, 5-trihydroxybenzoic acid, it has been reported to exhibit various pharmacological properties, including antibacterial, antiviral and antitumor activities [55]. In addition, this compound isolated from Alectryon serratus leaves possessed antiplasmodial activity against chloroquine-sensitive 3D7 strain of P. falciparum with IC50 value of 0.0722 μM [56].
Regarding pyrogallol or 1, 2, 3-benzenetriol which is an organic phenol compound that exists naturally in many plants such as Terminalia chebula, Myriophyllum spicatum and Diospyros chamaethamnus. It possesses antibacterial, antipsoriatic, antifungal properties, and revealed antimalarial activity against P. falciparum chloroquine-sensitive strain [57, 58]. The potential of pyrogallol to exert antimalarial activity was supported by its property that it is autoxidised rapidly in solutions ranging from pH 3.5–4.5 and generates various free radicals such as peroxide nitrite, hydrogen peroxide, and hydroxyradical. These free radicals may enable the inhibition of parasite growth [57].
Conclusions
A total of 10 plants from 23 medicinal plant ingredients of Prabchompoothaweep remedy showed high in vitro antimalarial activity. Among these, the aqueous fruit extract of T. arjuna possessed potent effect approximately 14.2–23.8 times compared to mildly active plants (IC50 ranged between 51 and 100 μg/ml). This extract exerts antimalarial activity against Plasmodium parasites found in humans (P. falciparum K1 strain) and mice (P. berghei ANKA strain). Acute toxicity studies revealed that the aqueous fruit extract of T. arjuna did not present any lethality or adverse effects up to a dose of 2000 mg/kg body weight. These results suggest that T. Arjuna extract has antimalarial activity that could be a promising starting point for the study of the antimalarial drug.
Therefore, further studies on the aqueous extract of T. arjuna should focus on its phytochemical contents to identify their bioactive constituents and mechanisms of parasite inhibition including studies on a synergistic effect of the combinations of the abundant phytochemicals identified in T. arjuna extracts.
Availability of data and materials
The data used to support the findings of this study have been included in this article. Additional files are available from the corresponding authors upon request.
Abbreviations
- pLDH:
-
Plasmodium lactate dehydrogenase enzyme
- GC-MS:
-
Chromatography-mass spectrometry
- IC50 :
-
Half-maximal inhibitory concentration
- CC50 :
-
50% cytotoxic concentration
- RBCs:
-
Red blood cells
- DMSO:
-
Dimethyl sulfoxide
- MTT:
-
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- SI:
-
Selectivity index
- NIST:
-
National Institute of Standards and Technology
- ICR mice:
-
Institute of Cancer Research mice
- MST:
-
Mean survival time
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- BUN:
-
Blood urea nitrogen
- Cr:
-
Creatinine
- SEM:
-
Standard error of the mean
- FL:
-
Flavonoids
- TN:
-
Terpenoids
- AL:
-
Alkaloids
- TA:
-
Tannins
- AN:
-
Anthraquinones
- CG:
-
Cardiac glycosides
- SA:
-
Saponins
- CM:
-
Coumarins
References
CDC. Malaria 2019 [cited 2022 12]. Available from: https://www.cdc.gov/malaria/index.html.
WHO. World malaria report. Geneva: World Health Organization; 2020.
Uwimana A, Legrand E, Stokes BH, Ndikumana J-LM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020;26:1602–8.
Stokes BH, Ward KE, Fidock DA. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med. 2022;386:1385–6.
Jai-aue A, Makchuchit S, Juckmeta T, Itharat A. Anti-allergic, anti-inflammatory and antioxidant activities of the different extracts of Thai traditional remedy called prabchompoothaweep for allergic rhinitis treatment. J Med Assoc Thail. 2014;97(Suppl 8):S140–8.
Onthong N, Chonpatathip U, Rajanivat Y, Patthananurak K, Sangvichien S, Kamoltham T. A comparative study on the effects of Prabchompoothaweep remedy and loratadine in treatment of patients with allergic rhinitis and upper respiratory tract infections at Pathumtani hospital. J Health Educ. 2019;42:135–45.
