Abstract
Background
Stephania yunnanensis H. S. Lo is widely used as an antipyretic, analgesic and anti-inflammatory herbal medicine in SouthWest China. In this study, we investigated the anti-inflammatory activity and mechanism of sinoacutine (sino), one of the primary components extracted from this plant.
Methods
A RAW264.7 cell model was established using lipopolysaccharide (LPS) induced for estimation of cytokines in vitro, qPCR was used to estimate gene expression, western blot analysis was used to estimate protein level and investigate the regulation of NF- κB, JNK and MAPK signal pathway. In addition, an acute lung injury model was established to determine lung index and levels of influencing factors.
Results
Using the RAW264.7 model, we found that sino reduced levels of nitric oxide (NO), tumour necrosis factor-α (TNF-α), interleukin (IL)-1β and prostaglandin E2 (PGE2) but increased levels of IL-6. qPCR analysis revealed that sino (50, 25 μg/ml) inhibited gene expression of nitric oxide synthase (iNOS). western blot analysis showed that sino significantly inhibited protein levels of both iNOS and COX-2. Further signalling pathway analysis validated that sino also inhibited phosphorylation of p65 in the NF-κB and c-Jun NH2 terminal kinase (JNK) signalling pathways but promoted the phosphorylation of extracellular signal regulated kinase (ERK) and p38 in the MAPK signalling pathway. In addition, in a mouse model induced by LPS, we determined that sino reduced the lung index and the levels of myeloperoxidase (MPO), NO, IL-6 and TNF-α in lung tissues and bronchoalveolar lavage fluid (BALF) in acute lung injury (ALI).
Conclusion
Taken together, our results demonstrate that sino is a promising drug to alleviate LPS-induced inflammatory reactions.
Similar content being viewed by others
Background
Inflammation, the basis of acute and chronic syndromes, often plays a crucial role in acute respiratory distress syndrome (ARDS), atherosclerosis, inflammatory bowel disease, el at [1].. Lipopolysaccharide (LPS) can stimulate macrophages to secrete various inflammatory cytokines, further causing systemic inflammatory reactions that lead to shock and sepsis in severe cases, eventually progressing to multiple organ dysfunction [2]. Drugs currently used for the treatment of inflammation are classified into steroidal anti-inflammatory drugs (SAIDs) and nonsteroidal anti-inflammatory drugs (NSAIDs). However, after many years of clinical use, it was found that long-term use of SAIDs could lead to complications such as adrenal cortex dysfunction [3], while NSAIDs, represented by aspirin, have good anti-inflammatory effects and do not cause some of the adverse reactions induced by SAIDs. However, NSAIDs also trigger a series of other adverse reactions such as damage to the liver and digestive tract after long-term use [4]. Therefore, identifying novel anti-inflammatory drugs with high efficacy and minimal side effects has become a hot spot in the research and development of lead compounds [5] .
Acute lung injury (ALI) and the more severe acute respiratory distress syndrome (ARDS), a subtype of ALI characterized by more severe hypoxemia, are the pulmonary manifestations of an acute systemic inflammatory process clinically characterized by pulmonary infiltrates, hypoxemia and oedema [6]. A prospective, population-based, cohort study in 21 hospitals demonstrated that the in-hospital mortality rate of ALI was 38.5% [7]. Studies have demonstrated that the development of ALI/ARDS leads to excessive production of proinflammatory cytokines, such as tumour necrosis factor (TNF)-n, interleukin (IL)-1rl IL-6, and IL-8, by immune cells [8], and chemotactic inflammatory cells excessively infiltrate lung tissue, resulting in oedema and gas exchange deterioration [9]. These phenomena indicate that acute inflammation plays an important role in the ALI/ARDS process16. In recent decades, mechanical ventilation has been conventionally used as a standard treatment method, but there is still no feasible or effective treatment method to treat ALI that reduces the mortality rate in critically ill patients [10]. Therefore, treatment of the primary disease and control of the systemic inflammatory response has become a major therapeutic strategy. Some natural ingredient have been reported to exert protective effects on LPS-induced ALI in mice and in RAW264.7 cells and have potential as therapies for the treatment of pulmonary inflammation. Such as Plantamajoside [11], Dehydrocostus lactone and Astaxanthin. Hence, developing more effective strategies to inhibit inflammatory responses and identifying new diagnostic and therapeutic targets are critical for improving patient outcomes [12, 13].
