Abstract
Background
The rise of multidrug-resistant (MDR) bacteria is a real public health problem worldwide and is responsible for the increase in hospital infections. Donella welwitschii is a liana or shrub belonging to the family Sapotaceae and traditionally used to cure coughs.
Objective
This study was conducted with the objective to validate the medicinal properties of this plant, the aerial part was studied for its phytochemical composition using column and PTLC chromatography and exploring its antibacterial and antibiotic-modifying activity as well as those of its phytochemicals.
Methods
The structures of the compounds were elucidated from their physical and spectroscopic data in conjunction with literature. The antibacterial activity of the isolated metabolites was performed toward a panel of MDR Gram negative and Gram-positive bacteria. The broth micro-dilution method was used to determine antibacterial activities, efflux pump effect using the efflux pump inhibitor (EPI) (phenylalanine-arginine-ß-naphthylamide (PAβN)), as well as the modulating activity of antibiotics. Monitoring the acidification of the bacterial growth medium was used to study the effects of the samples on the bacterial proton-ATPase pumps and cellular ATP production.
Results
Eleven compounds were isolated including pentacyclic triterpenes, C-glucosyl benzophenones. With a MIC value < 10 μg/mL, diospyric acid (7) significantly inhibited the growth of Escherichia coli AG102, Enterobacter aerogenes ATCC13048, Klebsiella pneumoniae KP55, Providencia stuartii NEA16 and Staphylococcus aureus MRSA3. 28-hydroxy-β-amyrin (8) significantly impaired the growth of Enterobacter aerogenes EA27, Klebsiella pneumoniae ATCC11296 and Staphylococcus aureus MRSA6; and oleanolic acid (9) strongly impaired the growth of Escherichia coli AG 102, Enterobacter aerogenes EA27 and Providencia stuartii PS2636. Diospyric acid (7) and 28-hydroxy-β-amyrin (8) induced perturbation of H+-ATPase pump and inhibition of the cellular ATP production. Moreover, at MIC/2 and MIC/4, compounds 7, 8, and 9 strongly improved the antibacterial activity of norfloxacin, ciprofloxacin and doxycycline with antibiotic-modulating factors ranging between 2 and 64.
Conclusion
The overall results of the current work demonstrate that diospyric acid (7), 28-hydroxy-β-amyrin (8) and oleanolic acid (9) are the major bioactive constituents of Donella welwitschia towards Gram-negative bacteria expressing MDR phenotypes.
Similar content being viewed by others
Introduction
According to the World Health Organization (WHO), high levels of resistance to many pathogenic bacteria remains a serious health concern globally [1]. The rapid emergence of bacterial multidrug-resistant (MDR) phenotypes is a critical issue in the fight against infectious diseases [2]. Consequently, there is a need to search for new antimicrobial agents that can efficiently tackle bacterial resistance. The WHO has stated that most of the developing world still benefits from the use of traditional medicines derived from medicinal plants [3]. Moreover, many studies revealed the antibacterial inhibition activity of botanicals from African medicinal plants against MDR bacteria [4,5,6,7,8,9]. Several constituents including triterpenoids, phenolics, as well as alkaloids isolated from African medicinal plants were also documented for their antibacterial activities [10,11,12,13]. The present study focusses on the antibacterial activity of the constituents of Donella welwitschii (Engl.) Pierre ex Aubrev. & Pellegr. (Sapotaceae). The antibacterial activity of many secondary metabolites from plants belonging to the Sapotaceae family such as Tridesmostemon omphalocarpoides [14], Omphalocarpum elatum [15], Chrysophyllum lacourtianum [16], Synsepalum msolo [17], and Manilkara zapota [18] have been reported. The infusion of the shrub of D. welwitschii is traditionally used in the Central African Region and is used as an antitussive to relieve coughs and a stiff neck [19]. Previous phytochemical studies on species of the genus Donella reported cyclopropane type triterpene di-acids [20]. Herein, we reported the isolation of 11 secondary metabolites from D. welwitschii, and their antibacterial potential against a panel of MDR bacteria.
Materials and methods
Chemicals for antibacterial assays
A few reference antibiotics (RA), namely chloramphenicol (CHL), streptomycin (STR), erythromycin (ERY), norfloxacin (NOR), ciprofloxacin (CIP), ampicillin (AMP), and doxycycline (DOX) (Sigma-Aldrich, St. Quentin Fallavier, France) were predominantly used for the sample association tests, and only one was used as a positive control. Dimethyl sulfoxide (Sigma-Aldrich) was used to dissolve the samples before any tests. p-Iodonitrotetrazolium chloride (INT; Sigma-Aldrich) and phenylalanine-arginine-ß naphthylamide (PAβN; Sigma-Aldrich) were used as microbial growth indicator and efflux pump inhibitor (EPI), respectively. Solvents used for extraction and purification of bioactive compounds were of analytical grade.
