Abstract
Background
Atopic dermatitis (AD) is a worldwide chronic skin disease which burden public health. Sea buckthorn (SBT) (Hippophae rhamnoides L., Elaeagnaceae) oil, as a traditional herbal medicine, has been used for disease treatment for many years. The effects of SBT oil on AD mouse model induced by repeated administration of 2,4-dinitrochlorobenzene (DNCB) in BALB/c mice was evaluated in this study.
Methods
Mice were divided into four groups including the normal control group, AD model group, AD model group treated with SBT oil (5 ml/kg) and AD model group treated with SBT oil (10 ml/kg). Same volume at different concentrations of SBT oil was applied daily on the latter two groups by gavage for 15 days following AD model induction. The function of skin barrier and the production of IL-4, IFN-γ, TNF-α and TSLP were examined after animal sacrifice. The migration and mature of langerhans cell (LCs) in lymph node was further assessed by flow cytometry.
Results
SBT oil alleviated dermatitis scores, decreased ear thickness, prevented infiltration of mast cell, reduced lymph node weight and depressed activity of Th2 cells. SBT oil also reduced the expression of IL-4, IFN-γ, TNF-α and TSLP in ear tissue, IgE level in serum and mRNA relative expression of IL-4, IFN-γ, TNF-α in lymph node. Moreover, SBT oil inhibited the migration of LCs cells from local lesions to lymph node and it’s mature in lymph node.
Conclusions
These results suggest SBT oil had a beneficial effect either systemic or regional on DNCB-induced AD mice via maintain the balance of Th1/Th2 and may be a potential complementary candidate for AD treatment.
Similar content being viewed by others
Background
AD is a chronic inflammatory skin disease characterized with eczematous pruritic rash which has high morbidity in children and could be recurrent in adulthood [1, 2]. As a general public health disease, the prevalence of AD has increased in recent years [3, 4]. AD affects nearly 20% of children and 3% of adults worldwide and the incidents become higher and higher [5]. Although the pathogenesis of AD is not explicit utterly, genetic risk, environmental factors, skin barrier dysfunction and immune dysregulation are thought to play important roles during the pathogenesis of AD [5,6,7,8]. As for immune dysregulation, Th2 skewing seems to be the key point of AD pathogenesis [9, 10]. Immunological disorder of Th1/Th2 balance due to strong type 2 immune responses characterized by over infiltration of mast cell, increased production of Th2 cytokines and IgE level in serum plays crucial role in the onset and process of AD. These Th2 cytokines subsequently induce the release of other proinflammatory cytokines such as IFN-γ through activating of Th1 cells [11].
Due to the heavy burden AD placed on society and patients [12, 13], treatment approaches are needed imperatively. In clinical practice, regional emollient and systemic corticosteroids were generally used to cure AD [14, 15]. However, experts of International Eczema Council reached on a conclusion that although the use of corticosteroids for AD is widespread, it is also discouraged due to the side effects and the risk of rebound. In consideration of potential fearful side effect of topical steroid and immunosuppressive application [14, 16], there is a strong enthusiasm in seeking alternative and complementary medication to treat AD. Recently, seeking new potential candidate from natural materials for AD management attracted greatly attention [11, 17,18,19,20].
SBT is a wild deciduous shrub or dwarf tree belonging to the Elaeagnaceae family which has been used in Tibetan, Mongolian and Chinese traditional medicine extensively for disease management [21,22,23]. According to many researchers SBT has various medicinal effects such as anti-tumor, anti-stress, anti-inflammatory, anti-ulcer, anti-oxidant, healing, regulation of cardiovascular and immune system [24,25,26,27,28]. SBT oil which contains rich fatty acids, tocopherols, ω3 and ω6 etc. is a main bioactive part of SBT and it has been proved to have beneficial effect on skin inflammation conditions and have the ability to improve the composition of fatty acid in skin [29, 30]. Therefore, this study carried out to explore the beneficial effects of SBT oil on DNCB-induced AD mouse model and its possible mechanism.
