Abstract
Background
Jianpi Qinghua Fomula (JPQHF), a clinically proven prescription,has been applied to cure insulin resistance(IR) and type 2 diabetes (T2DM) for more than 20 years. Here, we will unravel the underlying molecular mechanisms relevant to the therapeutic actions of JPQHF.
Methods
High-fat(HF)diet-induced obesity(DIO)mouse were established in our research, along with insulin resistance. After the administration of JPQHF 5 or 6 weeks, the parameters of the glucose and lipid metabolism were measured. Flow cytometry and Luminex were utilized to assess the inflammation in small intestine,whilst Western blot was used to determine the relative expression levels of the MAPK pathway-related proteins. The glucose and lipid transporter of small intestine was assessed by immunofluorescence and ELISA, and the expression of insulin signaling pathway was detected by Western blot.
Results
The metabolic phenotypes of DIO mouse were ameliorated after 6-week oral administration of JPQHF; Meanwhile,JPQHF downregulated levels of IL-1β,IL-6, TNF-α and IFN-γ but upregulated the ratio of M2/M1 macrophages in the small intestine. The elevated expressions of p-P38 MAPK/P38 MAPK、p-JNK/JNK and p-ERK1/2/ERK1/2 were reversed by JPQHF. Moreover, JPQHF enhanced expression of PI3K,p-AKT/AKT, p-IRS1/ IRS1, p-IRS2/ IRS2 and apoB48 in small intestine, and facilitated the translocation of GLUT2 to the basal side of small intestine epithelial cells.
Conclusion
JPQHF alleviates insulin resistance in DIO mice, and this effect may be associated with its restraining of inflammation of small intestine via attenuating MAPK pathway, and then diminishes small intestinal glucose and lipid absorption.
Similar content being viewed by others
Background
Type 2 diabetes is a grim health burden on a global scale. Obesity-induced insulin resistance is implicated as the initiating factor for type 2 diabetes, which can accelerate the progression to various metabolic disorders and cardiovascular diseases [1], wherefore ameliorating insulin resistance is becoming an urgent need.
Chronic state of low-grade inflammation is the pivotal pathological basis of obesity and insulin resistance. High-fat-diet-induced inflammatory pathological shift in the intestine occur prior to the development of obesity [2,3,4], accompanied by macrophages in intestinal lamina propria producing pro-inflammatory cytokines [5, 6]. The energy imbalance in which energy intake exceeds dissipation is considered as the foremost cause of obesity-induced insulin resistance. As the main organ of glycolipid absorption, the small intestine has essential roles in the pathogenesis of obesity-induced insulin resistance [3, 7]. Intestinal inflammation impaires epithelial insulin signaling pathway which in turn triggers glycolipid intake disorder [7, 8], accumulates excessive amounts of glucose and free fatty acids(FFA)in the circulation and exacerbates systemic insulin resistance [9,10,11].Consequently, intestinal immune system may be a novel therapeutic target in the treatment of insulin resistance.
Recently, accumulating evidence has shown the preventive and curative effect of traditional Chinese medicine (TCM) in T2DM and associated complications [12, 13]. The JPQHF is adapted from ‘Bupiwei Xieyinhuo Shengyang Decoction’ which derived from the < Theory of Spleen and Stomach > . JPQHF has been applied in the treatment of T2DM and insulin resistance in Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine( Shanghai, China) for more than two decades. JPQHF enhances metabolism of lipids and improves insulin sensitivity [14,15,16], but the potential pharmacodynamic mechanism is still remains incompletely clear. Therefore, the aim of our research was to investigate weather the JPQHF would improve insulin resistance via ameliorating intestinal inflammation.
Material and method
Preparation of JPQHF
The JPQHF consists of Codonopsis pilosula (Franch.) Nannf (15 g), Astragalus membranaceus (Fisch.) Bunge (15 g), Dioscoreae rhizoma(15 g), Polygonatum sibiricum (15 g),Coptis chinensis Franch (3 g), Scutellaria baicalensis Georgi (9 g), Radix Puerariae (15 g) and Euonymus alatus (Thunb.) Sieb (15 g). The herbs were obtained from Lei-Yun-Shang Pharmacy(Shanghai,China) and were identified by Dr Guanglin Xu from Pharmacy Department of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine. The herbs fulfilled the standard requirements of the 2015 version of the Chinese Pharmacopoeia.