Singh N, Kapur KK, Singh SP, Shanker K, Sinha JN, Kohli RP. Mechanism of cardiovascular action of Terminalia arjuna. Planta Med. 1982;45:102–4.
Dwivedi S, Jauhari R. Beneficial effects of Terminalia arjuna in coronary artery disease. Indian Heart J. 1997;49:507–10.
Kusumoto IT, Nakabayashi T, Kida H, Miyashiro H, Hattori M, Namba T, et al. Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1 (HIV-1) protease. Phytother Res. 1995;9:180–4.
Ram A, Lauria P, Gupta R, Kumar P, Sharma VN. Hypocholesterolaemic effects of Terminalia arjuna tree bark. J Ethnopharmacol. 1997;55:165–9.
Kumar P, Katram N, e M R, Mudili DV, Anand T, Anilakumar K. DNA damage protecting and free radical scavenging properties of Terminalia arjuna bark in PC-12 cells and plasmid DNA. Free radic antioxid. 2013;3:35–9.
Bachaya H, Iqbal Z, Khan M, Jabbar A, Gilani A-u, Islam UD. In vitro and in vivo anthelmintic activity of Terminalia arjuna bark. Int J Agric Biol. 2009;11:273–8.
Chusri S, Sinvaraphan N, Chaipak P, Luxsananuwong A, Voravuthikunchai SP. Evaluation of antibacterial activity, phytochemical constituents, and cytotoxicity effects of Thai household ancient remedies. J Altern Complement Med. 2014;20:909–18.
Senguttuvan J, Paulsamy S, Karthika K. Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. for in vitro antioxidant activities. Asian Pac. J Trop Biomed. 2014;4:S359–67.
Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5.
Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–10.
Chaniad P, Phuwajaroanpong A, Techarang T, Horata N, Chukaew A, Punsawad C. Evaluation of the antimalarial activity and toxicity of Mahanil-tang-thong formulation and its plant ingredients. BMC Complement Med Ther. 2022;22:51.
Koch A, Tamez P, Pezzuto J, Soejarto D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J Ethnopharmacol. 2005;101:95–9.
Peters W. The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol. 1975;69:155–71.
Chaniad P, Techarang T, Phuwajaroanpong A, Punsawad C. Antimalarial activity and toxicological assessment of Betula alnoides extract against Plasmodium berghei infections in mice. Evid Based Complement Altern Med. 2019;2019:2324679.
Muluye AB, Desta AG, Abate SK, Dano GT. Anti-malarial activity of the root extract of Euphorbia abyssinica (Euphorbiaceae) against Plasmodium berghei infection in mice. Malar J. 2019;18:261.
Misganaw D, Engidawork E, Nedi T. Evaluation of the anti-malarial activity of crude extract and solvent fractions of the leaves of Olea europaea (Oleaceae) in mice. Evid Based Complement Altern Med. 2019;19:171.
Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18:e3000411.
Organisation for Economic Co-operation and Development (OECD). Test No. 425: acute oral toxicity: up-and-down procedure. 2008.
Wichapoon B, Punsawad C, Chaisri U, Viriyavejakul P. Glomerular changes and alterations of zonula occludens-1 in the kidneys of Plasmodium falciparum malaria patients. Malar J. 2014;13:176.
Viriyavejakul P, Khachonsaksumet V, Punsawad C. Liver changes in severe Plasmodium falciparum malaria: histopathology, apoptosis and nuclear factor kappa B expression. Malar J. 2014;13:106.
Kigondu EVM, Rukunga GM, Gathirwa JW, Irungu BN, Mwikwabe NM, Amalemba GM, et al. Antiplasmodial and cytotoxicity activities of some selected plants used by the Maasai community. Kenya S Afr J Bot. 2011;77:725–9.
Berthi W, González A, Rios A, Blair S, Cogollo Á, Pabón A. Anti-plasmodial effect of plant extracts from Picrolemma huberi and Picramnia latifolia. Malar J. 2018;17:151.
