Abstract
Background
Diazinon (DZN), a widely used chemical herbicide for controlling agricultural pests, is an important organophosphorus pesticide and an environmental pollutant which induces toxic effects on living organisms during long-term exposure. Thymoquinone (TQ) is a phytochemical bioactive compound with antioxidant and anti-inflammatory properties. We aimed to evaluate the protective effects of TQ against DZN-induced hepatotoxicity through alleviating oxidative stress and enhancing cholinesterase (ChE) enzyme activity.
Methods
Rats were randomly divided into six groups (n = 8); a negative control group receiving corn oil; a group only receiving DZN (20 mg/kg/day); a group treated with TQ (10 mg/kg/day), and three treatment groups as TQ + DZN, receiving different doses of TQ (2.5, 5, and 10 mg/kg/day). All experimental animals were orally treated for 28 consecutive days. The levels of superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA), alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and lactic acid dehydrogenase (LDH) were determined. In addition, ChE activity and histopathological changes were evaluated.
Results
The results showed that DZN decreased GSH level (p < 0.01) and SOD activity (p < 0.01) in parallel to an increase in MDA level (p < 0.01) and increased the activity of AST, ALT, ALP, and LDH (p < 0.01) in comparison to the negative control group. Our findings demonstrated that TQ administration could diminish hepatotoxicity and reduce oxidative damage in DZN-treated rats, which could be linked to its antioxidant and free radical scavenging properties. It was also observed that TQ 10 mg/kg remarkably increased the activity of acetylcholinesterase, butyrylcholinesterase, and SOD enzymes, elevated GSH, decreased MDA, and reduced pathological alternations of the liver induced by DZN.
Conclusion
Thymoquinone 10 mg/kg increased the activity of plasma and blood cholinesterases and reduced DZN-induced alternations of the liver. Improvement of butyryl- and acetylcholinesterase activity suggests that maybe TQ supplement could be beneficial as pre-exposure prophylaxis among farm workers spraying pesticides.
Similar content being viewed by others
Background
Extensive quantities of organophosphate (OP) pesticides are used in many developing countries to maintain food supplies [1, 2]. The excessive use of OPs is a global issue that causes vast environmental pollution, which can affect humans and other species’ health [3]. There are various types of OPs with different brands in the market which are classified based on toxicity potential. Among the OPs, diazinon (DZN) is applied more than others due to its less toxic properties on mammals to control and protect agricultural and horticultural products from pests. The main environmental advantage of this compound could be photochemical inactivation by sunlight, which limits the chronicity of environmental damage and makes it a favored product for homeowners to be used for lawns, gardens, and interior spaces. The entrance of DZN into food chains and its accumulation in the body may cause adverse reactions such as heart, pancreas, kidney, brain, and liver dysfunctions [4, 5]. Health Canada's screening values has identified a drinking water screening limit of 0.015 mg/L (15 µg/L) for DZN. Besides, the maximum residue limits established for DZN in foods is 0.75 ppm [6].
Cholinesterase (ChE) is an important enzyme of the functioning of the nervous system which might be modulated by chemical compounds and pharmaceuticals [7]. The foremost mechanism of action of DZN (and its metabolites) is the inhibition of acetylcholinesterase (AChE), which causes an increase in acetylcholine neurotransmitter in synaptic space, over-stimulation of cholinergic receptors at the postsynaptic membrane, and consequent muscarinic and nicotinic complications [8, 9]. DZN-induced phosphorylation of the esteratic site of AChE produces irreversible inhibition of the enzyme unless a reactivator such as pralidoxime be used. However, the oxime antidote can only reactivate the enzyme, if given in time, before loss of an alkyl group from phosphorylated AChE could age the enzyme [10]. Previous studies have reported that the toxic effects of DZN and its potent metabolites are not limited to the hyperactivity of cholinergic receptors, but it also induces oxidative stress through generating reactive oxygen species (ROS) [11,12,13]. ROS are chemically electrophile, reactive molecules [14,15,16], and their increase can result in the disturbance of the balance between the production of free radicals and antioxidant defenses, which causes oxidative damage to cellular structures [17,18,19,20]. It has been revealed that bioactive molecules and natural antioxidants can effectively scavenge free radicals and diminish damages associated with oxidative stress. Currently, natural compounds have attracted many researchers to counteract certain types of toxins and pathogenic factors [21, 22]. Nigella sativa, also known as black seed or black cumin, is an annual flowering plant that belongs to the family Ranunculaceae. As a natural compound with remedial properties, it has been used for a variety of health conditions in many Middle Eastern and Asian countries for centuries. The seeds are traditionally used for the treatment of pulmonary, cardiovascular, and gastrointestinal diseases, as well as inflammation, diabetes, nervous disorders, rheumatism, and various cancers [23]. There are many organic, mineral, and vitamin compounds in N. sativa seeds, which each of these plant's constituents can exhibit multiple pharmacological or therapeutic effects.