JPQHF was prepared by Pharmacy Department of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine. All above eight herbs were submersed in 1000 ml of distilled water and extracted in a ceramic clay pot at 100 ℃ for 30 min with continuously stirred twice. The mixed extract was concentrated at 100 ℃ for 30 min and centrifuged at 13,000 rpm for 30 min at 4 ℃.Then the supernatant was filtered through a sieve until each milliliter contains 1.5 g drug.
Animal groups and treatment
Six-week-old healthy male C57BL/6 mice weighted 18 ± 2 g were acquired from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd. Shanghai Branch, (Shanghai, China), certification no. SCXK(HU)2017–0011. Mice were housed under controlled conditions: 12 h light/12 h dark cycle, ambient temperature of 23 ± 3 °C and humidity 55 ± 15%. All experimental procedures involving animals were approved by the Animal Experiment Ethics Committee of Shanghai University of Traditional Chinese Medicine, and the approval no.PZSHUTCM 190,823,004.
The mice were randomly divided into normal group (NOR) and the high-fat diet group, and were fed a normal diet (1,010,009; Nanjing Synergy Biology Co., Ltd.; Nanjing, Jiangsu province, China) or the HFD containing 60 kJ % fat (D12492; Research Diets, New Brunswick, NJ, USA) respectively for 12 weeks. After continuous feeding of 12 weeks, the weight of mice in the high-fat diet group was 1.2 times of the normal group were considered as DIO mice. The DIO mice were then divided into three groups according to body weight via SPSS 25.0 (SPSS Inc., Chicago, USA): DIO group (DIO), Metformin group (MET) and JPQHF group (JPQH),and daily treated with oral normal saline, metformin suspension and JPQHF individually. The metformin tablets (Merck Serono, Geneva, Switzerland) was dissolved in physiological saline at a density of 300 mg/kg body weight.
Metabolic phenotypes correlated parameters analysis
The body weight of mice was measured by the same electronic scale at the same time every week (Saturday morning 9:00–11:00).
After administration of 5 weeks, both IPGTT and IPITT were performed after 12 h of fasting. Glucose(1.5 g/kg) or insulin(0.5U/10 g,D134876A,Eli Lilly and Company, Indianapolis, Indiana, U.S.) was administered intraperitoneally respectively. Glucose levels were monitored by glucometer (ACCU-CHEK Active, Roche, Mannheim, Germany) via sampling from the end of the caudal vein at 0, 30, 60, 90 and 120 min after glucose or insulin injection. Finally the area under the curve (AUC) was calculated by the trapezoidal rule.
Levels of serum fasting insulin(FINS) were determined by ELISA(CSB-E05071 m, Wuhan Huamei Biological Engineering Co., Ltd., Wuhan, Hubei province, China).The cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) concentration was tested by the fully automated biochemical analyzer(7020, Hitachi High-Tech, Tokyo, Japan).
Morphological analyses
The small intestine was separated and fixed in 2% neutral buffered formalin for 8 h, then transferred to 20% glucose, 30% glucose, and embedded with OCT. The tissue specimens were applied to analysis the transposition of GLUT2 using the anti-GLUT2 antibody (Ab54460,1:200, Cambridge, UK) by fluorescence microscope (80i, Nikon, Tokyo, Japan).