Plant materials and extraction procedure
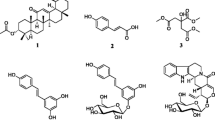
The aerial part of D. welwitschii was collected from Manjo in Bertoua, the East region of Cameroun on May 2018. The appropriate authorisation has been obtained for the collection of the plant and its use has been carried out in accordance with the relevant guidelines. The identification of the plant was carried out by Dr. Tchiengue Barthelemy at the Cameroon National Herbarium (Yaoundé) where a voucher specimen was conserved under specimen No: 10708SFC / 56630HNC. The air-dried and powdered twig and leaf (1160 g) of D. welwitschii were macerated in a mixture of CH2Cl2 – MeOH (1:1, 5 L) for 48 h, three times. The filtrate was evaporated at 40 °C under vacuum to yield 42.7 g of dark brown extract. The structures of compounds (Fig. 1) were determined by means of modern spectroscopic techniques (NMR and MS) and comparison with available literature (SM1). All 1H and 13C NMR spectra and major chemical shifts of these compounds are shown in the supplementary file (SM1).
Compounds isolated from D. welwitschia. 1: 3-β-D-glucopyranosyl-2,4,6-trihydroxyl (4-phenyl) methanone, 2: 3-α-D-glucopyranosyl-2,4,6-trihydroxyl (4-phenyl) methanone, 3: vanillic acid, 4: taraxerol, 5: taraxeryl acetate, 6: ursolic acid, 7: diospyric acid, 8: 28-hydroxy-β-amyrin, 9: oleanolic acid, 10: spinasterol 11: 3-O-β-D-glucopyranosyl spinasterol
Separation and purification of extracts of D. welwitschii
Forty-gram (40.0 g) of the crude extract was partitioned by solid-liquid process into hexane (DWa 12.2 g), ethyl acetate (DWb 11.7 g), and methanol (DWc 25.6 g) extracts. Part of the methanol extract was suspended in distilled water (200 mL) and extracted with n-butanol, this yielded n-BuOH extract (DWd 10.5 g) and aqueous extract (DWe 9.2 g). DWa and DWb were combined based on their TLC profiles to give 23.5 g of combined extract (DWab). The combined hexane and ethyl acetate extracts (DWab, 23.5 g) adsorbed on 30 g silica gel was applied to column packed with silica gel 60 (63–43 um Merck, particle size between 0.043 and 0.063 mm in diameter and porosity 230–400 mesh ASTM) eluting with hexane and ethyl acetate, ethyl acetate and methanol under gradient conditions. 189 fractions of 150 mL each were collected and pooled based on their TLC profiles. Hex: frs 1–15, Hex-EtOAc (95:5) frs 16–33, Hex-EtOAc (90:10) frs 34–50, Hex-EtOAc (85:15) frs 51–70, Hex-EtOAc (80:20) frs 71–92, Hex-EtOAc (75:25) frs 93–117, Hex-EtOAc (70:30) frs 118–132, Hex-EtOAc (60:40) frs 133–148, Hex-EtOAc (25:75) frs 149–158, EtOAc frs 159–171, EtOAc (85:15) frs 172–184. Further purification of sub fractions 1–50 led to the compound taraxeryl acetate, 5 (23 mg); 51–117 yielded spinasterol 10 (80 mg) and taraxerol 3 (35 mg) Ursolic 6 (27 mg) and oleanolic 9 (17 mg) acids respectively. Diospyric acid 7 (45 mg), 28-hydroxyolean-12-ene 8 (23 mg) and 3-O-β-D-glucopyranosyl spinasterol 11 (65 mg) were isolated from subfractions 118–132, 133–148 and 149–171 by purification on silica gel columns. The n-BuOH fraction (DWd, 9.32 g) was adsorbed on 20 g of fine silica, dried and applied to silica gel column eluted with DCM and Methanol under gradient conditions. 150 subfractions of 150 mL each were collected and pooled based on their TLC profiles. Subfractions 1–20 and 97–120 were purified by preparatory TLC yielding vanillic acid 3 (3 mg), 3-α-D-glucopyranosyl-2,4,6-trihydroxyl (4-phenyl) methanone 2 (80 mg) and 3-α-D-glucopyranosyl-2,4,6-trihydroxyl (4-phenyl) methanone 1 (60 mg) respectively.
Bacteria strains, culture media and growth conditions
Thirteen pathogenic strains consisting of Gram-negative and Gram-positive bacteria were recruited to measure the bacteriostatic and bactericidal power of the samples used in the study. Gram-negative bacteria included multidrug-resistant (MDR) isolates (laboratory collection) and reference strains of Escherichia coli (ATCC 10536, and AG102), Enterobacter aerogenes (ATCC 13048 and EA27), Klebsiella pneumoniae (ATCC 11296 andKP55), Providencia stuartii (ATCC 29916 and NEA16) and Pseudomonas aeruginosa (PA01 and PA124). Note that the clinical strains came from the laboratory collection of the UMR-MD1, University of Marseille, France. The Gram-positive bacterial strains were specifically Staphylococcus aureus including a reference strain obtained from the American Type Culture Collection (ATCC) (ATCC 25923), and a methicillin-resistant S. aureus isolate (MRSA3 and MRSA6) obtained from the culture collection of the Laboratory of Microbiology, Graduate School of Pharmaceutical Sciences, University of Tokyo, Japan, and was provided by Prof. Dr. Dzoyem of the University of Dschang [21, 22]. Susceptibility patterns of the tested bacteria are given in the Supplementary file (Table S1, SM2).