Methods
SBT oil
SBT oil was provided by Liaoning Dongning Pharmceutical Co. LTD. The oil was extracted from the dried press residue (including berry flesh, seeds and peel) of SBT juice processing by aseptic supercritical carbon dioxide process. Analysis of samples was performed using a HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm, Agilent technologies Inc., Santa Clara, CA, USA) in a GC/MS (5975C, Agilent technologies Inc). Sample was injected into the column and ran using split mode (split ratio = 10:1). The helium carrier gas was programmed to maintain a constant flow rate of 1 ml/min. Oven was initially 80 °C for 3 min, then finally raised to 300 °C at 4 °C/min. Fatty acids were identified by a reference standard mixture FAME (Supelco, Bellefonte, PA, USA) analyzed under the same operating conditions as those employed for FAME of the samples. The components in SBT oil are exhibited in Table 1.
Animals and animal treatment
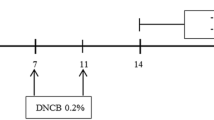
Female healthy specific pathogen-free BALB/c mice, aged 6-8 weeks, weighted 20 ± 2 g, were provided by Liaoning Changsheng Biotechnology Co., Ltd. (Benxi, China). All mice were housed in groups of 6 mice per cage waiting to be grouped in a specific pathogen-free environment in 12 h light-dark cycle and allowed free to water and food. Mice were acclimatized for 1 week before AD model induction. Mice were randomly divided into 4 groups: the normal control group, AD model group, AD model group treated with SBT oil (5 ml/kg) and AD model group treated with SBT oil (10 ml/kg), each group with 6 mice. The dorsal skin of each mouse was shaved following DNCB (200 μl,1%) sensitization three times in total from day 1 to day 7 and the skin of left ear was challenged by DNCB (20 μl,0.5%) seven times in total from day 14 to day 29. AD model group treated with SBT oil (5 ml/kg) was given 0.1 ml SBT oil plus 0.1 ml olive oil per mice, AD model group treated with SBT oil (10 ml/kg) was given 0.2 ml SBT oil per mice. Oil was applied by intragastric administration once a day from day15 to day 29, olive oil (0.2 ml) was given for the normal control group and AD model group at the same time. At the same time the thickness of left ear was measured every 3 days. All animals were sacrificed at day 30 and samples including blood, left ear tissues and submaxillary lymph nodes were collected, the mice were anesthetized with 1.2% avertin solution (0.5 g 2,2,2-tribromoethanol powder dissolved into 1 ml 2-methyl-2-butanol and 39 ml PBS at 55 °C) which was filtered using a Nalgene 0.22 μm filter (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and sacrificed via exsanguination [31]. The full-scale procedures of AD model induction and treatment are shown in Fig. 1. All experimental procedures performed were approved by the Ethical Committee of Experimental Animal Care at Liaoning University of Traditional Chinese Medicine (Shenyang, PR China).
Evaluation of AD severity
Dermatitis severity was assessed by ear thickness and dermatitis scores through the method of blind. Ear thickness was measured every 3 days since day 15 with a digital thickness gauge (Mitutoyo Co., Kanagawa, Japan). Dermatitis scores was calculated according to 4 main characteristics: dryness/crusting, hemorrhage/erythema, erosion/excoriation and edema [32]. Each one was marked on a scale from 0 (none), 1 (mild), 2 (moderate), to 3 (severe). The overall dermatitis score was consist of the sum of individual scores which range from 0 to 12 [33].
Histopathological analysis
The left ear samples of mice were collected on day 30, then fixed in 10% formalin and embeded in paraffin. The sections (5 μm thick) were stained either with hematoxylin and eosin (H&E) for visualizing dermal and epidermal thickness or with toluidine blue (TB) for visualizing mast cell numbers. The mast cells were counted in 3 sections of power fields at 200 × magnification.
Immunohistochemistry
In short, after deparaffinization and rehydration the ear tissue slides were treated with 0.3% hydrogen peroxide-methanol for inhibiting endogenous peroxidase and with high pressure for antigen retrieval. Then the slides were incubated with sheep serum for 30 min at 37 °C, with primary antibodies (Abcam) for overnight at 4 °C, with secondary antibodies provided by Zhongshanjinqiao (Beijing, China) for 1 h at 37 °C. At last the slides were stained with diaminobenzidine (DAB) provided by Zhongshanjinqiao (Beijing, China) for coloration. Result was analyzed by ImageJ.