Western Blotting
Total protein was extracted from small intestine and quantified by BCA assay in accordance with the manufacturers protocol (23,225, Thermo Fisher Scientific, MA, USA). Immunoblotting was performed as described previously [17]. The primary antibodies p-P38 MAPK (4613S,1:1000), P38 MAPK (9212S,1:1000), p-JNK (4668S, 1:1000), JNK (9252S,1:1000), p-ERK1/2(4370S,1:1000), ERK1/2 (4695S,1:1000), PI3K (4249S,1:1000), Akt(3063S,1:1000), p-Akt (4060S, 1:1000), IRS1 (2382S,1:10 00), p-IRS1 (3203S,1:1000), p-IRS2 (4502S,1:1000) and secondary antibody were purchased from Cell Signaling Technology (MA, USA).Chemiluminescent images were acquired by imaging system (5200, Tonan, Shanghai, China) and analyzed using the Protein Array Analyzer plugin for ImageJ.
Enzyme-linked Immunosorbent Assays (ELISA)
Concentrations of apoB48 in small intestinal tissue in serum were evaluated by ELISA (CSB-E16506m, Wuhan Huamei Biological Engineering Co., Ltd., Wuhan, Hubei province, China) according to kit instructions. The OD value was read at 450 nm by a microplate (ELX800, Biotek Instruments, Vermont, USA) after incubation at 37 °C for 20 min.
Flow cytometry
Separation and digestion of small intestinal tissue were operated under the guidance of instructions(Lamina Propria Dissociation Kit, mouse,130–097-410, Miltenyi Biotec, Bergisch Gladbach, Germany). Intestinal immune cell single cell suspension was placed in blank, compensation control and sample tube. The corresponding cell surface fluorescent antibody was added in compensation control tube, meanwhile, Fc Block, Fixable Viability Stain 510 and cell-surface fluorescent antibodies such as CD45 (557, 659), F4/80 (565, 411), CD11b (552, 850), CD206 (565 250) and CD86 (553,692) which were purchused from Becton, Dickinson and Company (New Jersey, USA) were put into sample tube in turn. Of note, incubating for 10 min at 4 °C in the dark and washing by FBS were needed after the reagent was added at above step. The flow cytometer (FACS Canto II, Becton, Dickinson and Company, New Jersey, USA) and software flow cytometer (V10, Becton, Dickinson and Company, New Jersey, USA) was utilized to detect and analysis the ratio of immune cells in the lamina propria of the small intestine.
Luminex
Total protein was extracted from small intestine and quantified by BCA assay in adherence to the the manufacturer,s protocol (23,225,Thermo Fisher Scientific, MA, USA). The standard and premixed beads mixture were diluted according to the method provided in the instructions (MTH17MAG-47 K,MILLIPLEX,Merck KGaA, Darmstadt, Germany). 25 μl of distilled premixed beads mixture and 25 μl of sample were added to sample well, while 25 μl standard and 25 μl of cell clesolution were put in each standard well.The 96-well plate was incubated at room temperature for 2 h on the shaker and then was placed on magnetic stand. Biotin-labeled antibody complex and diluted streptavidin-labeled PE were put in each well sequentially. Finally, the beads was detected by Luminex(X-200, Luminex Corporation, Texas, USA). The concentration ratio of cytokine to sample was applied to statistics.
Statistical analysis
All data were expressed as the means ± SD. One-way ANOVA test was applied for the comparison between groups using SPSS 25.0 (SPSS Inc., Chicago, USA). P < 0.05 was considered to indicate statistical significance.
Results
JPQHF ameliorated metabolic phenotype in DIO mice
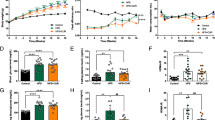
To elucidate the contribution of JPQHF to metabolic phenotype, the DIO mouse model was utilized in this study. As shown in Fig. 1, the DIO mouse expressed elevated body weight, blood glucose at all time-point and AUC of IPGTT and IPITT, FINS, TC, TG, LDL-C and decreased HDL-C, which indicated the attack of hyperglycemia, hyperinsulinemia, insulin resistance and lipid metabolism disorder. After JPQHF intervention for 6 weeks, these damages was improved significantly. The JPQHF diminished the body weight (P < 0.05), blood glucose at various time points and AUC of IPGTT (P < 0.01) and IPITT (P < 0.01), FINS (P < 0.01), TC (P < 0.01), TG (P < 0.01), LDL-C (P < 0.01) and enhanced HDL-C (P < 0.01). Consistent with above findings, these results collectively corroborated that JPQHF ameliorated insulin resistance with glucose and lipid metabolism.
