Abstract
Background
Despite the remarkable progress in cancer therapy in recent years, this disease still remains a serious public health concern. The use of natural products has been and continues to be one of the most effective ways to fight malignancies. The cytotoxicity of 14 compounds from African medicinal plants was evaluated in four human carcinoma cell lines and normal fibroblasts. The tested samples included: β-spinasterol (1), friedelanone (2), 16β-hydroxylupeol (3), β-amyrin acetate (4), lupeol acetate (5), sequoyitol (6), rhamnitrin (7), europetin 3-O-rhamnoside (8), thonningiol (9), glyasperin F (10), seputhecarpan B (11), seputhecarpan C (12), seputhecarpan D (13) and rheediaxanthone A (14).
Methods
The neutral red uptake (NR) assay was used to evaluate the cytotoxicity of samples; caspase-Glo assay, flow cytometry for cell cycle analysis and mitochondrial membrane potential (MMP) as well as spectrophotometry to measure levels of reactive oxygen species (ROS) were performed to detect the mode of action of compounds 9 and 13 in MCF-7 breast adenocarcinoma cells.
Results
Compounds 3, 9–13 displayed cytotoxic effects against the four tested cancer cell lines with IC50 values below 85 μM. Compounds 9 and 13 had IC50 values below 10 μM in 4/4 and 3/4 tested cell lines respectively. The IC50 values varied from 0.36 μM (against MCF7 cells) to 5.65 μM (towards colon carcinoma DLD-1 cells) for 9, from 9.78 μM (against MCF7 cells) to 67.68 μM (against HepG2 cells) for 13 and 0.18 μM (towards HepG2 cells) to 72 μM (towards Caco-2 cells) for the reference drug, doxorubicin. Compounds 9 and 13 induced cell cycle arrest in Go/G1 whilst doxorubicin induced arrest in G2/M. The two molecules (9 and 13) also induced apoptosis in MCF-7 cells through activation of caspases 3/7 and 9 as well as enhanced ROS production.
Conclusion
Compounds 9 and 13 are good cytotoxic phytochemicals that should be explored more in future to develop a cytotoxic drug to fight human carcinoma.
Similar content being viewed by others
Background
Malignant diseases still constitute one of the major health problems worldwide, despite considerable progress in cancer therapy in recent years. Globally, about 14.1 million new cancer cases and 8.2 million deaths were reported in 2012 [1, 2], with lung, breast, colon, prostate and liver cancers being the most occurring neoplasia [3]. The mortality related to breast cancer reached 459,000 victims in 2008 [4] meanwhile colorectal cancer was reported as second killing cancer [5]. Every year, lung adenocarcinoma also kills more than one million persons and appears in top in cancer-related death [6]. The mortality assigned to liver cancer was 696,000 deaths in 2008 [7]. The fight against these types of cancers as well as many others is therefore of interest and should be intensified. The use of natural products has been and continues to be one of the most effective ways to fight cancers. Various compounds from African flora displayed prominent cytotoxic effects in vitro in many cancer cell lines [8, 9]. Within our drug discovery research program, the present study was planned to investigate the cytotoxicity of a panel of 14 natural products previously isolated from the Cameroonian medicinal plant Garcinia epunctata Stapf (Guttiferae) [10], Ptycholobium contortum (N.E.Br.) Brummitt (Leguminosae) from Botswana [11, 12], and freshly isolated from Synsepalum zenkeri Engl. ex Aubrév. & Pellegr. (Sapotaceae) harvested in Cameroon. The study was extended to the analysis of the mode of action of the two most active samples, namely the flavonoid thonningiol and the pterocarpan isoflavonoid, seputhecarpan D. Various flavonoids and pterocarpan isoflavonoids from African medicinal plants previously showed antiproliferative effects. Some prominent anticancer flavonoids of the flora of Africa previously identified in our cancer research program include isobavachalcone, 4-hydroxylonchocarpin [13, 14], 6,8-diprenyleriodictyol [13], cycloartocarpesin [14] and gancaonin Q [13] meanwhile examples of good cytotoxic pterocarpan isoflavonoids are sophorapterocarpan A and isoneorautenol [15, 16].