Real-time polymerase chain reaction (RT-PCR)
Mouse submaxillary lymph nodes were collected and weighted while sacrifice and total RNA was extracted from lymph node tissues using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer’s protocols and reverse transcribed with Prime ScriptTMRT reagent kit (TaKaRa Biotechnology Co., Ltd., Dalian, China). Real-time polymerase chain reaction analyses were performed under the protocols of SYBR®Premix Ex TaqTM II (TaKaRa Biotech Co., Ltd., Dalian, China) and the primers used in this study were designed as shown in Table 2. Relative quantities of all targets in test samples were normalized to their corresponding GAPDH levels. The 2-ΔΔCt method was used to calculate relative expression quantify.
Flow cytometry
The antibodies used for flow cytometry were provided by BD (USA) and the scheme was performed follow induction. About 1 × 106–5 × 106 cells of submaxillary lymph node was collected in EP tube, centrifuged at 1500 rpm and 4 °C for 5 min. One hundred μl PBS was mixed with cells after discarding the supernatant, then another 100 μl PBS containing fluorescent antibodies was added for staining at 4 °C for 20 min and the process was kept out of the sun. Five hundred μl PBS was added and blended repeatedly for washing then centrifuged at 5000 rpm and 4 °C for 5 min. The supernatant was discarded carefully and another 500 μl PBS was added and mixed. Finally, the mixture was transferred to flow tube for flow cytometry.
Statistical analysis
The data is presented as mean ± SD. The significance of differences of different groups was evaluated by One-way analysis of variance (ANOVA) followed by the Dunnett t test. P<0.05 was considered statistically significant.
Results
SBT oil has a beneficial effect on skin against the development of DNCB-induced AD models in BALB/c mice
To investigate the effect of SBT Oil on AD-like skin lesions in our model, SBT Oil (5 ml/kg,10 ml/kg) was applied by gastric administration once a day following the AD model induction by DNCB showed in Fig. 1. Topical application of DNCB including sensitization and challenge induced AD-like skin lesions, presenting as erythema, itching and hemorrhage companied by abnormal scratching marks and dryness. Dermatitis severity was assessed by ear thickness and dermatitis scores. Ear thickness was measured every 3 days since day 15 and the dermatitis scores was evaluated according to 4 main characteristics as described previously. After SBT Oil administration for 15 days, the ear thickness and dermatitis scores were significantly decreased in a dose-dependent manner compared to the AD model group (Fig. 2).
Effects of SBT oil on AD model skin induced by DNCB. a Examples of characteristic of AD-like skin lesions. b The ear thickness of the mice. c The dermatitis scores were summarized by the sum of scores according to various AD symtoms. ## P < 0.01, # P < 0.05vs. the control group; ** p < 0.01, * p < 0.05 vs. the AD model group
SBT oil contributed to the skin barrier repair and decreased infiltration of mast cell in AD model mice induced by DNCB
To evaluate the effect of SBT oil on AD-like skin lesions histologically, H&E and TB staining were performed on tissue slides. Repetitive application of DNCB induced dermal thickening, epidermal hyperplasia and increased mast cell infiltration in AD model group. While according to H&E staining slides the dermal and epidermal thickness were both decreased and the epidermal hyperplasia was suppressed after SBT oil administration for 15 days which related to dosage (Fig. 3a, b). According to TB staining slides the infiltration numbers of mast cell were also decreased in mice treated with SBT oil (Fig. 3b, c).
SBT oil decreased the lymph node weights in AD model mice induced by DNCB
The submaxillary lymph nodes were collected and weighted after mice sacrifice to estimate whether SBT oil play a part in the process of AD induction. The results indicated an increase in submaxillary lymph node weights in AD model group which was decreased by SBT oil administration (Fig. 4).
Weights of submaxillary lymph node. A submaxillary lymph node. B lymph node weight. (a). Normal control group, (b). AD model group, (c). Treated with SBT oil (5 ml/kg), (d). Treated with SBT oil (10 ml/kg). ## P < 0.01, # P < 0.05 vs. the control group; ** p < 0.01, * p < 0.05 vs. the AD model group
SBT oil inhibited the expression of IL-4, IFN-γ, TNF-α and TSLP in ear tissue in AD model mice induced by DNCB
In order to evaluate the effects of SBT oil on regulation of Th1/Th2 cytokines, the expression of IL-4, IFN-γ, TNF-α and TSLP in AD model mice was examined by immunohistochemistry staining on ear tissue slides. Results demonstrated an up-regulation expression of IL-4, IFN-γ, TNF-α and TSLP in AD model group which was inhibited dose-dependent by application of SBT oil (Fig. 5).