JPQHF ameliorated metabolic phenotype in DIO mice. A Body weight were measured weekly after JPQHF administration. B-C IPGTT and (D-E) IPPTT after 5 weeks’treatment of JPQHF. F fasting insulin (FINS), (G) cholesterol (TC), (H) triglycerides (TG), (I) low density lipoprotein cholesterol (LDL-C) and (J) high density lipoprotein cholesterol (HDL-C) concentration were detected at the 6th week. (n = 6) Values are mean ± SD. NOR: normal control; DIO: HFD induced obesity mouse; MET: metformin group(DIO mouse treated with metformin at a dose of 300 mg/kg/d; JPQH: DIO mice treated with JPQHF(20.961 g/kg/d). *P < 0.05 vs. the NOR group; **P < 0.01 vs. the NOR group; #P < 0.05 vs. the DIO group; ##P < 0.01 vs. the DIO group
JPQHF attenuated inflammation of small intestine in DIO mice
Obesity-related intestinal inflammation is accompanied by the increased production of pro-inflammatory mediators and less anti-inflammatory cytokines [4, 18, 19]. In this study, we discussed the role of JPQHF in intestinal inflammation. As it can be discerned from the Fig. 2, the the proportion of macrophage (CD45+CD11b+ F4/80+) exhibited no comparable difference (P > 0.05), conversely, the ratio of M2 macrophage(CD45+CD11b+ F4/80+CD206+)/M1 macrophage(CD45+CD11b+ F4/80+ CD86+) was significantly elevated (P < 0.05). Additionally, the pro-inflammatory cytokines such as IL-1β,IL-6, TNF-α and IFN-γ were increased. The above results implied that high-fat diet feeding resulted in intestinal inflammation. Reciprocally, JPQHF prominently shifted the macrophage polarization toward a higher M2/M1 ratio(P < 0.01) critically, lessened the levels of IL-1β (P < 0.01), IL-6 (P < 0.01), TNF-α (P < 0.01) and IFN-γ (P < 0.05). Accordingly, the JPQHF attenuated the pro -gression of intestinal inflammation of DIO mice.
JPQHF attenuated inflammation of small intestine in DIO mice. A-C Flow cytometry analyzed the proportion of macrophage and the ratios of M2/M1 macrophage. Luminex evaluated the density of (D) IL-1β, (E) IL-6, (F) TNF-α, (G) IFN-γ (n = 6) of small intestine. Values are mean ± SD. *P < 0.05 vs. the NOR group; **P < 0.01 vs. the NOR group; #P < 0.05 vs. the DIO group; ##P < 0.01 vs. the DIO group
JPQHF decreased MAPK activation in the small intestine of DIO mice
MAPK signaling pathway plays an important role in macrophage activation, polarization and proliferation, which is the key regulator of intestinal inflammatory diseases simultaneously [20,21,22]. As presented in the Fig. 3, the JPQHF inhibited the activation of MAPK by suppressing of the ratio of p-P38 MAPK/P38 MAPK (P < 0.01)、p-JNK/JNK (P < 0.01) and p-ERK1/2/ERK1/2(P < 0.01) in small intestine tissue of DIO mice.
JPQHF diminished glucose and lipid absorption in the small intestine of DIO mice
Pro-inflammatory cytokines are pivotal factors of disrupting insulin signaling which interferes nutrient absorption in the small intestine through its impact on glucose and lipid transporters, which are essential for the uptake of glucose and lipid. This, in turn, lead to insulin resistance [23,24,25]. From Fig. 4 it is clear that JPQHF diminished the expression of PI3K (P < 0.01) and the the ratio of p-AKT/AKT (P < 0.01), p-IRS1/IRS1(P < 0.01), p-IRS2/ IRS2 (P < 0.01) considerably upregulated in DIO mice. The JPQHF lowered the concentration of apoB48 in small intestine (P < 0.01) and facilitated the translocation of GLUT2 from the brush border to the basal side of small intestine epithelial cells. Together, the JPQHF reduced glucose and lipid absorption of small intestine predominantly via IRS/PI3K/Akt activation.