Evaluation of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The ability of the isolated compounds to stop growth and kill bacteria was achieved through the determination of minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC). This was possible using microdilution methods as described by Eloff (1998) [23] with some modifications and improvements [22, 24]. Briefly, series of dilutions of the samples were performed in a 96-well microplate after dissolving the samples in 10% dimethyl sulfoxide (DMSO)/Mueller Hinton broth (MHB). Then 100 μL of bacterial inoculum (2 × 106 colony forming units (CFU)/mL) was added to each well. Chloramphenicol was used as a reference substance. The final concentrations ranged 4–512 μg/mL. Plates were then covered with a sterile plate sealer and incubated at 37 °C for 18 h. Revelation was done by adding 40 μL of INT (0.2 mg/mL) followed by incubation at 37 °C for 30 min. The MIC of each sample was defined as the lowest concentration of the sample that completely inhibited the growth of the bacteria. The MICs obtained were classified as follows: significant activity MIC < 100 μg/mL (MIC < 10 μg/mL for pure compounds), moderate activity 100 μg/ mL < MIC ≤625 μg/ mL (10 < MIC ≤100 μg/mL for pure compounds), weak activity MIC> 625 μg/mL (MIC> 100 μg/mL for pure compounds) [22, 25].
After MIC readings, minimum bactericidal concentrations (MBC) were determined by introducing 150 μL of MHB into a new 96-well microplate, after addition of 50 μL of the microplate contents, where no microbial growth was observed, and which did not receive INT during the MIC reading. The MBC of each sample was determined after 48 h of incubating at 37 °C, by adding 40 μL of 0.2 mg/mL INT, as previously described [22]. MBC was the lowest concentration of the samples, which did not produce a colour change after adding INT. Each experiment was performed in triplicate and repeated three times.
Evaluation of the role of efflux pumps in the antibacterial activity of the samples
After testing the isolated compounds alone, the same compounds were put in the presence of PaβN (at 30 μg/mL) against 13 bacterial strains including five MDR phenotypes (E. coli AG102, E. aerogenes EA27, K. pneumoniae KP55, P. stuartii PS2636 and S. aureus MRSA3) as previously described [25, 26]. The activity improvement factor (AIF) or fold increase of the activity was determined as ratio of MIC (sample alone)/MIC (sample + PAβN) [22]. Each assay was performed in duplicate and repeated three times.
Assessment of the antibiotic-modifying activity of the samples
The combination assay was performed as previously described, with some modifications made during the procedure [4, 7]. The test was briefly performed by introducing 100 μL of MHB into the wells of a 96-well microplate, followed by the addition of 100 μL of the test antibiotics in the first well of each column and progressive dilution. 50 μL of solution of test compounds at sub-inhibitory concentrations (MIC/2 and MIC/4) were then introduced into the wells, followed by the addition of 50 μL of bacterial inoculum. The microplates were sealed and incubated for 18 h at 37 °C. MICs were determined using the INT as previously described. The antibiotic modulation factor (AMF), calculated as MIC (antibiotic alone)/− MIC (antibiotic + compound), was used to express the modulating effects of compounds on antibiotics [5, 22, 27]. Each test was performed in duplicate and repeated three times.
H + -ATPase-mediated proton pumping assay
The ability of compounds 7 and 8 to inhibit the H + −ATPase mediated proton pumps of E. coli AG102 was assessed by monitoring the acidification of the bacterial growth medium as previously described [28] with slight modifications [22]. Briefly, 100 mL of bacteria suspension was cultured in MHB for 18 h at 37 °C. The resulting culture was centrifuged at 3000 tr/min for 10 min at 4 °C. The pellet was first washed twice in distilled water, then in 50 mM KCl and suspended in 50 mL of 50 mM KCl. Then, the cell suspension was incubated overnight (18 h) at 4 °C for glucose starvation. In 4 mL of the cell medium, 0.5 mL of the tested samples (MIC/2, MIC, and 2 × MIC) were added, and pH adjusted to 6.8 with 1 M HCl or 0.1 M NaOH. Upon 10 min pre-incubation at 37 °C, medium acidification was initiated by adding 0.5 mL of 20% glucose, followed by pH measurement every 10 min for 1 h using a pH-meter. Tube containing MHB, inoculum, and DMSO was used as negative control. Each essay was performed in triplicates.
Measurement of cellular ATP concentration
The ability of compounds 7 and 8 to inhibit the cellular ATP production of E. coli AG102 was assessed as previously described [28] with slight modifications. Briefly, 100 mL of bacterial culture (1:100 v/v) was grown in MHB culture medium for 18 h at 37 °C, followed by centrifugation (3500×g, 10 min). The pellet was washed twice with distilled water, then with 50 mM KCl and suspended in 50 mL of 50 mM KCl. The cell suspension (1.5–2 × 108 CFU/mL) was incubated overnight (18 h, 4 °C) for glucose starvation and then centrifuged. To 4 mL of the reaction medium, 0.5 mL of compounds 7 and 8 corresponding to MIC/2, MIC, and 2 × MIC was added, and the pH was adjusted to 6.4. After 10 min of pre-incubation under shaking at 37 °C, acidification of the medium was initiated after addition of 0.5 mL of 20% (w/v) glucose. The pH measurement was recorded every 20 min for a total period of 160 min. The experiment was conducted in the presence of DMSO (control) at a final concentration of 2.5%, to measure the degree of acidification of the external medium in the absence of compounds 7 and 8. The experiment was performed in triplicate and repeated twice. The measured pH was used to plot the pH versus time curve [pH = f (time)]. Any inhibition of acidification of the medium in the presence of samples was attributed to an inhibitory effect of the H + -ATPase pumps.