SBT oil down-regulated IgE level in serum and mRNA relative expression of IL-4, IFN-γ and TNF-α in lymph node
We next measured IgE level in serum by ELISA and mRNA relative expression of IL-4, IFN-γ and TNF-α in lymph node by RT-PCR. We found that IgE level in serum was increased in AD model mice induced by DNCB. The increase was suppressed significantly in groups treated with SBT oil in a dose-dependent manner. The same as above, mRNA relative expression of IL-4, IFN-γ and TNF-α in lymph node was increased in AD model group and decreased in groups treated with SBT oil (Fig. 6).
SBT oil decreased numbers of LCs in draining lymph node and the expressions of CD86, OX40L and MHCII on LCs
In order to assess the effect of SBT oil on the maturity and migration of LCs cell in submaxillary lymph node, we detected cell numbers expressing CD207/CD326, CD86, CD80, OX40L and MHC II by Flow Cytometry. Results as shown in Fig. 7 indicated that the expressions of CD207/CD326, CD86, OX40L and MHCIIon LCs cell in submaxillary lymph node were all increased in AD model groups induced by DNCB and decreased in groups treated with SBT oil.
Effect of SBT oil on the maturity and migration of LC cell. a The scatter diagram which indicate the proportion of cells in lymph node expressing CD207+CD326+, MHC II, CD86, CD80 and OX40L. b The histogram of above-mentioned results. ## P < 0.01, # P < 0.05vs. the control group; ** p < 0.01, * p < 0.05 vs. the AD model group
Discussion
AD is a skin inflammatory disease induced by hapten and mediated by T cells. Clinically the main characteristics of AD are erythema, edema, papule, blister, bleb, bullous reaction and even necrosis. The pathological changes of AD were infiltration of inflammatory cells and tissue edema. In this study, we employed DNCB-induced AD model using BALB/c mice which has been proposed as an appropriate representative of human AD because of similar symptoms including skin erosion, hemorrhage, epidermal hyperplasia, mast cell infiltration and increased IgE level in serum etc. SBT oil, as a traditional herbal extracts, have been proved diversified pharmacological actions such as anti-inflammatory, relieve the pressure, protecting vascular endothelial cell and immunomodulatory effects [25, 26]. The main constituents of SBT oil include Fatty acids (such as Myristic acid, Palmitic acid, Palmitoleic acid, Oleic acid, etc), Sitosterol and β-carotene. Fatty acids are crucial components of cell membranes and play important role in biological function of cells [34]. Some of the Fatty acids are required for innate immune activation and pathogen defense [35]. Sitosterol is the main constituent of many plants and vegetables and has the ability to modulate the functions of macrophages and anti-inflammation [36, 37]. β-carotene also has been shown to suppress the cellular and tissue response to inflammation [38, 39]. In view of the immunoregulation and anti-inflammatory actions of SBT oil, we assessed the anti-AD effects of SBT oil in vivo.
Topical application of DNCB followed schedule including sensitization and challenge induced AD-like skin lesions, presenting as erythema, itching and hemorrhage companied by abnormal scratching marks and dryness. The ear thickness and dermatitis scores were all significantly increased in AD model group compared to control group. After SBT Oil administration for 15 days, the ear thickness and dermatitis scores in groups treated with SBT oil were significantly decreased in a dose-dependent manner compared to the AD model group which indicate that SBT oil administration suppressed the development of AD-like skin lesions.