Thymoquinone (TQ; 2-isopropyl-5-methylbenzo-1, 4-quinone) is the most bioactive component of the volatile oil of N. sativa seeds that has potent pharmacological properties including antioxidant, anti-inflammatory and immunomodulatory, antimicrobial, neuroprotective, nephroprotective as well as hepatoprotective effects along with other beneficial effects [24,25,26,27]. Administration of N. sativa oil can possess hepatoprotective activity against carbon tetrachloride (CCl4) induced hepatotoxicity in male Wistar rats which provides a rationale to the medicinal use of this herbal supplement [28]. TQ as the main constituent of N. sativa has the potential benefits in the prevention of the onset and progression of cisplatin-induced hepatotoxicity [29]. TQ as a relatively safe compound and the combination with Vit D has anti-fibrogenic properties and hepato-protective effects against previously established liver fibrosis [30]. TQ administration can shows lower the levels of oxidative stress and elevation of the total antioxidant capacity indices in diabetic rats [31]. Furthermore, TQ treatment may improve AChE activity in by chlorpyrifos toxicity and alleviate neuronal injuries and oxidative stress [32, 33]. In this context, earlier studies have demonstrated that presence of many quinones especially thymoquinone as natural antioxidants in N. sativa and its extract is responsible for the improvement of oxidative stress and the subsequent organ damages induced by some xenobiotic and chemicals. Recent researches on TQ in rats and mice have shown that this bioactive compound has cardioprotective, neuroprotective, and nephroprotective effects based on its anti-inflammatory properties [34,35,36,37,38,39,40]. Some animal studies have demonstrated that TQ has therapeutic benefits against brain injury, ovarian cyst formation, and tumor growth [34, 39, 41, 42]. The hepatoprotective effects of TQ have also been suggested in rat models so that TQ may inhibit oxidative damage and might be able to prevent liver lesions through prevention of free radicals damage, lipid peroxidation, and by improving antioxidant sources [39, 43, 44]. However, there is still a lacking of knowledge of this effect on liver and the activity of ChE enzymes. Therefore, in this investigation, the effectiveness of oral supplementation of TQ as a potent natural antioxidant against DZN-induced hepatotoxicity in rats was examined on liver, blood, and biochemical factors.
Materials and methods
Chemical
Diazinon (purity ≥ 96%) was purchased from Ariashimi Co., Iran. Thymoquinone (C10H12O2; CAS number 490–91-5), dithiobisnitrobenzoic acid (DTNB), and acetylthiocholine iodide (ATCI) were procured from Sigma-Aldrich, Germany. Corn oil was obtained from Zarrin, Iran. Morin Biochemical colorimetric kits and antioxidant kits were purchased from BioLabo and Diaclone, France.
Animals and experimental design
All experiments were approved by the animal ethics committee of Mazandaran University of Medical Science, Sari, Iran (IR.MAZUMS.172319). Male Wistar rats weighing 150–200 g were obtained from the laboratory animal center of. The animals were acclimatized for 1 week prior to experimentation. The animals were maintained in propylene cages in an air-conditioned room (temperature 24 ± 3 °C; relative humidity 50 ± 10%; 12 h dark/light cycle), which had free access to standard diet pellets and drinking water ad-libitum.
The animals were randomly divided into six groups (8 rats in each group). Group 1 was considered to be the control group and received corn oil. Groups 2–6 received DZN (20 mg/kg/day), TQ (10 mg/kg/day), TQ (2.5 mg/kg/day) + DZN, TQ (5 mg/kg/day) + DZN, and TQ (10 mg/kg/day) + DZN, respectively. All animals were treated orally once a day for four weeks. Applied doses of DZN and TQ were selected for this experiment based on previous studies [9, 21]. Administration of TQ solution in corn oil was done by gavage. At the end of the treatment (28 days), the animals were sacrificed. Hence, an i.p. injection of the anesthetic cocktail including ketamine-xylazine-acepromazine (50–10-1.5 mg/kg, respectively) was used before scarifice. Then, blood samples were withdrawn through cardiac puncture for the examination of biochemical parameters and cholinesterase activity assay. Liver tissues were used to assess markers of oxidative stress and histopathological changes.
