Abstract
Background
Vitamin D (Vit D) supplementation and Aerobic Training (AT) exert several beneficial effects such as antioxidant and anti-inflammatory actions. The literature on the effects of AT and Vit D supplementation on the oxidative stress biomarkers and gene expression of inflammatory cytokines in patients with Type 2 Diabetes Mellitus (T2DM) is limited. The present study aimed to examine the effects of AT and Vit D supplementation on inflammation and oxidative stress signaling pathways in T2DM patients.
Materials and methods
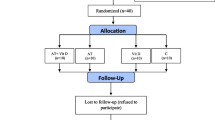
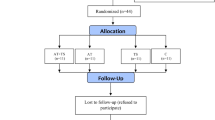
In this single-blinded, randomized, placebo-controlled trial, 48 men with T2DM (aged 35–50 years with Body Mass Index (BMI) of 25–30 kg/m2) were randomly allocated into four groups: AT+Vit D (n = 10); AT + placebo (AT; n = 10); Vit D (n = 10), and Control + placebo (C; n = 10). The eight-week AT program was executed for 20–40 min/day, at 60–75% of heart rate maximum (HRmax), for 3 days/wks. The Vit D group received 50,000 IU of Vit D supplement capsules per week for 8 weeks. The serum levels of oxidative stress biomarkers and gene expression of inflammatory cytokines in the Peripheral Blood Mononuclear Cells (PBMCs) were evaluated using the RT-PCR method. To analyze the data, paired t-tests and one-way analysis of variance and Tukey’s post hoc test were used at the significance level of P < 0.05.
Results
The result shows that serum 25-OH-Vit D, total nitrite, Total Glutathione (GSH), Total Antioxidant Capacity (TAC), Superoxide Dismutase (SOD), Catalase (CAT), and Glutathione Peroxidase (GPX) increased; and insulin, Fasting Blood Glucose (FBG), Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), High Sensitivity C-Reactive Protein (hs-CRP), Malondialdehyde (MDA), glycated albumin, and Urinary 8-hydroxydeoxyguanine (8-OHdG) decreased significantly in all groups after 8 weeks, except for C. In addition, results of RT-PCR showed that AT+Vit D, Vit D, and AT significantly downregulated the gene expression of Tumor Necrosis Factor-Alpha (TNF-α), Interleukin-1 Beta (IL-1β), Mitogen-Activated Protein Kinases 1 (MAPK1), Nuclear Factor Kappa B (NF-κB) 1 (p50). It also upregulated Interleukin-4 (IL-4) gene expression, Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ) in T2DM patients compared to the C.
Conclusion
Additionally, the AT+Vit D group showed significantly lower insulin, FBG, HOMA-IR, hs-CRP, MDA, glycated albumin, urinary 8-OHdG, IL-1β, TNF-α, MAPK1, and NF-κB1 (p50) levels and significantly higher serum 25-OH-Vit D, total nitrite, GSH, TAC, CAT, SOD, GPX, IL-4, and PPAR-γ levels compared to the AT and Vit D groups. In T2DM patients, 8 weeks of AT+Vit D had a more significant impact on certain gene expressions related to inflammation and oxidative stress than Vit D or AT alone.
Similar content being viewed by others
Introduction
Type 2 Diabetes Mellitus (T2DM) is a chronic disorder leading to hyperglycemia resulting from abnormal insulin function and secretion. According to the World Health Organization (WHO), the number of T2DM patients is expected to reach 642 million by 2040 [1]. Increased inflammatory cytokine is an inseparable part of T2DM and is associated with protein-energy malnutrition, cardiovascular disease (CVD), and all-cause mortality [2]. Increased inflammatory biomarkers, such as Tumor Necrosis Factor-Alpha (TNF-α), Interferon Gamma (IFN-g), and IL-1 Beta (IL-1β), Mitogen-Activated Protein Kinases 1 (MAPK1), were associated with T2DM [3, 4]. Furthermore, various factors, including lack of antioxidant vitamins and microelements, increased oxygen metabolism, inflammatory factors, and uremic toxicity, would result in oxidative stress in patients with T2DM [5]. Lifestyle alterations, i.e., exercise and supplementation, are complementary treatment approaches in T2DM patients [6]. Evidence has indicated that Aerobic Training (AT) exercise intervention is strongly recommended for T2DM patients to boost weight loss, improve lipid profile, facilitate skeletal muscle glucose uptake, and increase the abundance of Glucose Transporter Type 4 (GLUT4) [7, 8]. According to Teixeira de Lemos’s review, regular moderate-intensity exercise, as a natural antioxidant and anti-inflammatory strategy, helped control oxidative stress and inflammation in diabetic Zucker Diabetic Fatty (ZDF) rats [9]. While acute or exhaustive exercise can be damaging, several studies showed positive alteration of the oxidative homeostasis of cells and tissues following regular exercise training, possibly via decreasing the basal levels of oxidative damage and increasing resistance to oxidative stress [10,11,12,13,14]. Nishida et al. demonstrated upregulation of antioxidant defenses in animal models of T2DM, increased Cu/Zn- Superoxide Dismutase (SOD) protein production after low-intensity exercise, and increased Mn-SOD as a result of moderate-intensity exercise [15]. Besides, other clinical and experimental studies also associated the exercise-induced reduction in oxidative stress with the increased nitric oxide bioavailability, endothelial nitric oxide synthase (eNOS) expression, and/or eNOS Ser phosphorylation consequent lower reactive oxygen species generation [14, 16, 17]. A single bout of exercise induces a transient increase in high sensitivity C-reactive protein (hs-CRP). However, regular moderate-intensity exercise increases anti-inflammatory biomarkers (e.g., Interleukin (IL); IL-4 and IL-10) [18], and decreases of hs-CRP, IL-6, and TNF-α protein content [19] that reinforces the anti-inflammatory characteristics of long-term exercise [20].