JPQHF diminished glucose and lipid absorption in the small intestine of DIO mice. A Western blot quantified the levels of (B) PI3K, (C) p-AKT/AKT, (D) p-IRS1/IRS1 and (E) p-IRS2/IRS2. F Immunofluorescence inspected the transpo -sition of GLUT2. 200 × magnification. (G) Serum concentration of apoB48. (n = 6) Values are mean ± SD. **P < 0.01 vs. the NOR group; ##P < 0.01 vs. the DIO group
Discussion
Low-grade chronic systemic inflammation is the underlying etiological reason for insulin resistance. The latest investigations reconfirmed the notion that abnormal composition of small intestinal immune system triggers the local intestinal inflammation, which in turn contributes to systemic inflammation in other downstream insulin-sensitive organ [2,3,4]. Of note, macrophage have been considered pivotal in adipose which as key role in the inflammatory process associated with metabolic disease [26, 27]. Intestinal inflammation precedes incidence of systemic metabolic disorders in diet-induced obesity [18, 28, 29]. The obesity in humans is characterized by gut inflammation as shown by accumulation of pro-inflammatory intestinal macrophages [6]. The high-fat diet (HFD) increases inflammatory intestinal macrophages [5] and increased pro-inflammatory macrophages in the gut [2]. This is similar to what has been described in IBD and suggests disrupted differentiation of intestinal macrophages towards an anti-inflammatory/resident state. In addition, MAPK signaling pathway is thought to be important in macrophages activation, polarization and proliferation, which is considered pivotal in the occur of intestinal inflammatory diseases [20,21,22]. In the present study, we found that JPQHF could prominently ameliorates insulin resistance, shifted the macrophage polarization toward a higher M2/M1 ratio and reduced the inflammation factors expression remarkably in intestine,whilst inhibited the activation of MAPK. We infer that JPQHF improves inflammation in small intestine tissue via suppressing MAPK signaling pathway,in turn mitigates insulin resistance.
The small intestine is the primary site for nutrient absorption by glucose and lipids transporters. Facilitated diffusion of GLUT2 are involved in the transmembrane carriage of glucose [30, 31]. In the case of obesity and insulin resistance, overabundance of glucose is translocated to epithelial cells substrata and feeded into the circulation, which may account for postprandial hyperglycemia of insulin resistance and diabetes patient [10, 32]. Dietary fat is absorbed into small intestinal epithelial cells and is packaged into chylomicron, furthermore, the gut-specific apolipoprotein apoB48 is considered as a crucial component of the chylomicron [33, 34]. Serum apoB48 in a fasting state is intimately associated with higher postprandial lipemia, hence, it is now recognized as an important independent marker of postprandial hyperlipidemia risk [35, 36]. Perturbed insulin signaling pathway, the PI3K/Akt pathway, in intestinal cells will initiate the excessive intestinal glucose and lipid uptake. Impaired phosphorylation of insulin receptor substrates 1, 2(IRS1,2) and Akt in intestinal epithelial cells of obese patient facilitates GLUT2 localizing to the brush border [7]. Additionally, the activation of PI3K increases GLUT2 transcription [8]. Alternatively, blunted insulin signaling pathways is a state in which insulin unable to inhibit apoB48 synthesis. Furthermore, in the metabolic inflammation state, pro-inflammatory cytokines (e.g. IFN-γ,TNF-α,IL-6 and IL-1β) have been suggested to play a causal role in insulin signaling pathways suppression [22,23,24,25]. Our results revealed that JPQHF lowered small intestinal pro-inflammatory cytokines secretion, raised the expression of PI3K and phosphorylated IRS1, IRS2, Akt, facilitated the dislodgment of GLUT2 and depressed serum apoB48 concentrations. Hence, we conjecture that JPQHF reactivates insulin signaling pathways may through relieving of intestinal inflammation, eventually results in mitigating levels of glucose and lipid.