Biochemical parameters measurement
Blood samples were collected into heparinized tubes and were centrifuged at 3000 rpm for 10 min at 4 °C, and plasma was separated to determine biochemical parameters, including alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and lactic acid dehydrogenase (LDH) using Biolabo kits according to the instructions of the manufacturer.
Cholinesterase activity assay
The activity of acetylcholinesterase (AChE) in compact RBCs of treated rats was determined using the Ellman method [45]. Collected blood samples were centrifuged at 5000 rpm for 10 min at 4 °C. The compacted lower layer (RBCs) was isolated and washed thrice with cold normal saline. Then, the compact RBCs were suspended in distilled water and incubated with DTNB + ATCI containing guanidine sulfate as the plasma cholinesterase inhibitor at 37 °C for 10 min. The reaction was terminated by adding 0.002% hyamine (benzethonium chloride), and the absorbance was measured at 440 nm using a spectrophotometer (Shimadzu; Japan). The AChE activity of blood samples was calculated by multiplying the optical density (OD) of the samples by a converting factor of 17.68 U/ml/pack cell.
The serum cholinesterase activity was measured using a colorimetric commercial kit (Biorexfars; Iran).
Assessment of oxidative stress markers
The biomarkers of oxidative stress were measured in liver tissue homogenate in all the experimental groups. The tissue samples were quickly removed and weighed. A part of the liver samples was minced into smaller pieces and homogenized gently for 2 min, and then the homogenates were centrifuged at 6000 rpm for 15 min at 4 °C. Levels of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH) in supernatant fractions were determined using commercial mouse kits (Zell Bio; Germany).
Histopathological examination
For histopathological examination, a portion of the liver samples was immersed in 10% neutral buffered formalin. The tissues were routinely processed, and paraffin-embedded samples were cut into sections at 4 µm thickness and stained with hematoxylin–eosin (H&E). A semi-automatic microtome, RMT-SA3315 (Japan) was used to generate tissue slides.
Statistical analysis
All values were expressed as Mean ± Standard Deviation for all groups. For comparison between groups, statistical analysis was performed using one-way ANOVA followed by the Tukey multiple comparison test. A p-value of < 0.05 was considered to be statistically significant.
Results
Biochemical parameters
As shown in Table 1, rats exposed to DZN had significantly increased levels of serum LDH (p < 0.01), AST (p < 0.001), ALT (p < 0.01), and ALP (p < 0.001) as compared to the control group. On the other hand, the DZN + 2.5 mg/kg TQ rat group recorded a similar increase in AST and ALP activity (p < 0.01 for both enzymes) in comparison with the control animals, whereas administration of TQ at the dose of 10 mg/kg significantly declined the activity of all four enzymes when compared to DZN-treated groups. Moreover, the rats receiving 5 mg/kg of TQ showed a significant decrease in ALP serum levels as compared to DZN-treated groups (p < 0.05).
Cholinesterase activity
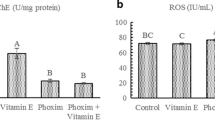
As demonstrated in Fig. 1, butyrylcholinesterase (plasma cholinesterase) and erythrocyte cholinesterase (RBC-cholinesterase) activity significantly decreased in the DZN-treated rats as compared to the corn oil control group (p < 0.001 and p < 0.01, respectively). Administration of TQ at the dose of 10 mg/kg remarkably increased the activity of the aforementioned enzymes as compared to DZN-treated rats, while groups that received TQ with lower doses (2.5 and 5 mg/kg) did not indicate any significant changes.