Statistical analysis
Data from each experimental group was expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test for multiple comparisons were used for statistical analysis of data using GraphPad Prism Version 8.1.0 (GraphPad Software, CA, USA). Differences were considered significant at a probability level of 5% (p < 0.05), ** p-values < 0.01, and *** p-values < 0.001.
Results
Phytochemistry
Column chromatography of the major fractions afforded eleven compounds, which were identified as 3-β-D-glucopyranosyl-2,4,6-trihydroxyl (4-phenyl) methanone (1), 3-α-D-glucopyranosyl-2,4,6-trihydroxyl (4-phenyl) methanone (2), vanillic acid (3), taraxerol (4), taraxeryl acetate (5), ursolic acid (6), diospyric acid (7), 28-hydroxy-β-amyrin (8), oleanolic acid (9), spinasterol (10) and 3-O-β-D-glucopyranosyl spinasterol (11). These compounds include two benzophenones: 1 and 2, one phenolic acid 3, six triterpenoids 4–9, one sterol 10, and one sterol saponin 11.
MICs and MBCs of phytochemicals
The antibacterial activity of the isolated compounds 1–4 and 7–10 has been evaluated against E. coli (ATCC 10536, and AG102), E. aerogenes (ATCC 13048 and EA27), K. pneumoniae (KP55 and ATCC 11296), P. stuartii (ATCC 29916, and NEA16), P. aeruginosa (PA01 and PA124), and S. aureus (ATCC 25923 MRSA3 and MRSA6). The results obtained show that phytochemicals 5 and 11 exhibited weak activities against all strains tested. However, the other phytochemicals showed variable antibacterial activities depending on the bacterial strains tested, with MICs ranging from 4 to 512 μg/mL (Table 1). Compounds 7 showed significant antibacterial activities (MIC < 10 μg/mL) against E. coli AG102, E. aerogenes ATCC 13048, K. pneumoniae KP55, P. stuartii NEA16 and S. aureus MRSA3, compound 8 (against E. aerogenes EA27, K. pneumoniae ATCC 11296 and S. aureus MRSA6); and compound 9 (against E. coli AG 102, E. aerogenes EA27 and P. stuartii PS2636). In the MBC determinations, the active phytochemicals exhibited bactericidal activity against at least one bacterial strain tested. Overall, the MBCs of the phytochemicals ranged from 32 to 128 μg/mL (Table 1). A closer look at the MBC/MIC ratios indicated that the tested samples had mainly bacteriostatic effects (MBC/MIC > 4).
Effect of PAβN on the antibacterial activity of the tested samples
The susceptibility of E. coli (ATCC 10536, and AG102), E. aerogenes (ATCC 13048 and EA27), K. pneumoniae (KP55 and ATCC 11296), P. stuartii (ATCC 29916, and NEA16), P. aeruginosa (PA01 and PA124), and S. aureus (ATCC 25923 MRSA3 and MRSA6) to compounds and chloramphenicol in the presence of PAβN, was evaluated. Phytochemicals 6, 7, 8 and 9 in the presence of PaβN showed improved antibacterial activity (MIC reduction) against the tested bacteria by 2 to > 64 times. These isolated compounds were more effective against MDR bacteria and mainly against Gram-negative bacteria. Moreover, PAβN did not affect the activity of chloramphenicol against Gram-positive bacteria (S. aureus: ATCC 25923 and MRSA3) (Table 2).
Antibiotic-modulating activity of compounds
The modulatory activity of active phytochemicals (7, 8 and 9) was investigated on six classical antibiotics including streptomycin (STR), erythromycin (ERY), norfloxacin (NOR), ciprofloxacin (CIP), ampicillin (AMP) and doxycycline (DOX). The overall results showed a considerable increase in the activity of the tested antibiotics. The best potentiating effects were recorded with NOR, CIP and DOX, in combination with phytochemicals 7, 8 and 9 at MIC/2 and MIC/4. It was found that the overall activity enhancement factors (AEFs) obtained with the antibiotics ranged from 2 to 64 (Table 3, Table 4 and Table 5), indicating a 2 to 64 fold increase in antibacterial activity of the antibiotics. These activities were predominantly recorded on the MDR strain E. aerogenes EA-CM64. The reference strain E. coli ATCC 10536 was also sensitive to the same combination (Table 3, Table 4 and Table 5). Combination of the other antibiotics and the compounds associations has mainly presented indifference or antagonistic effects.