Stephania yunnanensis H. S. Lo is a plant of Subgen. Tuberiphania Lo et M. Yang, Genus Stephania from Yunnan province, China. Many species of plants in Subgen. Tuberiphania are traditional Chinese herbal medicine and ethnic medicines with a long history of medical application and are widely distributed throughout South and West China, especially in Yunnan, Guizhou and Sichuan provinces. They have served as ethnic drugs in Yi (Biwugao), Dai (Bobohan) and other nationalities in Southwest Chinese populations with antipyretic, analgesic and anti-inflammatory actions [14,15,16]. Sinoacutine (sino) is a morphinoid alkaloid extracted from S. yunnanensis S. H. Lo [17] and S. epigaea H.S.Lo [14]. It has been reported that sino reduces articular swelling in an anti-rheumatoid arthritis model caused by type II collagen in rats [18], validating that sino exert an anti-inflammatory effect in vivo. Another study [19] found that sino plays an analgesic role by increasing the hot plate-induced pain threshold in mice, reducing the twisting times of mice caused by acetic acid, and increasing the pain threshold in response to electrical stimulation in the mice toes. At the same time, it also coordinates and enhances the sedative and hypnotic effects of sodium pentobarbital [19]. The structure of sino is similar to that of sinomenine (sin), a marketed medication in China used for the treatment of osteoarthritis and rheumatoid arthritis. It was reported that sin has strong anti-inflammatory and immunosuppressive effects, as well as analgesic and sedative effects [20], through inhibiting the NF-κB [21] and MAPK [22] signal transduction pathways. In addition, Liu et al. [14] found that sin attenuates ALI by suppressing inflammation. Therefore, it has been speculated that sino has similar effects as sin with respect to inhibiting the inflammatory signalling pathway and the expression of inflammation factors, and it is necessary to compare the difference in activity and mechanism between these two compounds toidentify new anti-inflammatory lead components with high efficiency and minimal side effects.
In this study, we first explored the anti-inflammatory effect and potential mechanism of sino in vitro using RAW264.7 mouse macrophages stimulated with LPS. Then, an ALI mouse model induced by LPS was established to investigate the anti-inflammatory effect in vivo to provide a theoretical basis for further research and novel drug development.
Methods
Drugs and reagents
The sino (96.46%) used in this experiment was prepared by our laboratory. Dexamethasone sodium phosphate injection (Dex) was purchased from Tianjin Suicheng Pharmaceutical Co., Ltd. (Tianjin, China). Zhengqingfengtongning Injection(sin) was produced by Zhengqing Pharmaceutical Co., Ltd. (Hunan, China). The NO Assay Kit (nitrate reductase method) and β-actin antibody were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu Sheng, China). High glucose Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), and phosphate-buffered solution (PBS) were obtained from Biological Industries (Kibbutz Beit Haemek, Israel). Cell Counting Kit-8 (CCK-8) was purchased from TransGen Biotech (Beijing, China). Dimethyl sulfoxide and LPS were purchased from Sigma (St. Louis, MO, USA). The antibodies specific for iNOS, phosphorylated p65 (p-p65), p65, p-IκB, p-ERK, ERK, P-P38, P38, P-JNK, JNK and horseradish peroxidase-conjugated goat anti-rabbit secondary antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). The enhanced BCA Protein Assay Kit was purchased from Beyotime Biotechnology (Beijing, China). The mice TNF-α, IL-6, and myeloperoxidase (MPO) enzyme-linked immunosorbent assay (ELISA) kits were purchased from multi sciences (Hangzhou, China). The Total RNA Extraction Kit, GoScript™ Reverse Transcription System, and GoTaq® qPCR Master Mix were purchased from Shanghai Promega (Shanghai, China).
Plant material
The plants of Stephania yunnanensis. Lo were collected from Yun county, Yunnan Province, China, and identified by Professor Yunshu Ma (College of Pharmaceutical Science, Yunnan University of Chinese Medicine) [23], which is not an endangered species, hence no special governmental permission was required for collection. The specimen was deposited in the College of Pharmaceutical Sciences, Yunnan University of Chinese Medicine. The root tuber of Stephania yunnanensis. Lo was used to isolated sino.
Extraction and separation of sino [24]
Collection of Stephania yunnanensis. Lo was sun-dried and pulverized into coarse powder, refluxed and extracted in 95% ethanol 3 times to obtain the extract. Extraction was performed with acid-soluble chloroform, and the PH value was adjusted to 8–9 with alkali solutions. The extract was dissolved and mixed with a silica gel column (petroleum ether-acetone system gradient elution, chloroform-methanol system gradient elution, repeated elution), and an LH-20 dextran gel column (chloroform-methanol 1:1) was separated and purified several times to obtain colourless crystals. The structure was identified using mass spectrometry, proton nuclear magnetic resonance (1H NMR), and carbon-13 NMR (13C NMR) analyses. Content determination was performed by HPLC.