Methods
General procedure
NMR spectra were recorded on Bruker DMX Avance 300 and 400 instruments equipped with an auto-tune probe and using the automation mode aided by the Bruker program, Icon-NMR using Acetone-d6, CDCl3 and CD3OD as solvents and internal standards. HR EISMS spectra were determined on a microTOF-Q 98 spectrometer. Infra-Red spectra were recorded as KBr disk. For column chromatography, silica gel 60 particles size 0.04–0.063 mm (Merck) or sephadex LH-20 (Sigma) were used. Analytical and Preparative TLC were performed respectively using silica gel 60 PF254 + 366 (Merck) and silica gel 60-F254precoated aluminum sheets (Merck). The plates were visualized using UV (254 and 366 nm) and revealed by spraying with vanillin-sulphuric acid.
Chemicals
The control drug, doxorubicin (98.0%) was purchased from Sigma-Aldrich (Munich, Germany). The 14 tested compounds were: β-spinasterol (1), friedelanone (2), 16β-hydroxylupeol (3), β-amyrin acetate (4), lupeol acetate (5), 5-O-methyl-myo-inositol or sequoyitol (6), rhamnitrin or 7-O-methylquercetin 3-O-rhamnoside (7), europetin 3-O-rhamnoside or 7-O-methylmyricetin 3-O-rhamnoside (8), thonningiol (9), glyasperin F (10), seputhecarpan B (11), seputhecarpan C (12), seputhecarpan D (13) and rheediaxanthone A (14). The isolation and identification of compounds 2, 3 and 14 from the Cameroonian medicinal plant Garcinia epunctata Stapf (Guttiferae) [10] and compounds 5, 9–13 from Ptycholobium contortum (N.E.Br.) Brummitt (Leguminosae) from Botswana [11, 12] were previously reported. Garcinia epunctata Stapf (Guttiferae) was collected in July 2011 at Eloumden inYaoundé (Centre Region of Cameroon) and identified by Mr. Victor Nana at the National Herbarium where a voucher specimen (19,534/SRFCam) was deposited [10]. Ptycholobium contortum was harvested around Maun, Ngamiland District in North-Western Botswana and identified by Joseph Madome of the Okavango Research Institute (ORI) Herbarium; voucher specimen (KM-2-Maun-2014) is available at at ORI Herbarium, respectively [11, 12]. Compounds 1, 4, 6, 7 and 8 were isolated from the bark, leaves and roots of Synsepalum zenkeri Engl. ex Aubrév. & Pellegr. (Sapotaceae) as described below.
Plant material
Synsepalum zenkeri Engl. ex Aubrév. & Pellegr. (Sapotaceae) was collected from Mont Kalla, Yaoundé, Cameroon, in April 2014. The sample was authenticated by Mr. Nana Victor of the National Herbarium of Cameroon, Yaoundé, Cameroon under the voucher number 21816/SRFCAM.
Extraction and isolation of compounds from Synsepalum zenkeri
Compounds isolated from leaves, bark and roots of Synsepalum zenkeri were β-amyrin acetate (4) [17], β-spinasterol (1) [18], rhamnitrin or 7-O-methylquercetin 3-O-rhamnoside (7) [19], europetin 3-O-rhamnoside or 7-O-methylmyricetin 3-O-rhamnoside (8) [19], and lupeol acetate 6 [20]. Details on extraction and their isolation are provided as (Additional file 1: S1).
Cell culture
Four human carcinoma cell lines and one normal cell line were used. They were: DLD-1 and Caco-2 colorectal adenocarcinoma cell lines, HepG2 hepatocarcinoma cells, MCF-7 breast adenocarcinoma cells and the normal CRL2120 human skin fibroblasts. Caco-2 cells (from the ŞAP Institute of Ankara,Turkey andMCF-7 cells (from ATCC) provided by Prof. Dr. Tansu Koparal (Anadolu University, Eskisehir, Turkey),HepG2, DLD-1 and CRL2120 cells were obtained from American Type Culture Collection (ATCC); DMEM medium (Sigma-aldrich, Munich, Germany) was used to maintain cells as a monolayer and was supplemented with 10% fetal calf serum and 1% penicillin (100 U/mL)-streptomycin (100 μg/mL) in a humidified 5% CO2 atmosphere at 37 °C.