Effect of TQ on butyryl (1A) and acetyl (1B) cholinesterase activity of different groups of rats. 1A aaaP < 0.001 indicate significant differences in comparison to control groups. bbP < 0.01 indicates significant differences in comparison to DZN-treated groups. 1B aaP < 0.01 indicate significant differences in comparison to control group. bP < 0.05 indicates significant differences in comparison to DZN-treated groups
Oxidant-antioxidant status
As shown in Table 2, oral administration of DZN caused a disturbance in the balance between the production of free radicals and the defense activity of antioxidants in rat liver tissue. DZN treatment resulted in a significant increase in MDA (p < 0.01) levels and a decrease in the level of GSH (p < 0.01) in comparison with the control group. There was a significant increase in the level of GSH (p < 0.01) and a decrease in the level of MDA (p < 0.05) in rats treated with DZN + TQ (10 mg/kg) as compared with the DZN–treated groups.
Histopathological examination
Histopathological findings related to the rats’ liver are portrayed in Table 3 and Fig. 2. DZN-treated groups showed various degrees of pathological lesions, including damage of liver structure, multifocal necrosis, apoptosis, hemorrhage, and edema when compared to the control group. Significant differences in histopathological characteristics were not observed in rats treated with the TQ dose of 2.5 and 5 mg/kg. In contrast, TQ at the dose of 10 mg/kg significantly reduced pathological alternations of the liver induced by DZN compared with the DZN group. Table 3 shows a dose–response gradient for the mitigating effect of TQ in the presence of the stressor DZN.
Discussion
The results of histopathological examinations, along with the activities of liver functional enzymes, indicated that subacute exposure to DZN could induce hepatotoxicity in rats. These alterations were accompanied by an increase in oxidative stress in liver tissue of the rats. Administration of TQ at the dose of 10 mg/kg remarkably ameliorated DZN-induced hepatotoxicity, reduced oxidative stress, and improved cholinesterase activity.
The signs and symptoms of acute OP poisoning have been well described, while the chronic effects of exposure to these compounds are not entirely clear. Many researchers postulate that the redox process in the organs of the body may be impaired due to OP toxic effects, thus leading to an increase in the level of lipid peroxidation [46,47,48]. As the enhanced generation of reactive oxygen species and induction of lipid peroxidation underlie many diseases, it is imperative to measure the effect of OP insecticides on the redox status of organs [48, 49], and it is equally vital to find protective mechanisms against pesticide-induced oxidative stress [48]. It is well understood that DZN increases the formation of ROS in tissues and erythrocytes, leading to oxidative stress [50, 51], which was confirmed in our study by the decrease in liver GSH level / SOD activity and an increase in MDA level. The present results support the hypothesis that oxidative stress and free radicals play an important role in DZN hepatotoxicity. One the other hand, TQ was found to be as potent antioxidant against DZN-induced hepatotoxicity.
Our findings showed that TQ and its metabolites, such as glutathione-dihydrothymoquinone and thymohydroquinone, as protective agents, were able to relieve oxidative stress induced by DZN due to having functional groups such as thiol (SH) and hydroxyl (OH) in their chemical structure [52]. The protective role of TQ can also be attributed to its potent phytochemical antioxidants that scavenge different types of oxygen radicals including superoxide anion, hydroxyl radical, and singlet molecular oxygen, and therefore, reduce ROS and oxidative stress levels [53]. Furthermore, as a quinone structure, TQ is able to cross the plasma membrane and thus reach the action-site in the intracellular organelles and scavenge free radicals and prevent them from causing oxidative damage to cellular components such as protein, DNA, and lipids [54]. Similar to our results, Maheswari et al., (2014) showed that while the level of ALT, AST and ALP increased by carbamazepine, administration of N-acetylcysteine, a known hepatoprotective drug, could decrease these parameters and increase the level of glutathione [55]. Similarly, N-acetyl-L-cysteine suppressed the increase in markers of liver functional state such as ALT, AST, and ALP and ameliorated GSH and SOD in liver damage induced by CCl4 [56].
OP toxicity is mainly attributed to the inhibition of acetylcholinesterase enzyme. Consistent with this fact, in our study, DZN-treated groups exhibited significant decreases in serum and RBC cholinesterase activity levels. On the other hand, TQ was found to have protective effects on both cholinesterases as our findings demonstrated an elevation in their activities when compared to DZN-treated groups. As an option, ROS and free radicals generated by DZN might disrupt the spatial conformation and integrity of cholinesterase, leading to lowered enzyme activity. Therefore, TQ as a potent antioxidant may scavenge and neutralize free radicals resulting in the improvement of enzyme inhibition. Apart from antioxidant capacity enhancement, TQ can reduce pro-inflammatory mediator TNF-α, revert the elevation of liver enzymes, and regulate pro and anti-apoptotic genes, and reduce the NF-kβ [57, 58]. To justify this phenomenon, we came across a study describing a reduction in cholinesterase activity of neuromuscular junction of the diaphragm among rats undergoing oxidative stress [59]. According to their findings, they considered that oxidative stress might decrease cholinesterase activity. In addition, TQ with an unknown mechanism might reactivate the inhibited enzymes. However, contrary to the present research, in a study performed by Hariri et al., TQ supplementation had no effects on cholinesterase activity [5].