Cell culture
RAW264.7 cells were purchased from the Cell Resource Center of Shanghai Institute of Life Science, Chinese Academy of Sciences (Shanghai, China). RAW264.7 cells were cultured in high glucose DMEM supplemented with 10% FBS and 1% penicillin-streptomycin in a humidified incubator at 37 °C and 5% CO2.
CCK-8 assay
RAW264.7 cells (2.5 × 105 cells/ml) were seeded in 96-well plates and cultured for 24 h in the presence of 5% CO2 at 37 °C. Sin (50 mg/2 ml) was diluted to 100 mg/ml in DEME medium. Since sino is difficult to dissolve in DMEM medium, 8 μg/ml DMSO was first used to dissolve it, and then DMEM medium was used to dilute it to the corresponding concentration. Then, the cells were treated with 100 μl sino at different concentrations (25, 50, 100 μg/ml) and DMSO (8 μg/ml) for 24 h, and 10 μl CCK-8 was added to each well and the cells were cultured for 3 h, the OD at 450 nm was measured by a microplate reader. and the cell viability and concentrations for experiment was calculated.
NO and inflammatory cytokine measurement
RAW264.7 cells (2.5 × 105 cells/well) were seeded into 24-well plates. Then, cells were treated with sin, Dex, and different concentration of sino for 2 h followed by incubation with 1 μg/ml LPS for 24 h. The concentrations of cytokines NO, TNF-α, IL-1β, IL-6 and PGE2 in the supernatant were detected according to the manufacturer’s instructions. All experiments were repeated for at least three times.
Quantitative real-time PCR
RAW264.7 cells (8 × 105 cells/well) were plated into 6-well plates. Then, the cells were treated with sin, Dex, and different concentrations of sino for 2 h followed by incubation with 1 μg/ml LPS for 6 h. Total RNA extraction and isolation was performed, and the mRNA levels were measured using quantitative real-time PCR (qPCR). Relative gene expression was normalized to GAPDH. Pre-denaturing was performed at 95 °C for 10 min followed by 40 cycles under conditions of denaturation at 95 °C for 15 s and annealing at 60 °C for 1 min. All experiments were repeated for at least three times. Primers are listed in Table 1.
Western blot analysis
RAW264.7 cells (8 × 105 cells/well) were plated into 6-well plates. Then,the cells were treated with sin, Dex, and different concentrations of sino for 2 h followed by incubation with 1 μg/ml LPS for 24 h. A cell lysis buffer (PMSF:RIPA = 1:100) was used to extract total proteins, and the protein concentrations were determined using a BCA protein assay kit. The protein extracts (40 μg) were separated using 10% SDS–PAGE and electrotransferred to PVDF membranes, which were incubated with primary antibody according to the conditions shown in Table 2. After incubation, the membranes were washed in TBST, and then incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody. The signals were detected using an enhanced chemiluminescence kit.
Animal experiment
Kunming (KM) mice (male, 20 ± 2 g) were purchased from Hunan Skolek Jingda Experimental Animal Co., Ltd. (Reg. No. SCXK (Hunan) 2016–0002) and fed a normal diet. All animal protocols were approved by the Yunnan University TCM Committee on Animal Care and Use (No. R-062016002).
One hundred and four mice were randomly divided into seven groups: control (saline), model (LPS, 10 mg/kg), sin (13 mg/kg), Dex (7 mg/kg), and 3 doses of sino (3, 6, and 12 mg/kg). LPS was injected into the tail vein except in the control group. After 12 h, the control and model groups were injected with the same dose of saline, while the 5 treatment groups were injected with their corresponding drugs. After 12 h, the trachea of six mice in each group was exposed, and their left lungs were lavaged with saline two times to obtain BALF sample [25], mice were inhaling isoflurane (2%) to anesthetize before sacrifice. The lung tissues of the remaining mice were collected, weighed and homogenized.
Measurement of lung index, NO, MPO, IL-6, TNF-α levels
The NO, MPO, IL-6, TNF-α contents in BALF and/or lung tissues were tested using kits according to the manufacturer’s protocols. After the lungs were weighed, the lung index (LI) was calculated according to the following formula to evaluate the degree of pulmonary edema.
Histopathological evaluation of the lungs
The right lungs of mice in each group were collected and fixed in 4% paraformaldehyde solution, embedded in paraffin, cut into 3 μm sections and stained with haematoxylin/eosin (H&E). Finally, the pathological changes in the tissues were microscopically examined.