Neutral red (NR) uptake assay
The cytotoxicity of compounds (1–14) and doxorubicin (positive control) was performed by NR uptake assay as previously described [21,22,23,24]. NR uptake assay is cheaper and more sensitive than other cytotoxicity tests and is based on the ability of viable cells to incorporate and bind the supravital dye NR in the lysosomes [25]. Dimethylsufoxide (DMSO) at less than 0.1% final concentration was used to dilute the tested samples. DMSO at 0.1% was used as solvent control. The incubation time was 72 h incubation in humidified 5% CO2 atmosphere at 37 °C, followed by NR coloration as previously described [23, 24]. ELx 808 Ultra Microplate Reader (Biotek) equipped with a 540 nm filter was used to measure the absorbance. Each experiment was performed at least three times, with three replicates each. The viability was evaluated based on a comparison with untreated cells. The IC50 values represented the sample’s concentrations required to inhibit 50% of cell proliferation and were calculated from a calibration curve by linear regression using Microsoft Excel [26] as follows: for each sample, the cell growth percentages at the different concentrations were used to draw the calibration curve with logarithmic regression obtained by 2 measuring points (one smaller and one larger than 50%) from where the IC50 value was deduced.
Cell cycle analysis and detection of apoptosis
The effect of compounds 9 and 13 and doxorubicin (positive control) in cell cycle distribution of MCF-7 cells was performed by flow cytometry using BD cycletest™ Plus DNA Kit Assay (BD Biosciences, San Jose, USA) as previously described [23]. Cells were tested in 6-well plates (3 mL, 1 × 105 cells/mL) and the incubation time was 72 h in humidified 5% CO2 atmosphere at 37 °C. The tested concentrations were ¼ × IC50, ½ × IC50 and IC50. Untreated cells (control) were used for comparison with treated cells. The BD FACS Aria I Cell Sorter Flow Cytometer (Becton-Dickinson, Germany) was then used for cell cycle analysis. For each sample, 104 cells were counted. For PI excitation, an argon-ion laser emitting at 488 nm was used. Cytographs were analyzed using BD FACSDiva™ Flow Cytometry Software Version 6.1.2 (Becton-Dickinson).
Caspase activity
Compounds 9 and 13 as well as doxorubicin (positive control) were used to treat MCF-7 cells for 6 h, followed by detection of caspase activity using Caspase-Glo 3/7 and Caspase-Glo 9 Assay kits (Promega, Mannheim, Germany) as previously reported [27,28,29]. Cells were incubated in humidified 5% CO2 atmosphere at 37 °C; the concentrations of samples used were ½ × IC50 and IC50 meanwhile DMSO was used as solvent control. The BioTek Synergy™ HT multi-detection microplate reader was used to measure the luminescence and Caspase activity was expressed as percentage of the untreated control.
Analysis of the integrity of mitochondrial membrane
The MCF-7 cells were treated with compounds 9 and 13 and doxorubicin, and the integrity of MMP was analyzed using 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1; Biomol, Hamburg, Germany) staining as previously reported [27,28,29]. Cells were tested in 6-well plates (3 mL, 1 × 105 cells/mL) and the incubation time was 72 h in humidified 5% CO2 atmosphere at 37 °C. The tested concentrations were ¼ × IC50, ½ × IC50 and IC50. Untreated cells (control) were used for comparison with treated cells. JC-1 staining was performed according to the manufacturer’s protocol as reported previously [23]. Cells were then measured in a BD FACS Aria I Cell Sorter Flow Cytometer (Becton-Dickinson, Germany). The JC-1 signal was measured at an excitation wavelength of 561 nm (150 mW) and detected using a 586/15 nm band-pass filter. The signal was analyzed at an excitation wavelength of 640 nm (40 mW) and detected using a 730/45 nm bandpass filter. Cytographs were analyzed using BD FACSDiva™ Flow Cytometry Software Version 6.1.2 (Becton-Dickinson). All experiments were performed at least in triplicates.