Some hospitals reportedly provide complementary and alternative medicine therapy and there are also reports of individuals using traditional medicine service as well. Such services support traditional indigenous healers for management of some diseases [60, 61]. Since co-exposure to TQ leads to the higher activity of the cholinesterase enzyme of rats receiving DZN in comparison to the group with exposure to DZN alone (Fig. 1), and also considering that cholinesterase is a main target in the DZN toxicity, it is conceivable that TQ supplement can be taken as pre-exposure prophylaxis among farm workers spraying pesticides. Previous studies have also indicated the success of TQ supplementation against DZN toxicity in rats’ model of cardio- and hemato-toxicity [62, 63].
Of course, further studies are required to investigate the aforementioned implications and to determine the most appropriate dose and form of TQ usage.
Conclusion
The findings of our study demonstrated that TQ and its metabolites were capable of countervailing DZN-induced hepatotoxicity in a dose-dependent manner and exerted the protective effects, probably through the deactivation of free radicals generated following exposure to DZN, to maintain the integrity of hepatocytes. TQ 10 mg/kg increased the activity of plasma and blood cholinesterases and reduced DZN-induced alternations of the liver. Improvement of butyryl- and acetylcholinesterase activity suggests that maybe TQ supplement could be beneficial as pre-exposure prophylaxis among farm workers spraying pesticides.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on request.
References
Razavi BM, Hosseinzadeh H, Movassaghi AR, Imenshahidi M, Abnous K. Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure. Chem Biol Interact. 2013;203(3):547–55.
Mehri N, Felehgari H, Harchegani AL, Behrooj H, Kheiripour N, Ghasemi H, et al. Hepatoprotective effect of the root extract of green tea against malathion-induced oxidative stress in rats [J]. J Herbmed Pharmacol. 2016;5(3):116–9.
Al-Haj M, Nasser A, Anis A. Survey of pesticides used in Qat cultivation in Dhale and Yafe and their adverse effects. J Nat Appl Sci. 2005;9:103–10.
Farkhondeh T, Aschner M, Sadeghi M, Mehrpour O, Naseri K, Amirabadizadeh A, et al. The effect of diazinon on blood glucose homeostasis: a systematic and meta-analysis study. Environ Sci Pollut Res. 2021;28(4):4007–18.
Gokcimen A, Gulle K, Demirin H, Bayram D, Kocak A, Altuntas I. Effects of diazinon at different doses on rat liver and pancreas tissues. Pestic Biochem Physiol. 2007;87(2):103–8.
Health Canada. Drinking water screening value for diazinon. 2022. Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-diazinon.html.
Aramjoo H, Riahi-Zanjani B, Farkhondeh T, Forouzanfar F, Sadeghi M. Modulatory effect of opioid administration on the activity of cholinesterase enzyme: a systematic review of mice/rat models. Environ Sci Pollut Res. 2021;28(38):52675–88.
Abdel-Daim MM, Taha R, Ghazy EW, El-Sayed YS. Synergistic ameliorative effects of sesame oil and alpha-lipoic acid against subacute diazinon toxicity in rats: hematological, biochemical, and antioxidant studies. Can J Physiol Pharmacol. 2015;94(1):81–8.
Hariri AT, Moallem SA, Mahmoudi M, Hosseinzadeh H. The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine. 2011;18(6):499–504.
Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11(3):315–35.
Boroushaki MT, Arshadi D, Jalili-Rasti H, Asadpour E, Hosseini A. Protective effect of pomegranate seed oil against acute toxicity of diazinon in rat kidney. Iranian journal of pharmaceutical research: IJPR. 2013;12(4):821.