Statistical analysis
SPSS19.0 was used to analyze the differences between values. All values were expressed as means ± SDs. The difference between two groups was determined to be statistically significant using one-way ANOVA (Dunnett’s t test). Statistical significance was accepted at p < 0.05 or p < 0.01.
Results
1H NMR and 13C NMR analyses
The substance is colorless massive crystal (methanol). mp195–198 °C, MSm/z327 (M+); C19H21O4N. 1H-NMR (CDCl3, 400 MHz): δH: 1.77 (1H, td, Ha-15), 2.37 (1H, J = 2.70 Hz, Hb-15), 2.51 (1H, d, J = 2.76 Hz, Ha-16), 2.64 (1H, dd, J = 3.40 Hz, J = 3.01 Hz, Hb-16), 2.46 (3H, s, N-CH3), 3.00 (1H, dd, J = 5.48 Hz, J = 5.50 Hz, Ha-10), 3.36 (1H, d, J = 17.71 Hz, Hb-10), 3.46 (1H, dd, J = 5.48 Hz, J = 5.50 Hz, Ha-10), 3.36 (1H, d, J = 17.71 Hz, Hb-10), 3.46 (1H, s, H-9), 3.74 (3H, s, 3-OCH3), 3.87 (3H, s, 6-OCH3), 6.31 (1H, s, 4-OH), 6.74 (1H, d, J = 8.31 Hz, H-1), 6.65 (1H, d, J = 8.28 Hz, H-2), 6.32 (1H, s, H-5), 7.53 (1H, s, H-8); 13C-NMR (CDCl3, 400 MHz): δc: 120.3 (d, C-1), 109.5 (d, C-2), 145.4 (s, C-3), 143.3 (s, C-4), 118.9 (d, C-5), 160.8 (s, C-6), 181.3 (s, C-7), 122.5 (d, C-8), 61.0 (d, C-9), 37.3 (t, C-10), 129.3 (s, C-11), 123.7 (s, C-12), 43.5 (s, C-13), 150.9 (s, C-14), 32.6 (t, C-15), 47.0 (t, C-16), 41.6 (q, N-CH3), 56.3 (q, 3-OCH3), 54.8 (q, 6-OCH3). the purity was 96.46% by HPLC. The chemical structure was listed in Fig. 1.
Effects of sino on cell viability
The potential cytotoxicity of sino was evaluated by the CCK assay. The result shoued that treatment with sino (25, 50, and 100 μg/ml) for 24 h did not cause significant cell viability change vs. normal control group (Fig. 2a). Thus, the effects of sino (25, 50, and 100 μg/ml) on RAW264.7 cells were not attributable to cytotoxic effects.
Effect of sino on the levels of inflammatory cytokines. a Cells viability at different concentrations of sino (25, 50, 100 μg/ml) was determined. Then, for the assay for NO (b), TNF-α (c), IL-6 (d), PGE2 (e) and IL-1β (f) with ELISA kits was determined. The values are presented are the mean ± SD (n = 3 in each group) of three independent experiments, *p < 0.05, **p < 0.01 compared with LPS group; △p < 0.05, △△p < 0.01 compared with con group
Effect of sino on levels of inflammatory cytokines
The cellular activity of sino is shown in Fig. 2a. In this study, we investigated the effects of sino on the production of inflammatory cytokines in LPS-stimulated RAW264.7 cells. The results showed that sino inhibited NO (Fig. 2b), TNF-α (Fig. 2c), IL-1β (Fig. 2d) and PGE2 (Fig. 2e) in LPS-stimulated RAW264.7 cells in a dose-dependent manner in contrast, the mid and high doses of sino significantly promoted the release of IL-6 (Fig. 2f).
Effect of sino on inflammatory response pathways
The MAPK and NF-κB signalling pathways have been reported to play a central role in the expression of proinflammatory cytokines such as TNF-α, IL-6, IL-1β and IL-1β in many cell types [26] . Another study also found that MAPK and NF-κB signalling pathways mediate the production of iNOS and COX-2 to affect the synthesis of NO and PGE2 [27]. Therefore, we investigated the effect of sino on the activation of NF-κB (p65 and IκB), MAPK (ERK, JNK and p38), iNOS and COX-2 by western blot, and detected the gene expression of iNOS and COX-2 by qPCR. Our results showed that sino significantly inhibited the phosphorylation of p65 in the NF-κB signalling pathway but had no significant effect on IκB phosphorylation (Fig. 3a). In the MAPK signalling pathway, sino (50, 25 μg/ml) promoted the phosphorylation levels of ERK and p38 (Fig. 2b), whereas it significantly inhibited JNK phosphorylation (Fig. 3b). Moreover, sino inhibited iNOS protein and gene expression levels (Fig. 3c, d), and COX-2 protein levels (Fig. 3e), while promoting COX-2 gene levels (Fig. 3f).