Measurement of reactive oxygen species production
The MCF-7 cells were treated with compounds 9 and 13 and doxorubicin, and ROS production was measured using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFH-DA) (Sigma-Aldrich) by OxiSelect™ Intracellular ROS Assay Kit (Green Fluorescence) as recommended by the manufacturer, Cell Biolabs Inc. (San Diego, USA) [23]. Cells were tested in 6-well plates (3 mL, 1 × 105 cells/mL) and the incubation time was 24 h in humidified 5% CO2 atmosphere at 37 °C. The tested concentrations were ¼ × IC50, ½ × IC50 and IC50. Untreated cells (control) were used for comparison with treated cells. The fluorescence was measured using SpectraMax® M5 Microplate Reader (Molecular Devices, Biberach, Germany) at 480/530 nm. All experiments were performed at least in triplicates.
Results
Tested compounds
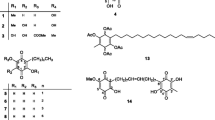
Compounds tested (Fig. 1) were five terpenoids including one steroid: β-spinasterol (1) and 4 triterpenoids: friedelanone (2), 16β-hydroxylupeol (3), β-amyrin acetate (4) and lupeol acetate (5), one cyclitol: 5-O-methyl-myo-inositol or sequoyitol (6), 3 flavonoids: rhamnitrin or 7-O-methylquercetin 3-O-rhamnoside (7) and europetin 3-O-rhamnoside or 7-O-methylmyricetin 3-O-rhamnoside (8) and thonningiol (9); one isoflavonid: glyasperin F (10), three pterocarpan isoflavonoids: seputhecarpan B (11), seputhecarpan C C21H20O5 (12) and seputhecarpan D (13) and one xanthone: rheediaxanthone A (14). The isolation and identification of compounds 2, 3 and 14 from Garcinia epunctata [10] and 5, 9–13 from Ptycholobium contortum [11, 12] were previously reported. Compounds 1, 4, 6, 7 and 8 were isolated from the bark, leaves and roots of Synsepalum zenkeri (Sapotaceae). Further details on the tested compounds are available in (Additional file 1: S2).
Chemical structures of tested compounds. 1: β-spinasterol; 2: friedelanone; 3: 16β-hydroxylupeol; 4: β-amyrin acetate; 5: 5-O-methyl-myo-inositol or sequoyitol; 6: lupeol acetate; 7: rhamnitrin or 7-O-methylquercetin 3-O-rhamnoside; 8: europetin 3-O-rhamnoside or 7-O-methylmyricetin 3-O-rhamnoside; 9: thonningiol; 10: glyasperin F; 11: seputhecarpan B; 12: seputhecarpan C; 13: seputhecarpan D; 14: rheediaxanthone A
Cytotoxicity
Compounds 3, 9–13 displayed antiproliferative effects against the four tested cancer cell lines with C50 values below 85 μM (Table 1). Other compounds showed selective cytotoxicity. The IC50 values varied from 9.12 μM (in MCF-7 breast adeconcarcinoma cells) to 35.64 μM (towards HepG2 hepatocarcinoma cells) for compound 3, from 0.36 μM (against MCF-7 cells) to 5.65 μM (towards colon carcinoma DLD-1 cells) for 9, from 11.14 μM (against MCF-7 cells) to 20.89 μM (towards DLD-1 colon carcinoma cells) for 10, from 11.92 μM (against MCF-7 cells) to 84.41 μM (against Caco-2 colon carcinoma cells) for 11, from 13.30 μM (in MCF-7 cells) to 76.72 μM (against HepG2 cells) for 12, from 9.78 μM (against MCF-7 cells) to 67.68 μM (against HepG2 cells) for 13 and 0.18 μM (towards HepG2 cells) to 72 μM (towards Caco-2 cells) for doxorubicin. Compounds 1–14 were in all cases less toxic in normal CRL2120 fibroblasts than in cancer cells, with IC50 values above 45 μM (Table 1). Compounds 9 and 13 had IC50 values below 10 μM in 4/4 and 3/4 tested cell lines respectively.