Conclusions
Generally, we demonstrate that JPQHF ameliorates insulin resistance in DIO mice,the speculated action mechanism as shown in Fig. 5. JPQHF suppresses intestinal inflammation,then diminishes glucose and lipid absorption,which eventually attenuates insulin resistance.
Availability of data and materials
All supporting data for this manuscript are in this article and supplementary Material. For further information, please contact the corresponding author.
Abbreviations
- DIO:
-
Diet-Induced Obesity
- HFD:
-
High Fat Diet
- T2DM:
-
Type 2 Diabetes Mellitus
- IR:
-
Insulin Resistance
- TCM:
-
Traditional Chinese Medicine
- MET:
-
Metformin
- MAPK:
-
Mitogen-Activated Protein Kinase
- P38 MAPK:
-
P38 Mitogen-Activated Protein Kinase
- ERK1/2:
-
Extracellular Regulated Protein Kinases
- JNK:
-
C-Jun N-terminal Kinase
- TC:
-
Cholesterol
- TG:
-
Triglycerides
- HDL-C:
-
High Density Lipoprotein Cholesterol
- LDL-C:
-
Low Density Lipoprotein Cholesterol
- PI3K:
-
Phosphatidylinositol-3 Kinase
- Akt:
-
Protein kinase B
- IRS:
-
Insulin Receptor Substrate
- apoB48:
-
Apolipoprotein B 48
- GLUT2:
-
Glucose Transporter type 2
References
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302.
Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5(8):e12191.
Amiya T, Nakamoto N, Irie J, Taniki N, Chu PS, Koda Y, Miyamoto K, Yamaguchi A, Shiba S, Morikawa R, Itoh H, Kanai T. C-C motif chemokine receptor 9 regulates obesity-induced insulin resistance via inflammation of the small intestine in mice. Diabetologia. 2021;64(3):603–17.
Khan S, Luck H, Winer S, Winer DA. Emerging concepts in intestinal immune control of obesity-related metabolic disease. Nat Commun. 2021;12(1):2598.
Kawano Y, Nakae J, Watanabe N, Kikuchi T, Tateya S, Tamori Y, Kaneko M, Abe T, Onodera M, Itoh H. Colonic Pro-inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-Dependent Manner. Cell Metab. 2016;24(2):295–310.
Rohm TV, Fuchs R, Müller RL, Keller L, Baumann Z, Bosch AJT, Schneider R, Labes D, Langer I, Pilz JB, Niess JH, Delko T, Hruz P, Cavelti-Weder C. Obesity in Humans Is Characterized by Gut Inflammation as Shown by Pro-Inflammatory Intestinal Macrophage Accumulation. Front Immunol. 2021;12:668654.
Monteiro-Sepulveda M, Touch S, Mendes-Sá C, André S, Poitou C, Allatif O, Cotillard A, Fohrer-Ting H, Hubert EL, Remark R, Genser L, Tordjman J, Garbin K, Osinski C, Sautès-Fridman C, Leturque A, Clément K, Brot-Laroche E. Jejunal T Cell Inflammation in Human Obesity Correlates with Decreased Enterocyte Insulin Signaling. Cell Metab. 2015;22(1):113–24.
Federico LM, Naples M, Taylor D, Adeli K. Intestinal insulin resistance and aberrant production of apolipoprotein B48 lipoproteins in an animal model of insulin resistance and metabolic dyslipidemia: evidence for activation of protein tyrosine phosphatase-1B, extracellular signal-related kinase, and sterol regulatory element-binding protein-1c in the fructose-fed hamster intestine. Diabetes. 2006;55(5):1316–26.
Vine DF, Takechi R, Russell JC, Proctor SD. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis. 2007;190(2):282–90.
Ait-Omar A, Monteiro-Sepulveda M, Poitou C, Le Gall M, Cotillard A, Gilet J, Garbin K, Houllier A, Château D, Lacombe A, Veyrie N, Hugol D, Tordjman J, Magnan C, Serradas P, Clément K, Leturque A, Brot-Laroche E. GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes. 2011;60(10):2598–607.