Effect of sino on inflammatory response pathways. Cells were pretreated with sin, Dex, and sino for 2 h, and then treated with 1 μg/ml LPS for 24 h. a Western blot analysis of phosphorylated NF-κB (a), phosphorylated MAPK (b), iNOS (c) and COX-2 (e) expression in RAW264.7 cells. qPCR analysis of iNOS (d) and COX-2 (f) in cells. The values are presented as the mean ± SD (n = 3), *p < 0.05, **p < 0.01compared with LPS group; △p < 0.05, △△p < 0.01 compared with con group, ;#p < 0.05, ##p < 0.01 compared with sin group
Effect of sino on LPS-induced in acute lung injury
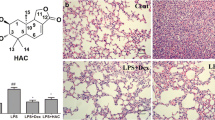
Recent studies have found that inflammation plays an important role in ALI, and the occurrence of ALI can increase the release of proinflammatory cytokines, such as TNF-α and IL-6 [28]. Here, we examined histopathological changes that occur in ALI by H&E staining. The control group exhibited normal lung tissue structure in response to saline treatment, while the alveolar cavity of the LPS treated group was narrowed with punctured haemorrhage and inflammatory cell infiltration. However, sino treatment significantly attenuated these LPS-induced histopathological changes, suggesting that sino exerts a protective effect (Fig. 4a). For further confirmation, cytokines were assessed, and our results showed that when LPS was administered to mice for 24 h, the cytokine indices increased substantially, indicating that the model was successfully constructed. After treatment with sino, the LI (lung index) (Fig. 4b) and the release of MPO (Fig. 4c), NO (Fig. 4d), IL-6 (Fig. 4e) and TNF-α (Fig. 4f) in lung tissue were drastically inhibited. At the same time, the contents of IL-6 (Fig. 4g) and TNF-α (Fig. 4h) in BALF were also notably decreased.
The protective effect of sino in ALI. After 12 h of LPS treatment in mice, sin, Dex, and sino were given for 12 h, pathological changes of lung tissue (a) were observed (200 ×), then LI (b) was calculated, moreover, the contents of MPO (c), NO (d), IL-6 (e) and TNF-α (f) in lung homogenate (n = 7 in each group) and the contents of IL-6 (g) and TNF-α (h) in BALF (n = 6 in each group) were determined according to the kits. Results are mean ± SD, *p < 0.05, **p < 0.01compared with LPS group; △p < 0.05, △△p < 0.01 compared with con group, ;#p < 0.05, ##p < 0.01 compared with sin group; +p < 0.05, ++p < 0.01 compared with Dex group
Discussion
Activation of the immune system leads to the occurrence of inflammatory reactions involving various mediators, such as NO, IL-1β, IL-6 and TNF-α [antipyretic, anti-inflammatory and ..]. Among them, IL-6 exerts both proinflammatory and anti-inflammatory effects in the inflammatory response. IL-6 is typically induced together with the proinflammatory cytokines TNF-α and IL-1β [29], but it is essential under normal physiological conditions and can inhibit the occurrence of various chronic liver diseases by selectively mediating signalling pathways [30]. IL-6 also plays a dual-functional role in the process of liver fibrosis-induced inflammation [31]. One study found that high levels of IL-6 in the circulation are a sign of poor prognosis in breast cancer, melanoma, and myeloma [32]. However, some studies have found that increasing the serum levels of IL-2, IL-6, IL-12, and TNF-α in tumour-bearing mice regulates the growth and differentiation of lymphocytes and activate macrophages, playing a regulatory role in antitumour immunity [33]. Meanwhile, Qinglong [34] studied the immunomodulatory effects of Polyporus umbellatus polysaccharide on macrophages and found that it promoted the secretion of inflammatory factors and induced significant immune enhancement. Their results revealed that sino promotes the production of IL-6, indicating that sino promotes immune enhancement, but the specific mechanism of action of sino in the immune pathway remains to be determined with respect to the interaction between immune signal molecules [34].
COX-2 expression in RAW264.7 cells at the protein level and gene level has been observed to be opposite to each other. The possible reasons for this phenomenon are as follows: (1) sino might act on protein translation rather than mRNA transcription in the process controlling COX-2 protein synthesis [35]. (2) sino may cause specific or non-specific degradation of protein, resulting in decreased protein content [36]. The specific reasons for these need to be further explored and analyzed by more experimental studies.