Cell cycle distribution and modes of action
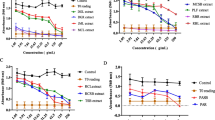
The MCF-7 cells’ cycle distribution after treatments with compound 9 and 13 and doxorubicin was analyzed, and the results are depicted in Fig. 2. The two phytochemicals as well as doxorubicin altered the distribution of various phases of cell cycle in MCF-7 cells, with pronounced concentration-dependent increase of cells in sub-G0/G1 phase. Compounds 9 and 13 induced cell cycle arrest in Go/G1 whilst the reference molecule, doxorubicin induced arrest in G2/M. As indication of apoptosis, percentages of cells in sub-G0/G1 phase were in the ranges of 11.6% (¼ × IC50) to 28.3% (2 × IC50) for 9, 7.6% (¼ × IC50) to 31.1% (2 × IC50) for 13, and from 27.6% (¼ × IC50) to 60% (2 × IC50) for the reference drug, doxorubicin. The percentage of cells in sub-G0/G1 phase in non-treated cells was 3.1%.
The activity of caspases in MCF-7 cells treated with compounds 9, 13 and doxorubicin are depicted in Fig. 3. Thonningiol (9), seputhecarpan D (13) as well as doxorubicin activated caspases 3/7 and 9 in MCF-7 cells, with optimal activities observed generally in cells treated with IC50. The increases were 3.97-fold, 4.66-fold and 2.02-fold for caspases 3/7 and 2.67-fold, 3.47-fold and 1.3-fold for caspase 9 respectively for compounds 9, 13 and doxorubicin.
The effects of compounds 9, 13 and doxorubicin in the integrity of MMP in MCF-7 cells are summarized in Fig. 4. Phytochemicals 9 (11.2%) and 13 (12.9%) slightly altered the MMP in MCF-7 cells, contrary to doxorubicin that induced up to 26.0% depletion at IC50.
The production of ROS in MCF-7 cells treated with compounds 9, 13 and doxorubicin is shown in Fig. 5. Thonningiol (9), seputhecarpan D (13) and doxorubicin induced increase in ROS levels respectively by 4.46-fold, 3.78-fold and 2.40-fold at IC50 as compared to non-treated cells.
Discussion
In the present investigation, we assessed the ability of 14 phytochemicals from African medicinal plants to prevent the proliferation of four cancer cell lines namely breast, colon, lung and liver cells. They are amongst the frequently diagnosed cancers globally [3]. The threshold recognized for a good phytochemical is the IC50 values around or below 4 μg/mL or 10 μM as defined by the National Cancer Institute (NCI) [8, 30, 31]. Amongst the 14 tested compounds, six (3, 9–13) had recordable IC50 values in all the four tested cancer cell lines.
To the best of our knowledge, the cytotoxicity of compounds 9 and 11 is being reported for the first time here. The triterpenoid16β-hydroxylupeol (3) is a hydroxylated derivative of the known cytotoxic compound, lupeol. In effect, the cytotoxicity of lupeol has been reported in several cell lines including colorectal cancer, gastric cancer or liver cancer cells [32,33,34,35]. The cytotoxicity of compound 3 is therefore in accordance with published literature and this work provides more information on the activity of lupeol derivatives. The cytotoxicity of compounds 10, 12 and 13 was reported against A549 and SPC-212 lung cancer cells [12]. The present work brings additional data on the cytotoxic potential of these compounds. In this study, IC50 values below 10 μM were obtained with compounds 9 and 13 in 4/4 and 3/4 tested cell lines respectively. This is an indication that these two molecules have promising cytotoxic potential.