Effect of compound 7 and 8 on proton-ATPase pumps
Figure 2a and b display the effects compounds 7 and 8 on the activity of proton-ATPase pumps. The differences observed in compounds 7 and 8 tested at MIC/2, MIC and 2MIC were significant compared to the control. In compound 7, a slight decrease in pH was observed, reaching 7.35 at a time equal to 20 min. Then a constant progression of pH to 7.35 until a time t = 60 min overall, with small variations of pH between 7.3 and 7.45 during the experiment, at concentrations equal to MIC/2, MIC and 2MIC. Contrary to the control whose pH decreases from the beginning of the experiment until reaching pH 6.2 at the end (T = 60 min) (Fig. 2a). In compound 8, a constant progression of pH (pH 7.3) can be observed until the end of the experiment, at MIC and 2MIC concentrations. At CMI/2, a decrease in pH is observed until reaching 6.4 at t = 20 min, then remains constant until the end of experiment. Contrary to the control whose pH decreases from the beginning of the experiment until reaching pH 6.2 at the end (t = 60 min) (Fig. 2b).
Effect of compound 7 and 8 on cellular ATP production
Figure 3a and b show the results of the effects of actives compounds 7 and 8 on the activity of ATP synthase. The differences observed in compounds 7 and 8 tested at MIC/2, MIC and 2MIC were significant compared to the control. For compound 7, an increase in pH can be observed from the second minute of the experiment (pH 7.9), at concentrations of MIC/2, MIC and 2MIC. Contrary to the control whose pH decreases from the beginning of the experiment until reaching pH 6.1 at the end (T = 160 min) (Fig. 3a). Virtually the same behaviour was observed with compound 8 (Fig. 3b).
(a) Effects of compound 7 and (b) compound 8 from D. welwitschia on cellular ATP production in E. coli AG102. MIC: Minimum inhibitory concentration. Results are expressed as Mean ± SD. Significantly different from the control, * p < 0.05, ** p-values < 0.01, and *** p-values < 0.001; ns: non-significant
Discussion
Chemical compounds produced by plants, generally help them to resist fungi, bacteria, and plant virus infections, and consumption by insects and other animals. However, some phytochemicals’ class have been used as medicine to cure various ailments. In the current work, we explored the anti-MDR bacteria’s chemicals of Donella welwitschii, as well as the mechanism of action through which they exhibited their antibacterial effects. The chemical investigation of the methylene chloride-methanolic extract of D. welwitschii aerial part, led to isolation of eleven compounds belonging to various class of secondary metabolites known for their antimicrobial activities through specific mechanisms of actions i.e. terpenoids, phenolic compounds and alkaloids [29, 30]. Vanillic acid 3 [31] is a phenolic acid belonging to phenolic compounds’ group, taraxerol 4 [32], taraxeryl acetate 5 [33], ursolic acid 6 [34], diospyric acid 7 [35], 28-hydroxy-β-amyrin 8 [36] and Oleanolic acid 9 [37] are all triterpenes belonging to terpenoids group.
Against a panel of MDR Gram-negative and Gram-positive bacteria, D. welwitschii’s compounds 5 and 11 were found to be inactive, while other compounds (1–4, 6–10) were selectively significantly active, moderately active and/or weakly active. Diospyric acid (7) significantly inhibited (MIC < 10 μg/mL) the growth of E. coli AG102, E. aerogenes ATCC 13048, K. pneumoniae KP55, P. stuartii NEA16 and S. aureus MRSA3. To the best of our knowledge, this study is illuminating for the first time the antibacterial activity of diospyric acid against the Gram-negative and Gram-positive MDR bacteria. The significant activity of 28-hydroxy-β-amyrin toward E. aerogenes EA27, K. pneumonia ATCC11296 and S. aureus MRSA6 is in accordance with the study of Abdel-Raouf et al. [38] which highlighted the interesting inhibition growth capacity of β-amyrin against Staphylococcus aureus NCTC 7447 and Salmonella typhi ATCC 19430. Oleanolic acid (9), one of the isolated triterpenes compounds, significantly impaired the growth of E. coli AG102, E. aerogenes EA27 and P. stuartii PS2636). The antimicrobial activity and mechanism of action of oleanolic acid is widely reported in the literature. Martins et al. [39] showed the antibacterial activity of oleanolic against E. coli AG100, Methicillin Resistant Staphyloccocus aureus COL, S. aureus HPH 107, and Mycobacterium tuberculosis H37Rv. Moreover, Fontanay et al. [40] showed the effect of oleanolic acid against E. faecalis ATCC 28212. In the present work, we also investigated the effect of diospyric acid (7) and 28-hydroxy-β-amyrin (8) on the functioning of H+-ATPase pump and the cellular ATP production of E. coli AG102. Let’s note that from the studies of Futai and Kanazawa [41] and those of Senior and Wise [42], it is well established that the H + -ATPase pump of Escherichia coli membranes catalyses ATP synthesis and the formation of ATP-driven proton electrochemical gradients, and resembles analogous enzymes from mitochondria, chloroplasts, and other bacteria. The D. welwitschii’s compounds might induce bacteria growth inhibition through significant perturbation of H+-ATPase pump and inhibition of the cellular production of ATP.