LPS binds to Toll-like receptor 4(TLR4) and transduces signals to activate multiple signalling pathways, including NF-κB and MAPK, ultimately leading to enhanced transcription of the proinflammatory cytokines TNF-α, IL-1β, and IL-6. IκB-α plays a key role in the NF-κB signal transduction pathway. When cells are stimulated by LPS, IκB kinase (IKK) is activated, IκB undergoes ubiquitination and exposes the binding site of IκB and NF-κB,at which point NF-κB is freed, the activity is enhanced, the expression of relevant inflammatory cytokines, inflammatory chemokines and inflammatory-related mediators is induced, and the range of inflammatory reactions is expanded [37, 38]. The NF-κB activation pathway described above, which relies on IKK to degrade IκB-α is referred to as the classical NF-κB activation pathway. Other unknown pathways such as the phosphoinositide-3-kinase (PI3K)/Akt pathway represent auxiliary activation pathways of NF-κB, which can bypass the phosphorylation of IκB-α and induce the dissociation of IκB-α from the trimer to activate part of NF-κB [39]. Our results revealed that sino did not inhibit IκB-α phosphorylation. We speculate that sino may achieve anti-inflammatory effect via other pathway rather than the classic IKK/IκB/NF-κB pathway, which is worth investigating. At the same time, studies have found that MAPK-specific inhibitors may alter the activity of other kinases, i.e., activation of different MAPK signalling pathways is dynamically regulated. When some of these signalling pathways are inhibited, other signalling pathways may be activated [40]. Therefore, it is possible that sino promotes the phosphorylation of ERK and p38 due to feedback regulation between different MAPK signalling pathways. Meanwhile, Lu [41] found that astilbin upregulates LPS-induced JNK phosphorylation, suggesting that it might be associated with M1 (classical activation) and M2 (alternative activation) polarization of RAW264.7 macrophages. However additional experiments are needed to elucidate the anti-inflammatory mechanism of sino.
At present, it is believed that the most essential pathogenesis of ALI is caused by uncontrolled inflammatory reactions in the lung and imbalances between the proinflammatory and anti-inflammatory systems. When inflammation occurs, neutrophils and monocytes infiltrate into inflammatory tissues in large quantities. MPO is a functional marker and activation marker of neutrophils, and its levels and activity represent the function and activation state of neutrophils [9]. In this study, sino significantly reduced MPO activity, indicating that sino might inhibit inflammation in ALI by simultaneously attenuating the accumulation of neutrophils. TNF-α and IL-6 not only directly affect cellular production of damage but also attract inflammatory cells such as monocytes, macrophages and neutrophils to aggregate inflammatory mediators such as prostaglandins, lysosomal enzymes and hydrogen peroxide, inducing or aggravating ALI, pulmonary oedema and sepsis [42, 43]. The results of this study revealed that sino exerts a significant inhibitory effect on TNF-α and IL-6, indicating that sino might achieve an anti-inflammatory effect on ALI in mice by inhibiting TNF-α and IL-6. However, these results were completely opposite in vitro. This may be because sino regulates the inflammatory response through a variety of mechanisms in vivo, but this needs further research in future experiments.
It is worth noting that sino exhibited stronger anti-inflammatory activity than sin based on NO, TNF-α, iNOS and MAPK results, which might be caused by the position exchange of methoxy and carbonyl groups in the structures. However, there are also some deficiencies. For example, ALI has a variety of pathogenetic mechanisms. Whether sino protects the lung from LPS-induced injury through other mechanisms requires further exploration. Overall, sino has been demonstrated to be a leading compound worthy of further study.
Conclusion
In summary, our demonstrated that sino effectively reduces the release of inflammatory factors in vitro and in vivo, potentially through the inhibition of the NF-kB and JNK signalling pathways. We also confirmed that sino attenuates the inflammatory response in an ALI mouse model induced by LPS, indicating that sino may be a potential therapeutic strategy for the treatment of other inflammatory response-related diseases.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Sino:
-
Sinoacutine
- LPS:
-
Lipopolysaccharide
- NO:
-
Nitric oxide
- TNF-α:
-
Tumor necrosis factor-α
- PGE2:
-
Prostaglandin E2
- iNOS:
-
Nitric oxide synthase
- COX-2:
-
Cyclooxygenase-2
- JNK:
-
c-Jun NH2 terminal kinase
- ERK:
-
Extracellular signal regulated kinase
- MPO:
-
Myeloperoxidase
- BALF:
-
Bronchoalveolar lavage fluid
- ALI:
-
Cute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- SAIDs:
-
Steroidal anti-inflammatory drugs
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- Dex:
-
Dexamethasone sodium phosphate injection
- Sin:
-
Zhengqingfengtongning injection
- LI:
-
Lung index
References
Chen X, Tang SA, Lee E, Qiu Y, Wang R, Duan HQ, et al. IVSE, isolated from Inula japonica, suppresses LPS-induced NO production via NF-κB and MAPK inactivation in RAW264.7 cells. Life Sci. 2015;124:8–15.