Cysteine-aspartic proteases commonly known as caspases are protease enzymes essential for programmed cell death and inflammation [36]. In this study, it was found that phytochemicals 9, 13 as well as doxorubicin induce apoptotic cell death in MCF-7 cells with increase in caspases 3/7 and 9 activities (Figs. 2 and 3). These data indicate that activation of caspases is one of the modes of action of compounds 9 and 13. Flavonol such as isorhamnetin was also shown to induce apoptosis in cancer cells by activation of caspases [37, 38], consolidating the results obtained with compound 9 belonging to the class of dihydroflavonol. However, isorhamnetin rather exerted cell cycle arrest in G2/M in HCT116 colon cancer cells contrary to compound 9 that exerted arrest in G0/G1 as observed in this study [38]. Also, pterocarpan isoflavonoids such as sophorapterocarpan A and isoneorautenol previously induced apoptosis in CCRF-CEM leukemia cells through activation of caspases 3/7, 8 and 9 as well as the loss of MMP and increased ROS production [15, 16]. In this study, it was also found that phytochemicals 9 and 13 slightly induced MMP alteration but enhanced ROS production in MCF-7 cells (Figs. 4 and 5). The activation of caspases 3/7 (effector caspases) and 9 (initiator caspases) (Fig. 3) as well as the low alteration of MMP, probably due to the low concentrations of compounds tested (¼ × IC50, ½ × IC50 and IC50), is an indication that intrinsic mitochondrial pathway could be involved in the cytotoxic effect of compounds 9 and 13 [39]. Mitochondria play a central role in cellular metabolism as main ATP source, and during ATP biosynthesis, ROS are generated. Mitochondria-targeting compounds kill cancer cells due to their ability to initiate mitochondrial outer membrane permeabilization [9, 40]. This also indicates that ROS production is another mode of apoptotic cell death induced these two phytochemicals.
Regarding the structure-activity relationship, it appears that the best spectra of activity were achieved with triterpenoid 3, flavonol 9 and the 4 tested isoflavonoids 10–13. Five terpenoids including one steroid (1) and four trierpenoids (2–5) were tested. The steroid 1 as well as triterpenoids 2, 4 and 5 had low and selective cytotoxic effects (Table 1). Only triterpenoid 3 with two hydroxyl (-OH) group in C3 and C16 was active towards the four tested cancer cell lines. Hydroxylation of triterpenoids therefore seems to increase the cytotoxic effect. However, steroid 1 with only one –OH group in C3 had poor activity. Flavonoids tested herein included glycosylated flavonols 7 and 8 as well as non glycosylated flavonol 9. Amongst them, compounds 7 and 8 had low and selective cytotoxicity, with a recordable IC50 values obtained only towards MCF-7 cells. In contrast, dihydroflavonol 9 had significant cytotoxic effect (IC50 values below 10 μM) in 4/4 tested cancer cell lines (Table 1). It can therefore be deduced that glycosylation of flavonoids in C3 significantly reduces their cytotoxic effect. The four tested isoflavonoids (10–13) were active in all cancer cell lines. Amongst them, pterocarpan 13 had the best activity with IC50 values below 10 μM against 3/4 cancer cells. Contrary to pterocarpans 11 and 12, pterocarpan 13 do not have an additional furo- cycle; this is an indication that additional furo- cycle of pterocarpans reduces the degree of activity. Between pterocarpans 11 and 12, the substitution of 4’-OH by 4’-OCH3 seems not to significantly influence their activity (Table 1). The only tested xanthone (14) displayed selective cytotoxicity. However, this effect was significant towards Caco-2 cells (IC50 value of 3.15 μM).
Conclusions
The present study highlights the good potential of the African flora as a source of cytotoxic phytochemicals to combat malignant diseases. Thonningiol (9) and seputhecarpan D (13) are good antiproliferative molecules that should be explored more in future to develop a cytotoxic drug to fight human carcinoma. Dihydroflavonol 9 and pterocarpan 13 induced apoptosis in MCF-7 cells through activation activator caspase 9 and effector caspases 3/7 as well as enhanced ROS production.