Clinically tripartite efflux systems, AcrAB-TolC for Enterobacteriaceae or MexAB-OprM for P. aeruginosa, are associated with multidrug resistance of pathogenic Gram-negative bacteria [43,44,45]. PAßN has been reported as a potent inhibitor of the RND efflux systems and is especially active on AcrAB-TolC and MexAB-OprM [46,47,48,49]. PAßN has been used to demonstrate the role of efflux pumps in MDR of the tested microorganisms. In presence of the efflux pump inhibitor PAβN, the MIC values of phytochemicals 7–9 decreased, showing that these compounds could be substrates of efflux pumps acting in resistant strains used in our study. These data suggested that combination of these compounds with antibiotics could improve the antibacterial activity of conventional antibiotics against MDR phenotypes. Combining phytoconstituents 7–9 with antibiotics against MDR bacteria at MIC/2 and MIC/4 strongly improved the antibacterial activity of norfloxacin, ciprofloxacin and doxycycline with antibiotic-modulating factors ranging between 2 and 64. This indicated that compounds 7, 8 and 9 behave as natural efflux pumps inhibitor. Martins et al. [39] revealed the antibiotic potentiating capacity (tetracycline) of β-amyrin (8) and Oleanolic acid (9) against E. coli AG100TET8. Moreover, our results are in accordance with those of Abreu et al.[50] which highlighted synergism effect against drug-resistant Staphylococcus aureus when oleanolic acid was combined to tetracycline or erythromycin, as well as those of Basri et al.[51] showing the synergistic interaction arising from the combination of the oleanolic acid and norfloxacin against SA1199B and MRSA274829 [51] .
Conclusion
The overall results of the current work provide baseline information for the possible use of some compounds, diospyric acid (7), 28-hydroxy-β-amyrin (8) and Oleanolic acid (9), of Donella welwitschii extract to fight against bacterial infections involving MDR phenotypes. In addition, the data reported herein indicated that compounds diospyric acid (7), 28-hydroxy-β-amyrin (8) and Oleanolic acid (9) behave like efflux pump inhibitors. They are the major antibacterial constituents of D. welwitschii against MDR Gram-negative bacteria as well as Staphylococcus aureus.
Availability of data and materials
All data generated or analysed during this study are included in this published article and the supporting file.
Abbreviations
- ATCC:
-
American-type culture collection
- ATP:
-
Adenosine triphosphate
- CFU:
-
Colony-forming unit
- CIP:
-
Ciprofloxacin
- DMSO:
-
Dimethyl sulfoxide
- EPIs:
-
Efflux pump inhibitors
- INT:
-
P-Iodonitrotetrazolium chloride
- MBC:
-
Minimal bactericidal concentration
- MDR:
-
Multidrug resistance
- MHA:
-
Mueller–Hilton agar
- MHB:
-
Mueller–Hinton broth
- MIC:
-
Minimal inhibitory concentration
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- NMR:
-
Nuclear magnetic resonance
- PaβN:
-
Phenylalanine arginine-β- naphthylamide
- TLC:
-
Thin-layer chromatography
References
World Health Organization (WHO). The World health statistic 2019a. 2019a.
Centres for Disease Control and Prevention, “Office of Infectious Disease Antibiotic resistance threats in the United States 2013. Apr, 2013.
World Health Organization. WHO global report on traditional and complementary medicine 2019. 2019b.
Guefack MGF, Tankeo SB, Ngaffo CMN, Nayim P, Wamba BEN, Bonsou IN, et al. Mbaveng AT antibiotic-potentiation activities of three animal species extracts, Bitis arietans, Helix aspersa, and Aristaeomorpha foliacea and mode of action against MDR gram-negative bacteria phenotypes. Invest Med Chem Pharmacol. 2021;4:000478.
Ngaffo CMN, Tankeo SB, Guefack MGF, Nayim P, Wamba BEN, Kuete V, et al. Phytochemical analysis and antibiotic-modulating activity of Cocos nucifera, Glycine max and Musa sapientum methanol extracts against multidrug resistant gram-negative bacteria. Invest Med Chem Pharmacol. 2021;4:53.
Dzotam JK, Touani FK, Kuete V. Antibacterial and antibiotic-modifying activities of three food plants (Xanthosoma mafaffa lam., Moringa oleifera (L.) Schott and Passiflora edulis Sims) against multidrug-resistant (MDR) gram-negative bacteria. BMC Complement Altern Med. 2016;16:9.
Manekeng HT, Mbaveng AT, Nguenang GS, Seukep AJ, Wamba BEN, Nayim P, et al. Anti-staphylococcal and antibiotic-potentiating activities of seven Cameroonian edible plants against resistant phenotypes. Invest Med Chem Pharmacol. 2018;1:7.
Fankam AG, Kuiate JR, Kuete V. Antibacterial activities of Beilschmiedia obscura and six other Cameroonian medicinal plants against multi-drug resistant gram-negative phenotypes. BMC Complement Altern Med. 2014;14:241.
Lacmata ST, Kuete V, Dzoyem JP, Tankeo SB, Teke GN, Kuiate JR, et al. Antibacterial activities of selected Cameroonian plants and their synergistic effects with antibiotics against bacteria expressing MDR phenotypes. Evid Based Complementary Altern Med. 2012;623723.
Kuete V, Sandjo LP. Isobavachalcone: an overview. Chin Jour Integ Med. 2012;18(2):543–7.