Schmitt CC, Aranias T, Viel T, Chateau D, Le Gall M, Waligora-Dupriet AJ, Melchior C, Rouxel O, Kapel N, Gourcerol G, Tavitian B, Lehuen A, Brot-Laroche E, Leturque A, Serradas P, Grosfeld A. Intestinal invalidation of the glucose transporter GLUT2 delays tissue distribution of glucose and reveals an unexpected role in gut homeostasis. Mol Metab. 2016;6(1):61–72.
Suthamwong P, Minami M, Okada T, Shiwaku N, Uesugi M, Yokode M, Kamei K. Administration of mulberry leaves maintains pancreatic β-cell mass in obese/type 2 diabetes mellitus mouse model. BMC Complement Med Ther. 2020;20(1):136.
Song D, Yin L, Wang C, Wen X. Zhenqing recipe attenuates non-alcoholic fatty liver disease by regulating the SIK1/CRTC2 signaling in experimental diabetic rats. BMC Complement Med Ther. 2020;20(1):27.
Zhu YH, Zhang XT, Lu H. Clinical observation of 60 cases of impaired glucose tolerance residents with traditional chinese medicine intervention for One Year. Lishizhen Medicine and Materia Medica Research. 2010;21(007):1674–5.
Xu JF, Hou RF, Gu YM, Tao LW, Zhang LQ, Yang XR, et al. Study on Influence of Jianpin Qinghua Recipe on Blood Glucose Fluctuations in Patients with Type 2 Diabetes. Liaoning J Traditional Chinese Med. 2015;042(009):1674–6.
Gong F, Chen QG, Han X, Liu YH, Lu H. Effects of Jianpi Qinghua Formula on glycolipid metabolism indexes and body mass in patients of type 2 diabetes mellitus with syndrome of deficiency of both qi and yin. Shanghai J Traditional Chinese Med. 2020;54(S1):65–6676.
Liu Y, Qiu Y, Chen Q, Han X, Cai M, Hao L. Puerarin suppresses the hepatic gluconeogenesis via activation of PI3K/Akt signaling pathway in diabetic rats and HepG2 cells. Biomed Pharmacother. 2021;137:111325.
Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, Lei H, Luk CT, Shi SY, Surendra A, Copeland JK, Ahn J, Prescott D, Rasmussen BA, Chng MH, Engleman EG, Girardin SE, Lam TK, Croitoru K, Dunn S, Philpott DJ, Guttman DS, Woo M, Winer S, Winer DA. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21(4):527–42.
Winer DA, Luck H, Tsai S, Winer S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab. 2016;23(3):413–26.
Guo HX, Ye N, Yan P, Qiu MY, Zhang J, Shen ZG, He HY, Tian ZQ, Li HL, Li JT. Sodium chloride exacerbates dextran sulfate sodium-induced colitis by tuning proinflammatory and antiinflammatory lamina propria mononuclear cells through p38/MAPK pathway in mice. World J Gastroenterol. 2018;24(16):1779–94.
Dong N, Xu X, Xue C, Wang C, Li X, Shan A, Xu L, Li D. Ethyl pyruvate protects against Salmonella intestinal infection in mice through down-regulation of pro-inflammatory factors and inhibition of TLR4/MAPK pathway. Int Immunopharmacol. 2019;71:155–63.
Kim N, Lertnimitphun P, Jiang Y, Tan H, Zhou H, Lu Y, Xu H. Andrographolide inhibits inflammatory responses in LPS-stimulated macrophages and murine acute colitis through activating AMPK. Biochem Pharmacol. 2019;170:113646.
Safi SZ, Batumalaie K, Qvist R, Mohd Yusof K, Ismail IS. Gelam Honey Attenuates the Oxidative Stress-Induced Inflammatory Pathways in Pancreatic Hamster Cells. Evid Based Complement Alternat Med. 2016;2016:5843615.