Khaksar MR, Rahimifard M, Baeeri M, Maqbool F, Navaei-Nigjeh M, Hassani S, et al. Protective effects of cerium oxide and yttrium oxide nanoparticles on reduction of oxidative stress induced by sub-acute exposure to diazinon in the rat pancreas. J Trace Elem Med Biol. 2017;41:79–90.
Al-Attar AM, Elnaggar MH, Almalki EA. Physiological study on the influence of some plant oils in rats exposed to a sublethal concentration of diazinon. Saudi J Biol Sci. 2018;25(4):786–96.
Mossa A-TH, Refaie AA, Ramadan A. Effect of exposure to mixture of four organophosphate insecticides at no observed adverse effect level dose on rat liver: the protective role of vitamin C. Res J Environ Toxicol. 2011;5(6):323.
Bhatti G, Sidhu I, Saini N, Puar S, Singh G, Bhatti J. Ameliorative role of melatonin against cypermethrin induced hepatotoxicity and impaired antioxidant defense system in Wistar rats. IOSR Journal of Environmental Science, Toxicology and Food Technology (IOSR-JESTFT). 2014;8(1):39–48.
Oruç EÖ, Usta D. Evaluation of oxidative stress responses and neurotoxicity potential of diazinon in different tissues of Cyprinus carpio. Environ Toxicol Pharmacol. 2007;23(1):48–55.
Cakici O, Akat E. Effects of oral exposure to diazinon on mice liver and kidney tissues: biometric analyses of histopathologic changes. Analytical and quantitative cytopathology and histopathology. 2013;35(1):7–16.
Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res Bull. 2008;75(1):166–72.
Jamialahmadi K, Arasteh O, Matbou Riahi M, Mehri S, Riahi-Zanjani B, Karimi G. Protective effects of glucosamine hydrochloride against free radical-induced erythrocytes damage. Environ Toxicol Pharmacol. 2014;38(1):212–9.
Rahnama M, Mahmoudi M, ZamaniTaghizadehRabe S, Balali-Mood M, Karimi G, Tabasi N, et al. Evaluation of anti-cancer and immunomodulatory effects of carnosol in a Balb/c WEHI-164 fibrosarcoma model. Journal of immunotoxicology. 2015;12(3):231–8.
Darakhshan S, Pour AB, Colagar AH, Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacol Res. 2015;95:138–58.
Azami S, Shahriari Z, Asgharzade S, Farkhondeh T, Sadeghi M, Ahmadi F, et al. Therapeutic potential of saffron (Crocus sativus L.) in ischemia stroke. Evid Based Complement Alternat Med. 2021;2021:6643950. https://doi.org/10.1155/2021/6643950.
Goreja WG. Black seed: nature's miracle remedy. 2003. Amazing Herbs Press.
Gali-Muhtasib H, El-Najjar N, Schneider-Stock R. The medicinal potential of black seed (Nigella sativa) and its components. Advances in Phytomedicine. 2006;2:133–53.
Laskar AA, Khan MA, Rahmani AH, Fatima S, Younus H. Thymoquinone, an active constituent of Nigella sativa seeds, binds with bilirubin and protects mice from hyperbilirubinemia and cyclophosphamide-induced hepatotoxicity. Biochimie. 2016;127:205–13.
Khan MA, Younus H. Thymoquinone shows the diverse therapeutic actions by modulating multiple cell signaling pathways: single drug for multiple targets. Curr Pharm Biotechnol. 2018;19(12):934–45.
Allemailem KS, Almatroudi A, Alrumaihi F, Aljaghwani A, Alnuqaydan AM, Khalilullah H, et al. Antimicrobial, Immunomodulatory and Anti-Inflammatory Potential of Liposomal Thymoquinone: Implications in the Treatment of Bacterial Pneumonia in Immunocompromised Mice. Biomedicines. 2021;9(11):1673.
Al-Seeni MN, El Rabey HA, Zamzami MA, Alnefayee AM. The hepatoprotective activity of olive oil and Nigella sativa oil against CCl4 induced hepatotoxicity in male rats. BMC Complement Altern Med. 2016;16(1):1–14.
Al-Malki AL, Sayed AAR. Thymoquinone attenuates cisplatin-induced hepatotoxicity via nuclear factor kappa-β. BMC Complement Altern Med. 2014;14(1):1–8.
Abdelghany AH, BaSalamah MA, Idris S, Ahmad J, Refaat B. The fibrolytic potentials of vitamin D and thymoquinone remedial therapies: insights from liver fibrosis established by CCl4 in rats. J Transl Med. 2016;14(1):1–15.