Furthermore, literature also showed the significant prevalence of hypovitaminosis D in T2DM patients, and both conditions are on the rise globally [21, 22]. Vit D has been linked to the development of T2DM, presumably by altering insulin secretion and, as a result, hyperglycemia [22, 23]. Hypovitaminosis D elevates the intracellular calcium levels by increasing the secretion of parathyroid hormone, which in turn impairs GLUT4 and inhibits the calcium-related insulin secretion and action [24]. There is limited literature on how Vitamin D (Vit D) affects gene expression related to oxidative stress biomarkers and inflammatory cytokines. Choi et al. (2013) reported reduced TNF-α and IL-4 gene expressions after treating rats with Vit D [25]. Additionally, calcitriol is known to inhibit TNF-α releasement in a dose-dependent manner [26]. In vitro animal studies have demonstrated that Vit D suppresses the activation of TNF-α-induced nuclear factor kappa B (NF-κB) and upregulates IL-4 through Vit D receptors (VDR) [27].
However, Carrillo et al. (2012) showed no significant alteration in TNF-α and IL-4 following a 12-week resistance training and high-dose Vit D in healthy overweight individuals [28]. The molecular mechanisms of AT and Vit D modulating inflammatory cytokines and oxidative stress are unknown. Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) and Mitogen-Activated Protein Kinase 1 (MAPK1) participate in signaling cascades induced by pro-inflammatory factors. In addition, NF-κB is hypothesized to control pro-inflammatory cytokines in cellular responses [29].
To our knowledge, controversial and limited data exist on the effects of AT and Vit D supplementation on the oxidative stress biomarkers and inflammation gene expression in T2DM. This study evaluated the effects of Vit D and AT on the oxidative stress biomarkers and gene expression of inflammatory cytokines in T2DM patients.
Methods
Experimental approach
This study was a single-blinded, placebo-controlled, randomized clinical trial among 40 T2DM patients aged 35–50 years, registered on the Iranian clinical trial website at http://www.irct.ir:IRCT20210811052151N1 on 01/09/2021. This research was conducted according to the Declaration of Helsinki. The Research Ethics Committees approved the study protocol of Kermanshah Razi University (IR.RAZI.REC.1400.044); informed consent was taken from all patients.
Subjects
The exclusion criteria were as follows: having a regular exercise program that might confound the results of the AT program; history of heart disease, hypertension, orthopedic disorders, and smoking; COVID-19 infection and muscle injuries during the study that enable subjects to perform the exercise training program; taking Vit D, antioxidant and anti-inflammatory supplements (e.g., omega-3 fatty acids, vitamins E and C) and immunosuppressive medications in the past 3 months before participating in the study that might affect the variables of the present study or confound the results. The 36-subject sample size was estimated for this study using G. POWER 3.1 software (power of 0.99, alpha error of 0.05, and effect size of 0.85). Given the probability of participants’ refusal, 40 middle-aged (35–50 years) males with T2DM were selected randomly from the Baxshin Medical & Health Center, Sulaymaniyah, Iraq. Then, using the Random Number Generator approach, they were randomly allocated to four groups: AT+Vit D (n = 10), AT+placebo (AT; n = 10), Vit D (n = 10), and Control+placebo (C; n = 10), all of which had the same chance of being chosen (Fig. 1).
Intervention
Aerobic training
The participants in the AT group were required to exercise at home three times a week for 8 weeks. All of the training protocols were supervised by the exercise physiologists.
According to the American Diabetes Association’s recommendation, the subjects were instructed to do AT for 20 minutes at 60% heart rate maximum (HRmax) per session, progressing to 40 minutes at 75% HRmax per session (ADA) [30] (Table 1). Also, 10 minutes of warming up and 10 minutes of cooling down were performed every session., The HRmax formula was used [HRmax = 220 − age] to determine the target heart rate [31]. Before the first training session, the participants learned the pulse palpation method to count their pulse and heart rates. Furthermore, the 6–20 Rating of Perceived Exertion (RPE) scale was utilized to ensure that the appropriate heart rate (exercise intensity) was obtained and sustained [32] (Table 1).
Supplementation
In the present study, Vit D and AT+Vit D received 50,000 IU/week of Vit D supplements (Zahravi Pharmaceutical Company, Tabriz, Iran), and AT and C received a placebo with the same color, taste, and shape (Barij Essence Pharmaceutical Company, Kashan, Iran). Additionally, 3-day food records (nutritionist IV; San Bruno, First Databank, CA) and physical activity levels were measured before the intervention in weeks 2, 4, 6, and 8.