AD is recognized as a Th2-midiated allergic disease with over expression of Th2 cytokines and increased serum IgE level [9]. Being the antibody synthesized by plasma cells, IgE plays an essential role in some hypersensitivity, such as AD, allergic asthma and allergic rhinitis. IgE has the capability of elevating the production of Th2 cytokines. Th2 cytokines IL-4 induced immunoglobulin switching in plasma cells and resulting in up-regulation of serum IgE level. Mast cell, as one of granular leukocytes, can release many cytokines to mediate inflammatory reaction and immune regulation. These cytokines also participate in pathological manifestations of many allergic disorders including AD [40]. The infiltration of mast cell which was activated by IgE is one of the key features of AD-like skin lesions [41, 42]. Cytokines released from activated mast cells attract eosinophils into the skin which give rise to skin tissue damage. Histologically, according to TB staining slides, the numbers of mast cell infiltration in ear skin tissue of AD model mice were increased by application of DNCB and were inhibited remarkably by SBT oil. The results indicated that SBT oil has beneficial effects on suppression of skin tissue mast cell accumulation in DNCB-induced AD mice. Our TB staining results indicated that mast cells in skin tissue were scarce in control group while abundance in AD model group which highly conform to the pathological changes of AD. The mast cell number was reduced remarkably after SBT oil administration in SBT oil treated group compared with AD model group. These results suggest that SBT oil has inhibiting effect of mast cell infiltration.
According to studies published, the over expression of Th2 cytokines go hand in hand with TSLP. TSLP, which can strongly promote the differentiation of Th0 cells to Th2 phenotype through activation of dendritic cell (DCs) [43], was determined as a crucial factor in the induction of Th2 skewing in AD. The expression of TSLP has been shown to be enhanced markedly in keratinocytes of AD lesions both in AD patients and in mouse models [44]. IL-4 can in turn induce the synthesis of TSLP by keratinocytes. Importantly, the migration of DCs to draining lymph node was triggered by TSLP. More interesting, Th1 cytokines IFN-γ which can activate keratinocytes was found also elevated in AD. IFN-γ and TNF-α can synergistically stimulate the release of cytokines and chemokines in chronic stage of AD. In our study SBT oil treatment reduced the increased serum IgE level which was induced by DNCB application. Moreover, SBT oil treatment also reduced DNCB-induced increases in expression of IL-4, IFN-γ, TNF-α and TSLP in ear tissue and mRNA relative expression of IL-4, IFN-γ and TNF-α in lymph node. These results suggest that SBT oil ameliorated AD symptoms partly through the activity suppression of Th1/Th2 cells. According to the down-regulated effects SBT oil did to the TSLP expression in ear tissue, we speculated that SBT oil may have the ability to suppress both the activation and migration of DCs cell. In order to clarify our speculation, flow cytometry was used to do further study about LCs.
Draining lymph node plays an important role in the pathogenesis of AD. The weight and volume of lymph node will increase company with strengthened function when it is active. We investigated the local lymph nodes through different means. First of all, the submaxillary lymph node weights of DNCB-induced AD model mice were increased significantly which were markedly reduced after intragastric application of SBT oil for 15 days in a dose-dependent manner. We further assessed the expressions of CD207/CD326, MHC class II, CD80, CD86 on LCs and OX40L on CD4+ T cells in lymph node by flow cytometry because the complex immune reaction of AD was mainly taken place in lymph node. Langerin (CD207), a type II trans-membrane protein, is a C-type lection of LCs [45]. LCs are virtual mediators of immune surveillance and tolerance which resided at epidermis as DC subpopulation [46]. Antigens both external and internal were captured by LCs and presented to Th0 cells within the skin draining lymph node. CD207 is the only surface antigen just restricted to LCs [47]. Epithelial cell adhesion molecule (EpCAM,CD326), a cell surface protein, is highly expressed on LCs and appears to stimulate LCs migration [48]. Since CD207+CD326+ as the main symbol of LCs migrated into lymph node, the proportion changes showed that SBT oil suppressed the migration of LCs cell from skin lesion to draining lymph node. After degrading proteins derive from extracellular environment were taken up by endocytosis or phagocytosis and captured by MHC class II molecules, then result in peptide-loaded MHC II and migrate to the surface of antigen presenting cell waiting for recognition by CD4+ T cells, finally activate adaptive immune response [49]. The increased cell proportion of MHC class II indicated the uptrend tendency of mature LCs in lymph node in DNCB induced AD model mice compared with normal control mice. While this uptrend tendency was inhibited by SBT oil application in mice treated with SBT oil. Constant epidermal LCs are immature normally, and barely express co-stimulatory molecules such as CD80 and CD86. While upon LCs maturation, the expression of these co-stimulatory molecules was enhanced. In this study, SBT oil down regulated the expression of CD86 on LCs in lymph node which was enhanced by DNCB in AD model mice. It suggested the effect of SBT oil on inhibiting LCs maturation. OX40(CD134) is a transmembrane protein of tumor necrosis factor receptor super-family member which mainly expressed on activated CD4+ T cells and upregulated within inflammatory lesions on the antigen-activated T cells [50, 51]. TSLP can stimulate the expression of OX40 ligand (OX40L) on LCs. LCs expressing OX40L migrate from skin lesion to local lymph node and induce the transformation of Th0 cells to Th2 cells. On one hand, the increased proportion of OX40L+ cells in lymph node of DNCB induced AD mice was suppressed by SBT oil administration which confirmed the effect of SBT oil on activation of CD4+ T cells, On the other hand, SBT oil conduced to the normal function of LCs through renovating the keratinocyte and suppressing TSLP release. As a result, the abnormal Th2 skewing inflammation was inhibited by SBT oil administration.