Bako HY, Ibrahim MA, Isah MS, Ibrahim S. Inhibition of JAK-STAT and NF-κB signalling systems could be a novel therapeutic target against insulin resistance and type 2 diabetes. Life Sci. 2019;239:117045.
Dwivedi DK, Jena GB. NLRP3 inhibitor glibenclamide attenuates high-fat diet and streptozotocin-induced non-alcoholic fatty liver disease in rat: studies on oxidative stress, inflammation, DNA damage and insulin signalling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(4):705–16.
Lee BC, Kim MS, Pae M, Yamamoto Y, Eberlé D, Shimada T, Kamei N, Park HS, Sasorith S, Woo JR, You J, Mosher W, Brady HJ, Shoelson SE, Lee J. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab. 2016;23(4):685–98.
Orliaguet L, Dalmas E, Drareni K, Venteclef N, Alzaid F. Mechanisms of Macrophage Polarization in Insulin Signaling and Sensitivity. Front Endocrinol (Lausanne). 2020;11:62.
Konrad D, Wueest S. The gut-adipose-liver axis in the metabolic syndrome. Physiology (Bethesda). 2014;29(5):304–13.
Gomes JMG, Costa JA, Alfenas RCG. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism. 2017;68:133–44.
Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35–54.
Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58(2):221–32.
Tobin V, Le Gall M, Fioramonti X, Stolarczyk E, Blazquez AG, Klein C, Prigent M, Serradas P, Cuif MH, Magnan C, Leturque A, Brot-Laroche E. Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice. Diabetes. 2008;57(3):555–62.
Hussain MM, Fatma S, Pan X, Iqbal J. Intestinal lipoprotein assembly. Curr Opin Lipidol. 2005;16(3):281–5.
Lo CC, Coschigano KT. ApoB48 as an Efficient Regulator of Intestinal Lipid Transport. Front Physiol. 2020;11:796.
Guo Q, Avramoglu RK, Adeli K. Intestinal assembly and secretion of highly dense/lipid-poor apolipoprotein B48-containing lipoprotein particles in the fasting state: evidence for induction by insulin resistance and exogenous fatty acids. Metabolism. 2005;54(5):689–97.
Masuda D, Sakai N, Sugimoto T, Kitazume-Taneike R, Yamashita T, Kawase R, Nakaoka H, Inagaki M, Nakatani K, Yuasa-Kawase M, Tsubakio-Yamamoto K, Ohama T, Nakagawa-Toyama Y, Nishida M, Ishigami M, Masuda Y, Matsuyama A, Komuro I, Yamashita S. Fasting serum apolipoprotein B-48 can be a marker of postprandial hyperlipidemia. J Atheroscler Thromb. 2011;18(12):1062–70.
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. The ARRIVE guidelines 20: Updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410.
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science Foundation of China (81874434, 82074381,81774235); Shanghai Municipal Science and Technology Commission Scientific Research Project (17401970100); Construction of the Alliance of Chinese Medicine Diabetes Specialist (ZY(2018–2020)-FWTX-4011); Shanghai Key Laboratory of Chinese Medicine Clinical Medicine (20DZ2272200); Shanghai Municipal Key Clinical Specialty (shslczdzk05401).
Author information
Authors and Affiliations
Contributions
LYH and HX contributed equally to this research. LH and CQG proposed the project notion. LYH,HX,CMJ,JSY,YZH designed and implemented experiments. LYH and HX analyzed data and writed manuscript. All participants discussed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The animal study was approved by Animal Experiment Ethics Committee of Shanghai University of Traditional Chinese Medicine which in compliance with both the laboratory animal-guidelines for ethical review of animal welfare, the National Standard of People,s Republic of China (GB/T 35892–2018) and the guidelines 2.0 of the ARRIVE [37].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Han, X., Cai, M. et al. Jianpi Qinghua Fomula alleviates insulin resistance via restraining of MAPK pathway to suppress inflammation of the small intestine in DIO mice. BMC Complement Med Ther 22, 129 (2022). https://doi.org/10.1186/s12906-022-03595-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03595-0