Yu R, Li Q, Feng Z, Cai L, Xu Q. m6A reader YTHDF2 regulates LPS-induced inflammatory response. Int J Mol Sci. 2019;20:1323.
Zhang NS. Clinical application and adverse reactions of non-steroidal anti-inflammatory drugs. Chin J Drug Eval. 2013;30(01):37–38+41.
Wang HW, Li JY, Yang YJ, Yu YG. Progress on non-steroidal anti-inflammatory drugs. Progress Vet Med. 2011;32(01):77–80.
Gralinski LE, Bankhead A, Jeng S, Menachery VD, Proll S, Belisle SE, et al. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. mBio. 2013;4:e00271–13.
Parekh D, Dancer RC, Thickett DR. Acute lung injury. Clin Med (Lond). 2011;11(6):615–8.
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–93.
Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202(2):145–56.
Chen Y, Dong J, Liu J, Xu W, Wei Z, Li Y, et al. Network pharmacology-based investigation of protective mechanism of Aster tataricus on lipopolysaccharide-induced acute lung injury. Int J Mol Sci. 2019;20:543.
Bakowitz M, Bruns B, McCunn M. Acute lung injury and the acute respiratory distress syndrome in the injured patient. Scand J trauma Resusc. Emerg Med. 2012;20:54.
Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu C, et al. Plantamajoside ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-kappaB and MAPK activation. Int Immunopharmacol. 2016;35:315–22.
Cai X, Chen Y, Xie X, Yao D, Ding C, Chen M. Astaxanthin prevents against lipopolysaccharide-induced acute lung injury and sepsis via inhibiting activation of MAPK/NF-kappaB. Am J Transl Res. 2019;11(3):1884–94.
Nie Y, Wang Z, Chai G, Xiong Y, Li B, Zhang H, et al. Dehydrocostus lactone suppresses LPS-induced acute lung injury and macrophage activation through NF-kappaB signaling pathway mediated by p38 MAPK and Akt. Molecules. 2019;24:1510.
Li L, Zuo AX, Rao GX. Studies on alkaloids from the Stephania epigaea of Dai traditional medicine. J Yunnan Univ Tradi Chin Med. 2012;35(03):14–9.
Gao L, Peng LF, Zhao CM, Fu DH, Wang JK, Zhu ZY. Medicinal experience, toxity and efficacy of five types of Yunnan ethnic medicine. Chin J Pharmacol Toxicol. 2017;31(06):503–7.
Wang GQ. National compendium of Chinese herbal medicine, vol. 2. Beijing: People’s Medical Publishing House; 2014. p. 411–2.
Yan H, Ma YS, Cheng X, Zhang XL. Studies on fingerprint and quantitative analysis method of main components of Stephania yunnanensis. J Yunnan College Trad Chin Med. 2007;03:9–14.
Shi KY. New use of sinoacutine in medicine. Chinese patent CN 103417540 A, 2013 Dec 04.
Ma YS, Yu ZF, Zhao ZX, Wang FC. Analgesic and sedative effects of sinoacutine. J Yunnan Univ Tradi Chin Med. 1992;04:9–11.
Liu Q, Zhou LL, Li R. Overview of sinomenine research. Chin Trad Herbal Drugs. 1997;04:247–9.
Jin XK, Li WD, Teng HL, Lin ZB. Effect of sinomenine on nuclear transcription factor κB and its inhibitory factor IκB. Chin Pharmacol Bull. 2004;07:788–91.
Li XJ, He LG, Hu YP, Duan H, Li XL, Liu SW. Sinomenine inhibited bone destruction and osteoclast formation in MT induced arthritis rats by regulating RANKL signaling pathway. Chin Soc Immunol. 2012;594.
Ma YS, Li YY, Yu H. Optimization of DALP reaction system by orthogonal experiment. J Kunming Med Univ. 2007;04:31–4.
Wang B. Preliminary study on chemical compositions of Stephania yunnanensis. Lo and structure-antiarrhythmic activity. Thesis, Yunnan University of Traditional Chinese Medicine, Yunnan, China, 2013.
Yao R, Zhang RH, Wang L, Chen ZY. Technical improvement of bronchoalveolar lavage in mice. Chin J Compar Med. 2017;27(11):80–3.