All in all, our results suggest that SBT oil inhibited both the migration of LCs to lymph node and its maturation in lymph node, thereby inhibited the transformation of Th0 cells to Th2 cells, and finally limited the occurrence of Th2 type inflammatory response.
Conclusion
In summary, our study results attested that SBT oil application suppressed DNCB-induced AD-like symptoms by down-regulating serum IgE level and the production of cytokines and chemokines, and regulated Th1/Th2 balance. In addition, our results also indicated that SBT oil treatment inhibited the migration of LCs to draining lymph node and its maturation. Taken together, SBT oil has excellent therapeutic effect on inflammatory skin diseases and might be a potential complementary candidate for AD treatment. In further studies, it will be worthwhile to explore the mechanism of SBT oil and its active constituent in the treatment of AD.
Availability of data and materials
All data and materials are contained and described within the manuscript.
Abbreviations
- AD:
-
Atopic dermatitis
- DAB:
-
Diaminobenzidine
- DCs:
-
Dendritic cell
- DNCB:
-
2,4-dinitrochlorobenzene
- HE:
-
Hematoxylin and eosin
- IFN-γ:
-
Interferon-γ
- IgE:
-
Immunoglobulin E
- IL-4:
-
Interleukin-4
- LCs:
-
Langerhans cell
- MHCII:
-
Major histocompatibility complex II
- RT-PCR:
-
Real-time polymerase chain reaction
- SBT:
-
Sea buckthorn
- TB:
-
Toluidine blue
- Th1:
-
T-helper 1
- Th2:
-
T-helper 2
- TNF-α:
-
Tumor necrosis factor-α
- TSLP:
-
Thymic stromal lymphopoietin
References
Klonowska J, et al. New cytokines in the pathogenesis of atopic dermatitis-new therapeutic targets. Int J Mol Sci. 2018;19(10):3086.
Choopani R, et al. Treatment of atopic dermatitis from the perspective of traditional Persian medicine: presentation of a novel therapeutic approach. J Evid Based Complementary Altern Med. 2017;22(1):5–11.
Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4S):S65–76.
Leung DYM, et al. New insights into atopic dermatitis. J Clin Investig. 2004;113(5):651–7.
Kim JE, et al. Molecular mechanisms of cutaneous inflammatory disorder: atopic dermatitis. Int J Mol Sci. 2016;17(8):1234.
Kim J, et al. Molecular mechanism of atopic dermatitis induction following sensitization and challenge with 2,4-Dinitrochlorobenzene in mouse skin tissue. Toxicol Res. 2018;34(1):7–12.
Hou DD, et al. Sea buckthorn (Hippophae rhamnoides L.) oil improves atopic dermatitis-like skin lesions via inhibition of NF-kappaB and STAT1 activation. Skin Pharmacol Physiol. 2017;30(5):268–76.
Campana R, et al. Molecular aspects of allergens in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2017;17(4):269–77.
Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2(3):110.
De Vuyst E, et al. Atopic dermatitis studies through in vitro models. Front Med (Lausanne). 2017;4:119.
Park JG, et al. Tabetri (Tabebuia avellanedae ethanol extract) ameliorates atopic dermatitis symptoms in mice. Mediat Inflamm. 2018;2018:9079527.