Kuete V, Tangmouo JG, MeyerJJM LN. Diospyrone, crassiflorone and plumbagin: three antimycobacterial and antigonorrhoeal naphthoquinones from two Diospyros spp. Int J Antimicrob Agents. 2009;34(8):322–5.
Nguemeving JR, Azebaze AG, Kuete V, Carly NNE, Beng VP, Meyer M, et al. Laurentixanthones a and B, antimicrobial xanthones from Vismia laurentii. Phytochem. 2006;67(6):1341–6.
Nono EC, Mkounga P, Kuete V, Marat K, Hultin PG, Nkengfack AE. Pycnanthulignenes A-D, antimicrobial cyclolignene derivatives from the roots of Pycnanthus angolensis. J Nat Prod. 2010;73:213–6.
Kuete V, Tangmouo JG, PenlapVB NFN, Lontsi D. Antimicrobial activity of the methanolic extract from the stem bark of Tridesmostemon omphalocarpoides (Sapotaceae). J Ethnopharmacol. 2006;104(11):5–11.
Sandjo LP, Fru CG, Kuete V, Nana F, Yeboah SO, Mapitse R, et al. Elatumic acid: a new ursolic acid congener from Omphalocarpum elatum Miers (Sapotaceae). Zeitschrift für Naturforschung C. Journ. Biosc. 2014;69(4):276–82.
Talla RM, Jouda JB, Mawabo IK, Tegasne C, Happi GM, Kapche G, et al. One new constituent from the stem bark of Chrysophyllum lacourtianum De wild. (Sapotaceae). Nat Prod Res. 2021:1986–193.
Ndifor AR, Stanislaus NN, Fru CG, Talontsi F, Tabopda TK, Menkem EZ, et al. Two new sphingolipids from the stem bark of Synsepalum msolo (Sapotaceae). Biochem Bioph Rep. 2021;27:101014.
Mourão MLC, Xavier-Júnior FH, Rodrigues AMS, Stien D, Allegretti SM, Garcia VL. Antimicrobial and anthelmintic activities of the ethanolic extract, fractions and isolated compounds from Manilkara zapota L. P Royen (Sapotaceae) Journ Pharm Pharmacol. 2021;73(2):377–87.
Aubreville A, du Cameroun F. familles des Sapotaceae 2. Musée National d’Histoire Naturelle, Laboratoire de pharmacopée, phamemegemee. Paris. 1964:3–21.
Djoumessi AVB, Sandjo LP, Liermann JC, Schollmeyer D, Kuete V, Rincheval V, et al. Donellanic acids A–C: new cyclopropanic oleanane derivatives from Donella ubanguiensis (Sapotaceae). Tetrahedron. 2012;68(1):4621–7.
Dzoyem JP, Hamamoto H, Ngameni B, Ngadjui BT, Sekimizu K. Antimicrobial action mechanism of flavonoids from Dorstenia species. Drug Discov Ther. 2013;7(2):66–72.
Demgne OMF, Damen F, Fankam AG, Guefack MF, Wamba BEN, Nayim P, et al. Botanicals and phytochemicals from the bark of Hypericum roeperianum (Hypericaceae) had strong antibacterial activity and showed synergistic effects with antibiotics against multidrug-resistant bacteria expressing active efflux pumps. J Ethnopharmacol. 2021;114257.
Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64(1):711–3.
Kuete V, Metuno R, Ngameni B, Tsafack AM, Ngandeu F, Fotso GW, et al. Antimicrobial activity of the methanolic extracts and compounds from Treculia obovoidea (Moraceae). J Ethnopharmacol. 2007;112(1):531–6.
Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010;76(1):1479–91.
Lorenzi V, Muselli A, Bernadini AF, Berti L, Pagès J-M. Geraniol restores antibiotic activities against multidrug resistant isolates from gram-negative species. Antimicrob Agents Chemother. 2009;53(2):2209–11.
Kovac J, Gavari N, Bucar F, Smole MS. Antimicrobial and resistance modulatory activity of Alpinia katsumadai seed extract, essential oil and post distillation extract. Food Technol Biotechnol. 2014;52(1):248–54.
Manavathu EK, Dimmock JR, Sarvesh CV, Chandrasekar PH. Inhibition of H+-ATPase-mediated proton pumping in Cryptococcus neoformans by a novel conjugated styryl ketone. J Antimicrob Chemother. 2001;47(3):491–4.
Cowan M. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(1):564–82.
Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines. 2015;2(1):251–86.
Chang SW, Kim KH, Lee IK, Choi SU, Ryu SY, Lee KR. Phytochemical constituents of Bistorta manshuriensis. Nat Prod Sci. 2009;15(4):234–40.
SangeethaK SS, Shanmuganathan V. 3 β -taraxerol of Mangifera indica , a PI3K dependent dual activator of glucose transport and glycogen synthesis in 3T3-L1 adipocytes. Biochim Biophys Acta. 2010;1800(3):359–66.
Díaz-Ruiz G, Hernández-Vázquez L, Luna H, Del Carmen W-RM, Navarro-Ocaña A. Growth inhibition of streptococcus from the oral cavity by α-amyrin esters. Molecules. 2012;17(11):12603–11.