Measurements
Anthropometric and body composition
The participants were familiarized with the study method 3 days before the beginning and end of the intervention, and anthropometric variables and body composition were measured. Height was measured using a stadiometer (DETECTO, USA; Model 3PHTROD-WM) and waist circumference with a non-elastic tape measure to the nearest 0.5 cm. The bioelectric impedance analysis (Zeus 9.9 PLUS; Jawon Medical Co., Ltd., Kungsang Bukdo, South Korea) was used to assess Body Mass Index (BMI), Waist–hip ratio (WHR), Body Fat Percentage (BFP), and Bodyweight (BW) at 8–9 A.M. after at least 12-hour fasting. The participants were requested to refrain from taking diuretics and participating in intensive exercise activities 48 hours before the tests.
Outcomes
The primary outcome included the difference in the mean of inflammatory markers, including TNF-α, IL-1β, MAPK1, NF-κB1 (p50), IL-4, and PPAR-γ. The secondary outcome included insulin, Fasting Blood Glucose (FBG), Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), serum 25-OH-Vit D, and oxidative stress biomarkers, such as Total Glutathione (GSH), Total Antioxidant Capacity (TAC), SOD, Catalase (CAT), and Glutathione Peroxidase (GPX), hs-CRP, Malondialdehyde (MDA), glycated albumin, and Urinary 8-hydroxydeoxyguanine (8-OHdG).
Biochemical assessment
Fasting insulin and glucose levels were evaluated by Enzyme-Linked Immunosorbent Assay (ELISA) (Sweden, Mercodia kits) and the enzymatic method (Iran, Pars Azmun Kit), respectively. The insulin resistance index was calculated using the HOMA-IR equation as follows: resistance (HOMA) = [glucose (mg/dL) × insulin (μU/mL)]/405 [33]. Moreover, the ELISA kits were used to determine serum 25-hydroxyvitamin D concentrations (IDS, Boldon, UK), serum hs-CRP concentration (LDN, Nordhorn, Germany) and plasma 8-OHdG (StressXpress ELA Kit, StressMarq Biosciences, Canada). An albumin assay reagent (Lucica GA-L kit, Asahi Kasei Pharma Corporation, Japan) determined serum albumin levels enzymatically. Also, the spectrophotometer was used to assess the SOD (Assay Kit, ZellBio GmbH, Ulm, Germany), CAT (Sigma-Aldrich kit, USA), MDA (Assay Kit, ZellBio GmbH, Ulm, Germany), GPX (Assay Kit, ZellBi, Germany), total nitrite (ZellBio assay kit, Germany), GSH (ZellBio assay kit, Germany), and TAC (Assay Kit, ZellBio, Germany).
Isolation of PBMCs
We examined the gene expressions associated with inflammation in Peripheral Blood Mononuclear Cells (PBMCs) from blood samples, which are the most available tissue for gene expression research, providing more information than plasma concentrations [34]. Before and after the study, 15 ml of fasting blood were drawn. For PBMCs isolation, 4 ml of blood was mixed through 3-part diluted blood to 2-part Ficoll-Hypaque and centrifuged for 30 min at 500×g, 30°. Then, 10 mL of phosphate-buffered saline (PBS) was added and centrifuged for 10 minutes at 400 g, 4C, followed by Hanks balanced salt solution and Percoll. After centrifuging for 25 minutes at 370 g, 25 °C, the cloudy layer in the top 5 mm was transferred into a separate tube using a sterile Pasteur pipet for further analysis.
RNA extraction and real-time PCR
Total RNAs were extracted from 5 mL peripheral blood with the trizol reagent (Invitrogen, USA) using an RNX-plus kit, Cinnacolon, Tehran, Iran. The RNA was quantified using a UV spectrophotometer, which revealed no contamination with protein or DNA (OD 260/280 ratio between 1.7 and 2.1), followed by reverse transcription to the cDNA library (via Moloney murine leukemia virus reverse transcriptase). Then, by using glyceraldehyde-3-phosphate dehydrogenase primers as housekeeping gene and quantitative RT-PCR method (LightCycler technology, Roche Diagnostics, Rotkreuz, Switzerland; SYBR green detection and Amplicon Kit), the gene expression of IL-1β, MAPK1, IL-4, PPAR-γ, NF-κB1 (p50), and TNF-α were evaluated. Additionally, the Primer Express (Applied Biosystems, Foster City) and Beacon designer (Takaposizt, Tehran, Iran) software were used to design the primers and Pffafi or 2 − 11CT method to calculate relative transcription levels (Table 2).
Statistical analysis
The data were analyzed by version 26.0 of SPSS software. The Shapiro–Wilk test was used to check the normality of the distribution in the continuous variables. The paired sample t-test was used for Within-group comparisons. Also, one-way ANOVA and Tukey’s posthoc were used for between-groups comparison at P < 0.05.
Results
Table 3 shows the results of the mean ± SD of the anthropometric variables. After 8 weeks, the t-test results indicate significant differences in the WHR, BMI, BW, and BFP. The variables mentioned above decreased significantly in all intervention groups but increased significantly in the C. Accordingly, a significant difference was found between the AT+Vit D, AT, and Vit D groups and the C group in terms of BW, BMI, BFP, and WHR. A significant difference was also observed in BW (p = 0.001; p = 0.001), BMI (p = 0.013; p = 0.001), BFP (p = 0.001; p = 0.00), and WHR (p = 0.017; p = 0.00) in the AT+Vit D group compared to the AT and Vit D groups alone, respectively. Furthermore, the results showed a significant difference between AT and TS groups regarding BW (p = 0.001), BMI (p = 0.003), BFP (p = 0.018), and WHR (p = 0.024) (Table 3).