Shrestha S, et al. Burden of atopic dermatitis in the United States: analysis of healthcare claims data in the commercial, Medicare, and Medi-Cal databases. Adv Ther. 2017;34(8):1989–2006.
Arima K, et al. Burden of atopic dermatitis in Japanese adults: analysis of data from the 2013 national health and wellness survey. J Dermatol. 2018;45(4):390–6.
Megna M, et al. Systemic treatment of adult atopic dermatitis: a review. Dermatol Ther (Heidelb). 2017;7(1):1–23.
Glatz M, et al. Emollient use alters skin barrier and microbes in infants at risk for developing atopic dermatitis. PLoS One. 2018;13(2):e0192443.
Drucker AM, et al. Use of systemic corticosteroids for atopic dermatitis: international eczema council consensus statement. Br J Dermatol. 2018;178(3):768–75.
Yang JH, et al. Ethanolic extracts of Artemisia apiacea Hance improved atopic dermatitis-like skin lesions in vivo and suppressed TNF-Alpha/IFN-Gamma(−)Induced proinflammatory chemokine production in vitro. Nutrients. 2018;10(7):806.
Ku JM, et al. The prevention of 2,4-dinitrochlorobenzene-induced inflammation in atopic dermatitis-like skin lesions in BALB/c mice by Jawoongo. BMC Complement Altern Med. 2018;18(1):215.
Ok S, et al. Effects of Angelica gigas Nakai as an anti-inflammatory agent in in vitro and in vivo atopic dermatitis models. Evid Based Complement Alternat Med. 2018;2018:2450712.
Han HM, et al. Ameliorative effects of Artemisia argyi Folium extract on 2,4dinitrochlorobenzeneinduced atopic dermatitislike lesions in BALB/c mice. Mol Med Rep. 2016;14(4):3206–14.
Boesi A. Traditional knowledge of wild food plants in a few Tibetan communities. J Ethnobiol Ethnomed. 2014;10:75.
Li M, et al. Traditional Mongolian, traditional Chinese, and Western medicine hospitals: system review and patient survey on expectations and perceptions of quality of healthcare in Inner Mongolia, China. Evid Based Complement Alternat Med. 2018;2018:2698461.
Li C, et al. Separation of the main flavonoids and essential oil from seabuckthorn leaves by ultrasonic/microwave-assisted simultaneous distillation extraction. R Soc Open Sci. 2018;5(7):180133.
Olas B, Skalski B, Ulanowska K. The anticancer activity of sea buckthorn [Elaeagnus rhamnoides (L.) A. Nelson]. Front Pharmacol. 2018;9:232.
Olas B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A.Nelson) oil. J Ethnopharmacol. 2018;213:183–90.
Diandong H, et al. Sea buckthorn (Hippophae rhamnoides L.) oil protects against chronic stress-induced inhibitory function of natural killer cells in rats. Int J Immunopathol Pharmacol. 2016;29(1):76–83.
Suchal K, et al. Seabuckthorn pulp oil protects against myocardial ischemia–reperfusion injury in rats through activation of Akt/eNOS. Front Pharmacol. 2016;7:155.
Patel CA, et al. Remedial prospective of Hippophae rhamnoides Linn. (sea buckthorn). ISRN Pharmacol. 2012;2012:436857.
Zielinska A, Nowak I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017;16(1):95.
Basu M, et al. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine. 2007;14(11):770–7.
Woo SM, et al. Sip-jeon-dea-bo-tang, a traditional herbal medicine, ameliorates cisplatin-induced anorexia via the activation of JAK1/STAT3-mediated leptin and IL-6 production in the fat tissue of mice. Mol Med Rep. 2016;13(4):2967–72.
Choi JH, et al. Cultivated ginseng inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice and TNF-alpha/IFN-gamma-induced TARC activation in HaCaT cells. Food Chem Toxicol. 2013;56:195–203.
Mizutani N, et al. Thymic stromal lymphopoietin-induced interleukin-17A is involved in the development of IgE-mediated atopic dermatitis-like skin lesions in mice. Immunology. 2015;146(4):568–81.