Wang Z, Xiao S, Wang Y, Liu J, Ma H, Wang Y, et al. Effects of light irradiation on essential oil biosynthesis in the medicinal plant Asarum heterotropoides Fr. Schmidt var. mandshuricum (maxim) Kitag. PLoS One. 2020;15:e0237952.
Saijo R. Biosynthetic pathways of gallic acid. Chagyo Kenkyu Hokoku (Tea Res J) 2014;2014:118_127-118_131.
Farag MA, Hegazi NM, Donia MS. Molecular networking based LC/MS reveals novel biotransformation products of green coffee by ex vivo cultures of the human gut microbiome. Metabolomics. 2020;16:86.
Ene AC, Ameh DA, Kwanashie HO, Agomo P, Atawodi SE. Preliminary in vivo antimalarial screening of petroleum ether, chloroform and methanol extracts of fifteen plants grown in Nigeria. J Pharmacol Toxicol. 2008;3:254–60.
Thiengsusuk A, Muhamad P, Chaijaroenkul W, Na-Bangchang K. Antimalarial activity of piperine. J Trop Med. 2018;2018:9486905.
Suganthy N, Muniasamy S, Archunan G. Safety assessment of methanolic extract of Terminalia chebula fruit, Terminalia arjuna bark and its bioactive constituent 7-methyl gallic acid: in vitro and in vivo studies. Regul Toxicol Pharmacol. 2018;92:347–57.
Indrayanto G, Putra GS, Suhud F. Validation of in-vitro bioassay methods: application in herbal drug research. Profiles Drug Subst Excip Relat Methodol. 2021;46:273–307.
Vitorino KA, Alfonso JJ, Gómez AF, Santos APA, Antunes YR, Caldeira CA, et al. Antimalarial activity of basic phospholipases A2 isolated from Paraguayan Bothrops diporus venom against Plasmodium falciparum. Toxicon: X. 2020;8:100056.
Fenta M, Kahaliw W. Evaluation of antimalarial activity of hydromethanolic crude extract and solvent fractions of the leaves of Nuxia congesta R. Br. Ex Fresen (Buddlejaceae) in Plasmodium berghei infected mice. J Exp Pharmacol. 2019;11:121–34.
Aragaw TJ, Afework DT, Getahun KA. Antimalarial activities of hydromethanolic crude extract and chloroform fraction of Gardenia ternifolia leaves in Plasmodium berghei infected mice. Evid based Complement Altern Med. 2020;2020:6674002.
Popa GL, Popa MI. Recent advances in understanding the inflammatory response in malaria: a review of the dual role of cytokines. J Immunol Res. 2021;2021:7785180.
Vasquez M, Zuniga M, Rodriguez A. Oxidative stress and pathogenesis in malaria. Front Cell Infect Microbiol. 2021:11.
Amalraj A, Gopi S. Medicinal properties of Terminalia arjuna (Roxb.) Wight & Arn.:a review. J Tradit Complement Med. 2017;7:65–78.
Bariweni M, Oboma Y, Ozolua R. Toxicological studies on the aqueous leaf extract of Pavetta crassipes (K. Schum) in rodents. J Pharm Pharmacogn Res. 2018;6:1–16.
Ezeja MI, Anaga AO, Asuzu IU. Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. J Ethnopharmacol. 2014;151:1155–64.
Elford BC. L-glutamine influx in malaria-infected erythrocytes: a target for antimalarials? Parasitol Today. 1986;2:309–12.
Al-Adhroey A, B.A.H Z-A. In vivo antimalaria tests of Nigella sativa (black seed) different extracts. Am J Pharmacol Toxicol. 2007;2:46–50.
Abdillah S, Tambunan R, Farida Y, Sandhiutami N, Dewi R. Phytochemical screening and antimalarial activity of some plants traditionally used in Indonesia. Asian Pac. J Trop Biomed. 2015:77.
Anuthakoengkun A, Itharat A. Inhibitory effect on nitric oxide production and free radical scavenging activity of Thai medicinal plants in osteoarthritic knee treatment. J Med Assoc Thail. 2014;97(Suppl 8):S116–24.