The results of between-group comparisons of the participants’ glycemic control, Vit D, biomarkers of inflammation, and oxidative stress are presented in Table 4. The t-test results showed significant differences in the mean of all the variables in the post-test compared to the pre-test. After 8 weeks, insulin, FBG, HOMA-IR, hs-CRP, MDA, glycated albumin, and 8-OHdG significantly decreased in all intervention groups, while these variables increased substantially in the C (Table 4). Serum 25-OH-Vit D, total nitrite, GSH, TAC, CAT, SOD, and GPX increased significantly after 8 weeks in all intervention groups except for C. After the intervention, AT+Vit D, AT, and Vit D all significantly lowered insulin, FBG, HOMA-IR, hs-CRP, MDA, glycated albumin, and urinary 8-OHdG. Serum 25-OH-Vit D, total nitrite, GSH, TAC, CAT, SOD, and GPX increased significantly compared to the C. Additionally, the AT+Vit D group had significantly lower insulin, FBG, HOMA-IR, hs-CRP, MDA, glycated albumin, and urinary 8-OHdG levels, and significantly higher serum 25-OH-Vit D, total nitrite, GSH, TAC, CAT, SOD, and GPX levels compared to the AT and Vit D groups. The results also showed a significant difference between the AT and Vit D groups in all the variables.
Furthermore, AT+Vit D, AT, and Vit D downregulated IL1-β, MAPK1, NF-κB1 (p50), and TNF-α. They also upregulated IL-4, and PPAR-γ in T2DM patients compared to the C (Fig. 2). Based on the results, AT+Vit D significantly downregulated IL1-β (p = 0.009; p = 0.001), MAPK1 (p = 0.014; p = 0.001), NF-κB1 (p50) (p = 0.023; p = 0.001), and TNF-α (p = 0.024; p = 0.001) and significantly upregulated IL-4 (p = 0.032; p = 0.014), and PPAR-γ (p = 0.026; p = 0.001), compared to AT and Vit D, respectively. There were significant differences between AT and Vit D in the IL1-β (p = 0.033), MAPK1(p = 0.007), NF-κB1 (p50) (p = 0.001), and TNF-α (p = 0.029) and upregulated IL-4 (p = 0.027), and PPAR-γ (p = 0.018) (Fig. 2).
Effect of aerobic training and vitamin d supplementation on IL1-β, MAPK1, IL-4, PPAR-γ, NF-κB1, and TNF-α in patients with T2DM. IL-1β: Interleukin 1 Beta; MAPK1: Mitogen-Activated Protein Kinases; IL-4: Interleukin-4; PPAR-γ: Peroxisome Proliferator-Activated Receptor Gamma; NF-kβ: Nuclear Factor Kappa B; and TNF-α: Tumor Necrosis Factor Alpha. Results from analysis of one-way analysis of variance (ANOVA), post-hoc Tukey’s test. β: Significant differences with C. μ: Significant differences with AT. €: Significant differences with Vit D
Discussion
The present study results indicated reduced BW, BMI, BFP, and WHR in AT; however, this reduction was more significant when combining AT and Vit D supplementation. Regular AT improves body composition, insulin resistance, lipolytic enzyme expression, mitochondrial density, daily energy expenditure, and fat oxidation [34,35,36,37,38]. BW, BMI, and BFP were reduced in Vit D after 8 weeks based on the results. Hoseini et al. (2017) also documented reduced BW, visceral fat, and BMI in rats’ model of metabolic syndrome following high doses of Vit D [39]. These results could be due to the roles of Vit D, parathyroid hormone (PTH), and intercellular Ca++ in closing the GLUT4 channel and impairing the adipose tissue metabolic processes, glucose metabolism, and insulin function [38]. A further reduction in anthropometric indices following combined AT+Vit D, compared to AT or Vit D, might be related to the fact that Vit D and AT benefited the indices through different pathways or by affecting other downstream targets. In this study, 8 weeks of AT down-regulated gene expression of hs-CRP, IL-1β, TNF-α, IFN-γ, MAPK1, and NF-κB1 (p50), and upregulated IL-4 and PPAR-γ decreased oxidative damage (urinary 8-oHdG, glycated albumin, MDA, and hs-CRP). They increased antioxidant defense (GPx, SOD, CAT, TAC, GSH, and total nitrite) concomitantly, which is consistent with the recent results of Shephardet et al. (2000) [40], Gielenet et al., (2003) [41], Stewart et al., (2004) [42], Petersen et al., (2005) [20] Devrieet et al., (2008) [43], and de Oliveiret et al., (2012) [44]. Although the underlying mechanisms are unknown, the improvement of inflammatory and oxidative stress indicators generated by AT is well established, as shown in this study [40, 45, 46]. Based on our findings and those of other studies, underlining the importance of concurrent reductions in insulin resistance, BW, and, in particular, BFP in improving inflammatory and oxidative stress indicators could be a plausible mechanism [47, 48]. Moreover, it has been reported that AT mediates the myokines and adipokines secretion by targeting skeletal muscle and adipose tissue, which improves the inflammatory and oxidative stress biomarkers [20, 41]. As an endocrine tissue that expresses both NADPH oxidase and adipokines diminished BFP and obesity may simply give Redox homeostasis and a balanced inflammatory profile in T2DM patients, lowering the risk of chronic illnesses [43, 44, 49]. AT reduces inflammation by presenting multiplied physiological benefits such as reducing toll-like receptors and NF-κB1 (p50) expression. It also increases lipolysis, down-regulates leukocyte migration, and enhances angiogenesis [50, 51]. Based on the results, AT can suppress the mRNA expression of pro-inflammatory cytokines and enhance anti-inflammatory cytokines production. The present study results also showed a cross-talk between the enhancement of the oxidative defense system and the improvement of the inflammatory biomarkers following AT. However, some studies demonstrated no change in the mRNA expression of some inflammatory biomarkers following long-term AT [52, 53]. Thus, it could be hypothesized that combining AT and Vit D might be a powerful strategy for T2DM patients to improve inflammatory and oxidative stress biomarkers.