Liu M, Yeng S, Jin L, Hu D, Wu Z, Yang S. Chemical constituents of the ethyl acetate extract of Belamcanda chinensis (L.) DC roots and their antitumor activities. Molecules. 2012;17(5):6156–69.
Thuong PT, et al. Triterpenoids from the leaves of Diospyros kaki (persimmon) and their inhibitory effects on protein tyrosine phosphatase 1B. J Nat Prod. 2008;71(1):1775–8.
Shashi BM, Asish AP. 13C Nmr spectra of pentacyclic triterpenoids-a and some salient features. Phytochemistry. 1994;37(6):1517–75.
Seebacher W, SimicN WR, Saf R, Kunert O. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn Reson Chem. 2003;41(8):636–8.
Abdel-Raouf N, Al-Enazi NM, Al-Homaidan AA, Ibraheem IBM, Al-Othman MR, Hatamley AA. Antibacterial β-amyrin isolated from Laurencia microcladia. Arab J Chem. 2015;81(1):32–7.
Martins A, Vasas A, Viveiros M, Molnár J, Hohmannc J, Amaral L. Antibacterial properties of compounds isolated from Carpobrotus edulis. Int J Antimicrob Agents. 2011;37(1):438–44.
Fontanay S, Grare M, Mayer J, Finance C, Duval RM. Ursolic, oleanolic and betulinic acids: antibacterial spectra and selectivity indexes. J Ethnopharmacol. 2008;120(2):272–6.
Futai M, Kanazawa H. Structure and function of proton-translocating adonesine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983;47(2):285–312.
Senior AE, Wise JG. The proton-ATPase of bacteria and mitochondria. J Membr Biol. 1983;73(1):105–24.
Blot S, Depuydt P, Vandewoude K, De Bacquer D. Measuring the impact of multidrug resistance in nosocomial infection. Curr Opin Infect Dis. 2007;20(2):391–6.
Pages J-M, Amaral L. Mechanisms of drug efflux and strategies to combat them: challenging the efflux pump of gram-negative bacteria. Biochim Biophys Acta, Proteins Proteomics. 2009;1794(1):826–33.
Papadopoulos CJ, Carson CF, Chang BJ, Riley TV. Role of the MexAB-OprM efflux pump of Pseudomonas aeruginosa in tolerance to tea tree (Melaleuca alternifolia) oil and its monoterpene components terpinen-4-ol, 1, 8-cineole and α-terpineol. Appl Environ Microbiol. 2008;74(6):1932–5.
Pietras A, Bavro VN, Furnham N, Pellegrini-Calace M, Milner-White EJ, Luisi BF. Structure and mechanism of drug efflux machinery in gram negative bacteria. Current Drug Target. 2008;9:719–28.
Lomovskaya O, Bostian KA. Practical applications and feasibility of efflux pump inhibitors in the clinic--a vision for applied use. Biochem Pharmacol. 2006;71(2):910–8.
Pagès J-M, Lavigne JP, Leflon-Guibout V, Marcon E, Bert F, Noussair L, et al. Efflux pump, the masked side of β-lactam resistance in Klebsiella pneumoniae clinical isolates. PLoS One. 2009;4(1):4817.
Kuete V, Ngameni B, Tangmouo JG, Bolla JM, Albert-Franco S, Ngadjui BT, et al. Efflux pumps are involved in the defense of gram-negative bacteria against the natural products isobavachalcone and diospyrone. Antimicrob Agents Chemother. 2010;54(1):1749–52.
Abreu AC, Paulet D, Coqueiro A, Malheiro J, Borges A, Saavedra JM, et al. Antibiotic adjuvants from Buxus sempervirens to promote effective treatment of drug-resistant Staphylococcus aureus biofilms. RSC Adv. 2016;6(3):95000–9.
Basri DF, Xian LW, Abdul-Shukor NJ. Bacteriostatic antimicrobial combination: antagonistic interaction between epsilon-viniferin and vancomycin against methicillin-resistant Staphylococcus aureus. Biomed Res Int. 2014;2014(16):461756.
Acknowledgements
ATM and VK are thankful to Alexander von Humboldt Foundation for the 3 months further research stay fellowship at home from November 2021 to January 31, 2022.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
M-GFG, MON, PN, CMNN, JRNK, GFC carried out the experiments and contributed to the data analysis; ATM, BN, BTN, VK designed the study; PN, M-GFG, GFC, VK drafted the manuscript; ATM, VK provided the bacterial strains and facilities for the antimicrobial testing; All authors read and agreed on the final version of the manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Ethics approval and consent to participate
The identification of the plant was carried out by Dr. Tchiengue Barthelemy at the Cameroon National Herbarium (Yaoundé) where a voucher specimen was conserved under specimen No: 10708SFC / 56630HNC.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. SM1
. Physical properties and NMR data of Compounds 1–10. SM2. Table S1: Bacterial features of the tested of microorganisms.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guefack, MG.F., Ngangoue, M.O., Mbaveng, A.T. et al. Antibacterial and antibiotic-potentiation activity of the constituents from aerial part of Donella welwitshii (Sapotaceae) against multidrug resistant phenotypes. BMC Complement Med Ther 22, 194 (2022). https://doi.org/10.1186/s12906-022-03673-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03673-3