Olajide OA, Aderogba MA, Fiebich BL. Mechanisms of anti-inflammatory property of Anacardium occidentale stem bark: inhibition of NF-kappaB and MAPK signalling in the microglia. J Ethnopharmacol. 2013;145(1):42–9.
Kang YJ, Wingerd BA, Arakawa T, Smith WL. Cyclooxygenase-2 gene transcription in a macrophage model of inflammation. J Immunol. 2006;177(11):8111–22.
Lee YC, Lin CY, Chen YH, Chiu WC, Wang YY, Hsu C, et al. Essential role of Visfatin in lipopolysaccharide and colon Ascendens stent peritonitis-induced acute lung injury. Int J Mol Sci. 2019;20:1678.
Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101(2):311–20.
Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64(6):1403–15.
Mai ZH, Huang D, Weng JF, Huang ZS, Huang Y, Zhang HY, et al. Effect and mechanism of reversin on hepatic stellate cell inflammatory response. Shandong Med J. 2018;58(24):31–3.
Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat. 2007;102(2):129–35.
Shui L, Liu MH, Shui PX, Sun Q, Zhuang YC. Effects of Dendrobium nobile lindl wall-broken powder on tumor growth and immune function of tumor-bearing mice. Chin J New Drugs. 2018;27(16):1896–901.
Tan QL, Zhou CY, Liu CP, Luo M, Xu WX, Li X, et al. Investigation on the effects of polyporus polysaccharides on morphological changes and immune function in human macrophages. Chin J Tradit Chin Med Pharm. 2018;33(05):1891–6.
Kang J, Li G, Wang Q, Yin Z. The effect of Lycorine on LPS-induced COX-2 invivo and vitro. J Nanjing Normal Univ (Natural Science Edition). 2016;39(1):86–9.
Kang J, Zhang Y, Cao X, Fan J, Li G, Wang Q, et al. Lycorine inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in RAW264.7 cells through suppressing P38 and STATs activation and increases the survival rate of mice after LPS challenge. Int Immunopharmacol. 2012;12(1):249–56.
Choi YS, Jeong S. PI3-kinase and PDK-1 regulate HDAC1-mediated transcriptional repression of transcription factor NF-kappaB. Mol Cell. 2005;20(2):241–6.
Kiemer AK, Hartung T, Huber C, Vollmar AM. Phyllanthus amarus has anti-inflammatory potential by inhibition of iNOS, COX-2, and cytokines via the NF-kappaB pathway. J Hepatol. 2003;38(3):289–97.
Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–71.
Fernandes A, Falcao AS, Silva RF, Brito MA, Brites D. MAPKs are key players in mediating cytokine release and cell death induced by unconjugated bilirubin in cultured rat cortical astrocytes. Eur J Neurosci. 2007;25(4):1058–68.
Lu CL, Zhu YF, Hu MM, Wang DM, Xu XJ, Lu CJ, et al. Optimization of astilbin extraction from the rhizome of Smilax glabra, and evaluation of its anti-inflammatory effect and probable underlying mechanism in lipopolysaccharide-induced RAW264.7 macrophages. Molecules. 2015;20(1):625–44.
Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992;145(5):1016–22.
Chang XJ, Zhang S, Jiang YP, Chen CM, Chen J, Liu BJ, et al. Mechanism of reduning injection on anti-acute lung injury in rats based on cytokine storm. Chin Trad Herbal Drugs. 2015;46(02):236–9.
Acknowledgments
The authors would like to thank all of the teachers and classmates who contributed to this study.
Research involving plants
Sinoacutine is from Stephania yunnanensis. Lo, which is not an endangered plant species. The collection of plant material complied with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. And the study protocol on plants was approved by the office of science and technology administration of Yunnan University of TCM.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81260651) and Traditional Chinese Medicine Joint Special Key Project of Yunnan Provincial Science and Technology Department (No. 2018FF001–008).
Author information
Authors and Affiliations
Contributions
YCZ and ZXY drafted the manuscript; YCZ, XQS and YKL performed main experiments; YSM, LLC and XXY were responsible for reviewing and editing the manuscript; YKL and JFC made a formula analysis; CRP and LYZ provided resources and literature. All authors reviewed and approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal experiments in this study were conducted according to the Guidelines for the Care and Use of Laboratory Animals, and were approved by Yunnan University TCM Committee on Animal Care and Use (No.R-062016002), and carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, Y., Cui, L., Yang, X.X. et al. Sinoacutine inhibits inflammatory responses to attenuates acute lung injury by regulating NF-κB and JNK signaling pathways. BMC Complement Med Ther 21, 284 (2021). https://doi.org/10.1186/s12906-021-03458-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-021-03458-0