To the best of our knowledge, data on AT and Vit D supplementation on inflammation-related gene expression and oxidative stress biomarkers among T2DM patients are limited. The results revealed 8 weeks of combined Vit D and AT downregulated hs-CRP, IL-1β, TNF-α, MAPK1, and NF-κB1 (p50) gene expressions. Also, upregulated IL-4 and PPAR-γ, decreased oxidative damage (urinary 8-oHdG, glycated albumin, MDA, and hs-CRP), and increased antioxidant defense (GPx, SOD, CAT, TAC, GSH, and total nitrate) were observed. Based on current evidence, Vit D supplementation reduced hs-CRP [54,55,56]. In line with our findings, Willis et al. (2012) documented an inverse relationship between TNF-α and Vit D levels [57], Schleithoff et al. (2006) reported the suppression of TNF-α production following 9 months of Vit D supplementation [58], Müller et al., (1992) showed suppressed IL-1β and TNF-α [59], and Irani et al., (2015) showed decreased TGF-b1 after 8 weeks of Vit D administration (50,000 IU/week) [60]. Furthermore, some cross-sectional studies demonstrated decreased MAPK1 gene expression [61, 62], suppressed NF-κB1 (p50) [63], and increased PPAR-γ expression [39] induced by 1,25-(OH)2D3. However, contradictory findings show no significant changes in various inflammation and oxidative stress indicator after Vit D supplementation in healthy overweight and obese people, which could be related to the wide range of supplementation dosages, periods, and subjects’ conditions [28, 64, 65]. Chronic inflammation in T2DM is associated with progressing cardiovascular risk factors and is also one of the leading players in insulin function, resulting in hyperinsulinemia [66, 67]. Hyperinsulinemia resulting from chronic inflammation is related to consequent hyperandrogenism, insulin resistance, oxidative stress, and cardiovascular events in the T2DM population [67]. Vit D might decrease clinical and metabolic symptoms in T2DM by affecting oxidative stress and inflammatory cytokines, given Vit D’s antagonism to calcium which plays a crucial role in inflammatory responses [68]. This study also showed that Vit D supplementation downregulated the MAPK1 gene expression. Moreover, the MAPK1 signaling pathway has known to be crucial in the transcription and translation of biomarkers of inflammation and oxidative stress [69, 70]. Jafari et al. (2021) reported that Vit D supplementation along with exercise modulates antioxidant defense and the expression of antioxidant genes [71]. Vit D is also considered an antioxidant that might cause the expression of several genes involved in the antioxidant defense system, including GPX, GSH, CAT, SOD, and the suppression of NADPH oxidase [72, 73]. Besides, increasing Vit D levels in individuals with insufficient Vit D levels (trained and untrained) has improved antioxidant capacity [74]. Overall, Vit D deficiency has adverse effects on antioxidant capacity, and Vit D supplementation upregulates the activity of SOD and CAT [75] (Fig. 3).
Role of aerobic training and vitamin D on inflammatory biomarker gene expression and oxidative stress in type 2 diabetes mellitus. GSH: Total Glutathione; TAC: Total Antioxidant Capacity; CAT: Catalase; SOD: Superoxide Dismutase; GPX: Glutathione Peroxidase; FBG: Fasting Blood Glucose; hs-CRP: High Sensitivity C-Reactive Protein; MDA: Malondialdehyde; 8-OHdG: Urinary 8-hydroxydeoxyguanine; IL-1β: Interleukin 1 Beta; TNF-α: Tumor Necrosis Factor-Alpha; MAPK1: Mitogen-Activated Protein Kinases 1; NF-κB1: Nuclear Factor Kappa B 1; IL-4: Interleukin-4; PPAR-γ: Peroxisome Proliferator-Activated Receptor Gamma
Strengths and limitations
This study’s strength was using a randomized, placebo-controlled, single-blind trial with a low dropout rate and evaluating the gene expression alterations in human subjects. Also, this study was conducted in the winter; thus, the seasonal changes played a minor role. However, considering the small sample size, it is premature to conclude a definitive answer. Also, using a self-reported questionnaire to measure and control daily diet and physical activity and not evaluating the alterations of transcriptional factors such as AP-1 and Nrf2/HO-1 signaling were among the limitations of the present study that is suggested to be considered in future studies.