Faisal Lutfi M, Abdel-Moneim A-MH, Alsharidah AS, Mobark MA, Abdellatif AA, Saleem IY, et al. Thymoquinone lowers blood glucose and reduces oxidative stress in a rat model of diabetes. Molecules. 2021;26(8):2348.
Aboubakr M, Elshafae SM, Abdelhiee EY, Fadl SE, Soliman A, Abdelkader A, et al. Antioxidant and anti-inflammatory potential of thymoquinone and lycopene mitigate the chlorpyrifos-induced toxic neuropathy. Pharmaceuticals. 2021;14(9):940.
Demiroren K, Basunlu MT, Erten R, Cokluk E. A comparison of the effects of thymoquinone, silymarin and N-acetylcysteine in an experimental hepatotoxicity. Biomed Pharmacother. 2018;106:1705–12.
Hamdan AM, Al-Gayyar MM, Shams ME, Alshaman US, Prabahar K, Bagalagel A, et al. Thymoquinone therapy remediates elevated brain tissue inflammatory mediators induced by chronic administration of food preservatives. Sci Rep. 2019;9(1):1–11.
Chen H, Zhuo C, Zu A, Yuan S, Zhang H, Zhao J, et al. Thymoquinone ameliorates pressure overload-induced cardiac hypertrophy by activating the AMPK signalling pathway. J Cell Mol Med. 2022;26(3):855–67.
Velagapudi R, Kumar A, Bhatia HS, El-Bakoush A, Lepiarz I, Fiebich BL, et al. Inhibition of neuroinflammation by thymoquinone requires activation of Nrf2/ARE signalling. Int Immunopharmacol. 2017;48:17–29.
Badary OA, Nagi MN, Al-Shabanah OA, Al-Sawaf HA, Al-Sohaibani MO, Al-Bekairi AM. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol. 1997;75(12):1356–61.
Xu J, Zhu L, Liu H, Li M, Liu Y, Yang F, et al. Thymoquinone reduces cardiac damage caused by hypercholesterolemia in apolipoprotein E-deficient mice. Lipids Health Dis. 2018;17(1):1–9.
Abdel-Daim MM, Abo El-Ela FI, Alshahrani FK, Bin-Jumah M, Al-Zharani M, Almutairi B, et al. Protective effects of thymoquinone against acrylamide-induced liver, kidney and brain oxidative damage in rats. Environ Sci Pollut Res. 2020;27(30):37709–17.
Lotfi M, Kazemi S, Ebrahimpour A, Pourabdolhossein F, Satarian L, Eghbali A, et al. Thymoquinone Improved Nonylphenol-Induced Memory Deficit and Neurotoxicity Through Its Antioxidant and Neuroprotective Effects. Mol Neurobiol. 2022;59(6):3600–16.
Arif M, Thakur SC, Datta K. Implication of thymoquinone as a remedy for polycystic ovary in rat. Pharm Biol. 2016;54(4):674–85.
Badary OA. Thymoquinone attenuates ifosfamide-induced Fanconi syndrome in rats and enhances its antitumor activity in mice. J Ethnopharmacol. 1999;67(2):135–42.
Bai T, Lian L-H, Wu Y-L, Wan Y, Nan J-X. Thymoquinone attenuates liver fibrosis via PI3K and TLR4 signaling pathways in activated hepatic stellate cells. Int Immunopharmacol. 2013;15(2):275–81.
Ghazwani M, Zhang Y, Gao X, Fan J, Li J, Li S. Anti-fibrotic effect of thymoquinone on hepatic stellate cells. Phytomedicine. 2014;21(3):254–60.
Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical pharmacology. 1961;7(2):88 IN 191-9095.
Fortunato JJ, Agostinho, RÉus GZ, Petronilho FC, Dal-Pizzol F, Quevedo J. Lipid peroxidative damage on malathion exposure in rats. Neurotox Res. 2006;9(1):23–8.
Abdollahi M, Mostafalou S, Pournourmohammadi S, Shadnia S. Oxidative stress and cholinesterase inhibition in saliva and plasma of rats following subchronic exposure to malathion. Comp Biochem Physiol C: Toxicol Pharmacol. 2004;137(1):29–34.