Lekana-Douki JB, Oyegue Liabagui SL, Bongui JB, Zatra R, Lebibi J, Toure-Ndouo FS. In vitro antiplasmodial activity of crude extracts of Tetrapleura tetraptera and Copaifera religiosa. BMC Res Notes. 2011;4:506.
Shaik MM. Phytochemistry and pharmacological potential of Terminalia arjuna L. J Med Plant Res. 2013;15:3.
Mandal S, Patra A, Samanta A, Roy S, Mandal A, Mahapatra TD, et al. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac J Trop Biomed. 2013;3:960–6.
Shimadzu corporation. A guide to GCMS sample introduction systems: choosing the best system for your analysis. 1st ed. p. 1–40.
Bai J, Wu Y, Liu X, Zhong K, Huang Y, Gao H. Antibacterial activity of shikimic acid from pine needles of Cedrus deodara against Staphylococcus aureus through damage to cell membrane. Int J Mol Sci. 2015;16:27145–55.
Zhang H, Jiang Z, Shen C, Zou H, Zhang Z, Wang K, et al. 5-Hydroxymethylfurfural alleviates inflammatory lung injury by inhibiting endoplasmic reticulum stress and NLRP3 inflammasome activation. Front Cell Dev Biol. 2021;9:782427.
Zhao L, Chen J, Su J, Li L, Hu S, Li B, et al. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J Agric Food Chem. 2013;61:10604–11.
Zhang T, Ma L, Wu P, Li W, Li T, Gu R, et al. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol Rep. 2019;41:1779–88.
Khasanah U, WidyaWaruyanti A, Hafid AF, Tanjung M. Antiplasmodial activity of isolated polyphenols from Alectryon serratus leaves against 3D7 Plasmodium falciparum. Pharm Res. 2017;9:S57–s60.
Alfaqih H, Abu BN. The potential of pyrogallol as a possible antimalarial drug candidate. Academic. J Microbiol Immunol Infect. 2020;1:2020.
du Preez-Bruwer I, Mumbengegwi DR, Louw S. In vitro antimalarial properties and chemical composition of Diospyros chamaethamnus extracts. S Afr J Bot. 2022;149:290–6.
Acknowledgments
We thank the staff at the Department of Tropical Pathology, Faculty of Tropical Medicine, Mahidol University, Thailand, for their help with histological preparation and staining. We would also like to thank Miss Rungruedi Kimseng from the Research Institute for Health Science, Walailak University, for supporting the animal experiments.
Funding
This research was supported by the Walailak University Plant Genetic Conservation Project under the Royal Initiation of Her Royal Highness Princess Maha Chakri Sirindhorn (RSPG) (contract no. WUBG-015/2564). The funders had no role in the study design, data collection, data analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
PC and CP developed the concepts for the research study and data curation. PC, TT, AP, WP, PV, AWS, and CP were responsible for study design, methodology, and investigation. Formal analyses were performed by TT, AP, WP, PC, and CP. PC and CP contributed to project administration and validation. PC, PV, and CP provided the required resources. CP supervised the project. PC, TT, and CP drafted the manuscript. PC, AWS and CP reviewed and edited the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Studies involving human participants were performed in accordance with relevant guidelines and regulations of the Declaration of Helsinki. The protocol was approved by the Human Research Ethics Committee of Walailak University prior to recruitment of all participants (approval number: WUEC-20-344-01). Written informed consent was obtained from the participants prior to obtaining blood specimens for in vitro cultivation of P. falciparum strains. The animal study by the Animal Ethics Committee of Walailak University, Thailand (certificate number: WU-AICUC-64027). All protocols in this study were performed in accordance with the relevant guidelines and regulations for using animals, in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines [23].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chaniad, P., Techarang, T., Phuwajaroanpong, A. et al. Antimalarial efficacy and toxicological assessment of medicinal plant ingredients of Prabchompoothaweep remedy as a candidate for antimalarial drug development. BMC Complement Med Ther 23, 12 (2023). https://doi.org/10.1186/s12906-023-03835-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-03835-x