Conclusions
This study showed the potential molecular benefits of combined AT+Vit D on improving anthropometric indices, inflammation, and oxidative stress biomarkers in T2DM patients. Further studies are needed to find the best dose and training approach.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AT:
-
Aerobic Training
- Vit D:
-
Vitamin D
- T2DM:
-
Type 2 Diabetes Mellitus
- GSH:
-
Total Glutathione
- TAC:
-
Total Antioxidant Capacity
- CAT:
-
Catalase
- SOD:
-
Superoxide Dismutase
- GPX:
-
Glutathione Peroxidase
- FBG:
-
Fasting Blood Glucose
- hs-CRP:
-
High Sensitivity C-Reactive Protein
- MDA:
-
Malondialdehyde
- 8-OHdG:
-
Urinary 8-hydroxydeoxyguanine
- IL-1β:
-
Interleukin 1 Beta
- TNF-α:
-
Tumor Necrosis Factor-Alpha
- MAP K1:
-
Mitogen-Activated Protein Kinases 1
- NF-κB 1:
-
Nuclear Factor Kappa B 1
- IL-4:
-
Interleukin-4
- PPAR-γ :
-
Peroxisome Proliferator-Activated Receptor Gamma.
References
Mirzaei M, Rahmaninan M, Mirzaei M, Nadjarzadeh A. Epidemiology of diabetes mellitus, pre-diabetes, undiagnosed and uncontrolled diabetes in Central Iran: results from Yazd health study. BMC Public Health. 2020;20(1):1–9.
Li J, Shen X. Effect of rosiglitazone on inflammatory cytokines and oxidative stress after intensive insulin therapy in patients with newly diagnosed type 2 diabetes. Diabetol Metab Syndr. 2019;11(1):1–9.
Chakraborty A, Chowdhury S, Bhattacharyya M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res Clin Pract. 2011;93(1):56–62.
Yu Y, Tian L, Xiao Y, Huang G, Zhang M. Effect of vitamin D supplementation on some inflammatory biomarkers in type 2 diabetes mellitus subjects: a systematic review and meta-analysis of randomized controlled trials. Ann Nutr Metab. 2018;73:62–73.
Lin J, Hu FB, Mantzoros C, Curhan GC. Lipid and inflammatory biomarkers and kidney function decline in type 2 diabetes. Diabetologia. 2010;53(2):263–7.
Khademi Z, Imani E, Heidary Khormizi M, Poordad Khodaei A, Sarneyzadeh M, Nikparvar M. A Study on The Variation of Medicinal Plants Used for Controlling Blood Sugar and Causes of Self–Medication by Patients Referred to Bandarabbas Diabetic Center. J Diabetes Nurs. 2013;1(1):12–20.
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–67.
Ghalavand A, Shakeriyan S, Monazamnezhad A, Dadvar N, Heidarnezhad M, Delaramnasab M. The effects of aerobic training on blood glycemic control and plasma lipid profile in men with type 2 diabetes. Sylwan. 2014;158(6):1–10.
Teixeira de Lemos E, Oliveira J, Páscoa Pinheiro J, Reis F. Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitus. Oxid Med Cell Longev. 2012;2012:741545.
Cooper CE, Vollaard NB, Choueiri T, Wilson M. Exercise, free radicals and oxidative stress. Biochem Soc Trans. 2002;30(2):280–5.
Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1–2):41–54.
Roberts CK, Won D, Pruthi S, Barnard RJ. Effect of a diet and exercise intervention on oxidative stress, inflammation and monocyte adhesion in diabetic men. Diabetes Res Clin Pract. 2006;73(3):249–59.
Nojima H, Watanabe H, Yamane K, Kitahara Y, Sekikawa K, Yamamoto H, et al. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2008;57(2):170–6.
Wycherley T, Brinkworth GD, Noakes M, Buckley J, Clifton P. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(11):1062–73.
Nishida Y, Iyadomi M, Higaki Y, Tanaka H, Hara M, Tanaka K. Influence of physical activity intensity and aerobic fitness on the anthropometric index and serum uric acid concentration in people with obesity. Intern Med. 2011;50(19):2121–8.
Lauer N, Suvorava T, Rüther U, Jacob R, Meyer W, Harrison DG, et al. Critical involvement of hydrogen peroxide in exercise-induced up-regulation of endothelial NO synthase. Cardiovasc Res. 2005;65(1):254–62.
Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107(25):3152–8.
Chen J-S, Huang P-H, Wang C-H, Lin F-Y, Tsai H-Y, Wu T-C, et al. Nrf-2 mediated heme oxygenase-1 expression, an antioxidant-independent mechanism, contributes to anti-atherogenesis and vascular protective effects of Ginkgo biloba extract. Atherosclerosis. 2011;214(2):301–9.
Das UN. Anti-inflammatory nature of exercise. Nutrition. 2004;20(3):323.
Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–62.