Tanaka H, et al. The delivery of mRNA to colon inflammatory lesions by lipid-nano-particles containing environmentally-sensitive lipid-like materials with oleic acid scaffolds. Heliyon. 2018;4(12):e00959.
Collins JJ, et al. The fatty acid oleate is required for innate immune activation and pathogen defense in Caenorhabditis elegans. PLoS Pathog. 2019;15(6):e1007893.
Lampronti I, et al. β-Sitosterol reduces the expression of chemotactic cytokine genes in cystic fibrosis bronchial epithelial cells. Front Pharmacol. 2017;8:236.
Liu R, et al. β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm Biol. 2019;57(1):161–8.
Vollmer D, West V, Lephart E. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19(10):3059.
Cicero AFG, Colletti A. Effects of carotenoids on health: are all the same? Results from clinical trials. Curr Pharm Des. 2017;23(17):2422–7.
Ito T, et al. IL-33 promotes MHC class II expression in murine mast cells. Immun Inflamm Dis. 2015;3(3):196–208.
Dubois A, et al. Regulation of Th2 responses and allergic inflammation through bystander activation of CD8+ T lymphocytes in early life. J Immunol. 2010;185(2):884–91.
Girtsman T, et al. Natural Foxp3(+) regulatory T cells inhibit Th2 polarization but are biased toward suppression of Th17-driven lung inflammation. J Leukoc Biol. 2010;88(3):537–46.
Wallmeyer L, et al. TSLP is a direct trigger for T cell migration in filaggrin-deficient skin equivalents. Sci Rep. 2017;7(1):774.
Leyva-Castillo JM, et al. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol. 2013;133(1):154–63.
Feinberg H, et al. Trimeric structure of langerin. J Biol Chem. 2010;285(17):13285–93.
Zhang X, et al. TIM-4 is differentially expressed in the distinct subsets of dendritic cells in skin and skin-draining lymph nodes and controls skin Langerhans cell homeostasis. Oncotarget. 2016;7(25):37498–512.
Kumkate S, et al. CD207+ Langerhans cells constitute a minor population of skin-derived antigen-presenting cells in the draining lymph node following exposure to Schistosoma mansoni. Int J Parasitol. 2007;37(2):209–20.
Gaiser MR, et al. Cancer-associated epithelial cell adhesion molecule (EpCAM; CD326) enables epidermal Langerhans cell motility and migration in vivo. Proc Natl Acad Sci U S A. 2012;109(15):E889–97.
Natarajan K, et al. The role of molecular flexibility in antigen presentation and T cell receptor-mediated signaling. Front Immunol. 2018;9:1657.
Weinberg AD, et al. Science gone translational: the OX40 agonist story. Immunol Rev. 2011;244(1):218–31.
Kinnear G, et al. A diametric role for OX40 in the response of effector/memory CD4+ T cells and regulatory T cells to alloantigen. J Immunol. 2013;191(3):1465–75.
Acknowledgements
Not applicable.
Funding
This project was supported by the National Natural Science Foundation of China (grant no. 81774023) which was granted to Diandong Hou. The results indicated in this manuscript were main achievements of the project.
Author information
Authors and Affiliations
Contributions
XXW carried out the experiment and drafting of the manuscript. SJL and JPL assisted to accomplish the experiment. DNK and XWH carried out statistical analysis, PL and MX did the interpretation work. HQG and DDH revised the research and manuscript. DDH designed the experiment and submitted the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experimental procedures were conducted according to the guidelines provided by the Ethical Committee of Experimental Animal Care at Liaoning University of Traditional Chinese Medicine (Shenyang, PR China).
Consent for publication
Not applicable.
Competing interests
The authors state no potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Li, S., Liu, J. et al. Ameliorative effects of sea buckthorn oil on DNCB induced atopic dermatitis model mice via regulation the balance of Th1/Th2. BMC Complement Med Ther 20, 263 (2020). https://doi.org/10.1186/s12906-020-02997-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-020-02997-2







 : epidermis;
: epidermis;  : dermis). b Dermal and epidermal thickness in H&E-stained tissue, mast cell number in TB- stained tissue. c TB staining. × 200(
: dermis). b Dermal and epidermal thickness in H&E-stained tissue, mast cell number in TB- stained tissue. c TB staining. × 200(  : mast cell)
: mast cell)