Al-Attar AM. Effect of grapeseed oil on diazinon-induced physiological and histopathological alterations in rats. Saudi journal of biological sciences. 2015;22(3):284–92.
Yagi K. Lipid peroxides and human diseases. Chem Phys Lipid. 1987;45(2–4):337–51.
Cha SW, Gu HK, Lee KP, Lee MH, Han SS, Jeong TC. Immunotoxicity of ethyl carbamate in female BALB/c mice: role of esterase and cytochrome P450. Toxicol Lett. 2000;115(3):173–81.
Suddek GM. Protective role of thymoquinone against liver damage induced by tamoxifen in female rats. Can J Physiol Pharmacol. 2014;92(8):640–4.
Flesar J, Havlik J, Kloucek P, Rada V, Titera D, Bednar M, et al. In vitro growth-inhibitory effect of plant-derived extracts and compounds against Paenibacillus larvae and their acute oral toxicity to adult honey bees. Vet Microbiol. 2010;145(1):129–33.
Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct. 2002;20(2):143–51.
Lotti M. Clinical toxicology of anticholinesterase agents in humans. Handbook of pesticide toxicology. 2001;2:1043–85.
Maheswari E, Saraswathy GRL, Santhranii T. Hepatoprotective and antioxidant activity of N-acetyl cysteine in carbamazepine-administered rats. Indian journal of pharmacology. 2014;46(2):211.
Berkeley LI, Cohen JF, Crankshaw DL, Shirota FN, Nagasawa HT. Hepatoprotection by L-cysteine-glutathione mixed disulfide, a sulfhydryl-modified prodrug of glutathione. J Biochem Mol Toxicol. 2003;17(2):95–7.
Farooq J, Sultana R, Taj T, Asdaq SMB, Alsalman AJ, Mohaini MA, et al. Insights into the protective effects of thymoquinone against toxicities induced by chemotherapeutic agents. Molecules. 2021;27(1):226.
Butt MS, Imran M, Imran A, Arshad MS, Saeed F, Gondal TA, et al. Therapeutic perspective of thymoquinone: A mechanistic treatise. Food Sci Nutr. 2021;9(3):1792–809.
Liu H, Wu J, Yao J-y, Wang H, Li S-t. The role of oxidative stress in decreased acetylcholinesterase activity at the neuromuscular junction of the diaphragm during sepsis. Oxid Med Cell Longev. 2017;2017:9718615. https://doi.org/10.1155/2017/9718615.
Kenu A, Kenu E, Bandoh DA, Aikins M. Factors that promote and sustain the use of traditional, complementary and integrative medicine services at LEKMA hospital, Ghana, 2017: an observational study. BMC complementary medicine and therapies. 2021;21(1):1–10.
Carrie H, Mackey TK, Laird SN. Integrating traditional indigenous medicine and western biomedicine into health systems: a review of Nicaraguan health policies and miskitu health services. International journal for equity in health. 2015;14(1):1–7.
Danaei GH, Memar B, Ataee R, Karami M. Protective effect of thymoquinone, the main component of Nigella Sativa, against diazinon cardio-toxicity in rats. Drug Chem Toxicol. 2019;42(6):585–91.
Danaei GH, Karami M. Protective effect of thymoquinone against diazinon-induced hematotoxicity, genotoxicity and immunotoxicity in rats. Environ Toxicol Pharmacol. 2017;55:217–22.
Acknowledgements
The authors are thankful to the Vice Chancellor of Research, Mazandaran University of Medical Sciences for financial support. The results of the present study are part of a PhD thesis.
Funding
Mazandaran University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
GHD performed the project and made the analysis as well as writing the manuscript; AA and MBK contributed in the writing of manuscript; MK advised on the research project contributed in the writing of manuscript; BRZ and MS contributed in data analysis and the writing of manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experiments were approved by the Ethics Committee of Mazandaran University of Medical Sciences. All protocols of this study were conducted after the approval of the Ethic Council of Mazandaran University of Medical Sciences, Sari, Iran. All experiments were performed in accordance with ARRIVE guidelines. All methods and experiments were carried out according to the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Danaei, GH., Amali, A., Karami, M. et al. The significance of thymoquinone administration on liver toxicity of diazinon and cholinesterase activity; a recommendation for prophylaxis among individuals at risk. BMC Complement Med Ther 22, 321 (2022). https://doi.org/10.1186/s12906-022-03806-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03806-8