Malik R, Farooq R, Mehta P, Ishaq S, Din I, Shah P, et al. Association of Vitamin D receptor gene polymorphism in adults with type 2 diabetes in the Kashmir Valley. Can J Diabetes. 2018;42(3):251–6.
Wang SY, Shen TT, Xi BL, Shen Z, Zhang X. Vitamin D affects the neutrophil-to-lymphocyte ratio in patients with type 2 diabetes mellitus. J Diabetes Investig. 2021;12(2):254–65.
Bhat MH, Mohd M, Dar IH, Bhat JA. Role of vitamin D deficiency in type 2 diabetes: association or coincidence? Clin Diabetol. 2021;10(2):188–94.
Nachankar A, Kotwal N, Upreti V, Verma V, Kumar KH. Association of vitamin D and parathyroid hormone with insulin sensitivity, beta cell function and gestational diabetes in pregnancy: a cross-sectional, observational study. Diabetes Ther. 2018;9(5):2081–90.
Choi M, Park H, Cho S, Lee M. Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high-intensity exercise in SD rats. Cytokine. 2013;63(1):27–35.
Zhou L, Wang J, Li J, Li T, Chen Y, June RR, et al. 1, 25-Dihydroxyvitamin D3 ameliorates collagen-induced arthritis via suppression of Th17 cells through miR-124 mediated inhibition of IL-6 signaling. Front Immunol. 2019;10:178.
Liu W, Chen Y, Golan MA, Annunziata ML, Du J, Dougherty U, et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest. 2013;123(9):3983–96.
Carrillo AE, Flynn MG, Pinkston C, Markofski MM, Jiang Y, Donkin SS, et al. Vitamin D supplementation during exercise training does not alter inflammatory biomarkers in overweight and obese subjects. Eur J Appl Physiol. 2012;112(8):3045–52.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20.
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33(12):2692–6.
Branco BHM, de Oliveira MF, Ladeia GF, Bertolini SMMG, Badilla PV, Andreato LV. Maximum heart rate predicted by formulas versus values obtained in graded exercise tests in Brazilian jiu-jitsu athletes. Sport Sci Health. 2020;16(1):39–45.
Rahimi E. Physical activity and type 2 diabetes: a narrative review. J Phys Activity Hormones. 2019;2(4):51–62.
Matthews DR, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Farrell C, Turgeon DR. Normal versus chronic adaptations to aerobic exercise. StatPearls; 2021.
Ghalavand A, Delaramnasab M, Ghanaati S. Comparison of the effect of telenursing and aerobic training on cardiometabolic and anthropometric indices in patients with type 2 diabetes. Razi J Med Sci. 2021;28(4):34–45.
Polak J, Klimcakova E, Moro C, Viguerie N, Berlan M, Hejnova J, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor α in obese women. Metabolism. 2006;55(10):1375–81.
Spriet LL. Regulation of skeletal muscle fat oxidation during exercise in humans. Med Sci Sports Exerc. 2002;34(9):1477–84.
Hoseini Z, Behpour N, Hoseini R. Co-treatment with vitamin D supplementation and aerobic training in elderly women with Vit D deficiency and NAFLD: a single-blind controlled trial. Hepat Mon. 2020;20(2):1–11.
Hoseini R, Damirchi A, Babaei P. Vitamin D increases PPARγ expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition. 2017;36:54–9.
Shephard RJ, Gannon G, Hay J, Shek P. Adhesion molecule expression in acute and chronic exercise. Crit Rev Immunol. 2000;20(3):245–66.
Gielen S, Adams V, Möbius-Winkler S, Linke A, Erbs S, Yu J, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42(5):861–8.
Stewart KJ. Role of exercise training on cardiovascular disease in persons who have type 2 diabetes and hypertension. Cardiol Clin. 2004;22(4):569–86.
Devries MC, Hamadeh MJ, Glover AW, Raha S, Samjoo IA, Tarnopolsky MA. Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free Radic Biol Med. 2008;45(4):503–11.
Oliveira VN, Bessa A, Jorge MLMP, Oliveira RJS, de Mello MT, De Agostini GG, et al. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl Physiol Nutr Metab. 2012;37(2):334–44.
Hvas A-M, Neergaard-Petersen S. Influence of exercise on platelet function in patients with cardiovascular disease. In: Seminars in thrombosis and hemostasis: Thieme Medical Publishers; 2018. p. 802–12.
Borges YG, Cipriano LHC, Aires R, Zovico PVC, Campos FV, de Araújo MTM, et al. Oxidative stress and inflammatory profiles in obstructive sleep apnea: are short-term CPAP or aerobic exercise therapies effective? Sleep Breathing. 2020;24(2):541–9.
Sjöholm Å, Nyström T. Endothelial inflammation in insulin resistance. Lancet. 2005;365(9459):610–2.
Giannopoulou I, Fernhall B, Carhart R, Weinstock RS, Baynard T, Figueroa A, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54(7):866–75.
Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911–9.
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–15.
You T, Arsenis NC, Disanzo BL, LaMonte MJ. Effects of exercise training on chronic inflammation in obesity. Sports Med. 2013;43(4):243–56.
Marcell TJ, McAuley KA, Traustadóttir T, Reaven PD. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism. 2005;54(4):533–41.
de Mello VD, Kolehmainen M, Schwab U, Mager U, Laaksonen DE, Pulkkinen L, et al. Effect of weight loss on cytokine messenger RNA expression in peripheral blood mononuclear cells of obese subjects with the metabolic syndrome. Metabolism. 2008;57(2):192–9.
Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complicat. 2017;31(4):766–72.
Chen N, Wan Z, Han S-F, Li B-Y, Zhang Z-L, Qin L-Q. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: a meta-analysis of randomized controlled trials. Nutrients. 2014;6(6):2206–16.
Mazidi M, Rezaie P, Vatanparast H. Impact of vitamin D supplementation on C-reactive protein; a systematic review and meta-analysis of randomized controlled trials. BMC Nutr. 2018;4(1):1–11.
Willis KS, Smith DT, Broughton KS, Larson-Meyer DE. Vitamin D status and biomarkers of inflammation in runners. Open Access J Sports Med. 2012;3:35.
Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9.
Müller K, Haahr P, Diamant M, Rieneck K, Kharazmi A, Bendtzen K. 1, 25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine. 1992;4(6):506–12.
Irani M, Seifer DB, Grazi RV, Julka N, Bhatt D, Kalgi B, et al. Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2015;100(11):4307–14.
Xu Q-a, Li Z-f, Zhang P, Cao LH, Fan MW. Effects of 1, 25-dihydroxyvitamin D3 on macrophage cytokine secretion stimulated by Porphyromonas gingivalis. Jpn J Infect Dis. 2016;69(6):482–7.
Marampon F, Gravina G, Festuccia C, Popov V, Colapietro E, Sanita P, et al. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J Endocrinol Investig. 2016;39(4):411–22.
Al-Rasheed NM, Al-Rasheed NM, Bassiouni YA, Hasan IH, Al-Amin MA, Al-Ajmi HN, et al. Vitamin D attenuates pro-inflammatory TNF-α cytokine expression by inhibiting NF-кB/p65 signaling in hypertrophied rat hearts. J Physiol Biochem. 2015;71(2):289–99.
Jamka M, Woźniewicz M, Walkowiak J, Bogdański P, Jeszka J, Stelmach-Mardas M. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: a systematic review with meta-analysis. Eur J Nutr. 2016;55(6):2163–76.
Gonzalez-Curiel I, Marin-Luevano P, Trujillo V, Enciso-Moreno JA, Gonzalez-Castillo C, Rivas-Santiago B. Calcitriol prevents inflammatory gene expression in macrovascular endothelial cells. Br J Biomed Sci. 2016;73(2):74–8.
Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004;44(1):6–11.
Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–9.
Aneiros E, Philipp S, Lis A, Freichel M, Cavalié A. Modulation of Ca2+ signaling by Na+/Ca2+ exchangers in mast cells. J Immunol. 2005;174(1):119–30.
Winkler C, Ferdous F, Dimmick M, Scott T. Lipopolysaccharide induced Interleukin-6 production is mediated through activation of ERK 1/2, p38 MAPK, MEK, and NFκB in chicken thrombocytes. Dev Comp Immunol. 2017;73:124–30.
Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754(1–2):253–62.
Jafari A, Sheikholeslami-Vatani D, Khosrobakhsh F, Khaledi N. Synergistic effects of exercise training and vitamin D supplementation on mitochondrial function of cardiac tissue, antioxidant capacity, and tumor growth in breast cancer in bearing-4T1 mice. Front Physiol. 2021;12:640237.
Wahba NS, Ghareib SA, Abdel-Ghany RH, Abdel-Aal M, Alsemeh AE. Vitamin D3 potentiates the nephroprotective effects of metformin in a rat model of metabolic syndrome: role of AMPK/SIRT1 activation and DPP-4 inhibition. Can J Physiol Pharmacol. 2021;99(6):685–97.
Tagliaferri S, Porri D, De Giuseppe R, Manuelli M, Alessio F, Cena H. The controversial role of vitamin D as an antioxidant: results from randomised controlled trials. Nutr Res Rev. 2019;32(1):99–105.
BaSalamah MA, Abdelghany AH, El-Boshy M, Ahmad J, Idris S, Refaat B. Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci Rep. 2018;8(1):1–13.
Boakye D, Jansen L, Schöttker B, Jansen EH, Halama N, Maalmi H, et al. The association of vitamin D with survival in colorectal cancer patients depends on antioxidant capacity. Am J Clin Nutr. 2021;113(6):1458–67.
Acknowledgments
The authors would like to thank the participants for their cooperation.
Funding
The authors declared that the research did not receive any financial grants.
Author information
Authors and Affiliations
Contributions
RH: designed the study. H.A.R: experimented. RH: analyzed the data and wrote the manuscript. J.K.A: was involved in interpreting data and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Razi University of Kermanshah (IR.RAZI.REC.1400.044) and was registered in the Iranian Clinical Trial Registration Center (code: IRCT20210811052151N1 on 01/09/2021).
All procedures were under the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients being included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hoseini, R., Rahim, H.A. & Ahmed, J.K. Concurrent alteration in inflammatory biomarker gene expression and oxidative stress: how aerobic training and vitamin D improve T2DM. BMC Complement Med Ther 22, 165 (2022). https://doi.org/10.1186/s12906-022-03645-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03645-7