Abbreviations
- 1:
-
β-spinasterol
- 10 :
-
glyasperin F
- 11 :
-
seputhecarpan B
- 12 :
-
seputhecarpan C
- 13 :
-
seputhecarpan D
- 14 :
-
rheediaxanthone A
- 2 :
-
friedelanone
- 3 :
-
16β-hydroxylupeol
- 4 :
-
β-amyrin acetate
- 5 :
-
5-O-methyl-myo-inositol or sequoyitol
- 6 :
-
lupeol acetate
- 7 :
-
rhamnitrin or 7-O-methylquercetin 3-O-rhamnoside
- 8 :
-
europetin 3-O-rhamnoside or 7-O-methylmyricetin 3-O-rhamnoside
- 9 :
-
thonningiol
- CH2Cl2 :
-
dichloromethane
- CHCl3 :
-
chloroform
- DMSO:
-
dimethylsufoxide
- EtOAc:
-
ethyl acetate
- H2DCFH-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- MeOH :
-
methanol
- MMP:
-
mitochondrial membrane potential
- NR:
-
neutral red
- ROS:
-
reactive oxygen species
- TLC:
-
thin layer chromatography
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. International Agency for Research on Cancer. In: GLOBOCAN 2012 v10, cancer incidence and mortality worldwide: IARC CancerBase no 11 globocaniarcfr; 2013. Accessed December 12, 2013.
Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–45.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36(3):237–48.
Basri DF, Alamin ZAZ, Chan KM. Assessment of cytotoxicity and genotoxicity of stem bark extracts from Canarium odontophyllum Miq. (dabai) against HCT 116 human colorectal cancer cell line. BMC Complement Altern Med. 2016;16(1):36.
Niu H, Wang J, Li H, He P. Rapamycin potentiates cytotoxicity by docetaxel possibly through downregulation of Survivin in lung cancer cells. J Exp Clin Cancer Res. 2011;30(1):28.
Qiao Y, Xiang Q, Yuan L, Xu L, Liu Z, Liu X. Herbacetin induces apoptosis in HepG2 cells: involvements of ROS and PI3K/Akt pathway. Food Chem Toxicol. 2013;51:426–33.
Kuete V, Efferth T. African flora has the potential to fight multidrug resistance of cancer. Biomed Res Int. 2015;2015:914813.
Mbaveng AT, Kuete V, Efferth T. Potential of central, eastern and western Africa medicinal plants for cancer therapy: spotlight on resistant cells and molecular targets. Front Pharmacol. 2017;8:343.
Fotso GW, Ntumy AN, Ngachussi E, Duber M, Mapitse R, Kapche DGFW, et al. Epunctanone, a new benzophenone, and further secondary metabolites from Garcinia epunctata Stapf (Guttiferae). Acta Chim Helvet. 2014;97:957–64.
Fotso GW, Maher FA, Ngnintedo D, Ango PY, Kapche DGFW, Ngameni B, et al. Three new isoflavonoids with antioxidant properties from Ptycholobium contortum (N.E.Br.) Brummitt (Leguminosae). Phytochem Lett. 2015;14:254–9.
Ngnintedo D, Fotso GW, Kuete V, Nana F, Sandjo LP, Karaosmanoglu O, et al. Two new pterocarpans and a new pyrone derivative with cytotoxic activities from Ptycholobium contortum (N.E.Br.) Brummitt (Leguminosae): revised NMR assignment of mundulea lactone. Chem Cent J. 2016;10:58.
Kuete V, Ngameni B, Wiench B, Krusche B, Horwedel C, Ngadjui BT, et al. Cytotoxicity and mode of action of four naturally occuring flavonoids from the genus Dorstenia: gancaonin Q, 4-hydroxylonchocarpin, 6-prenylapigenin, and 6,8-diprenyleriodictyol. Planta Med. 2011;77(18):1984–9.
Kuete V, Mbaveng AT, Zeino M, Fozing CD, Ngameni B, Kapche GD, et al. Cytotoxicity of three naturally occurring flavonoid derived compounds (artocarpesin, cycloartocarpesin and isobavachalcone) towards multi-factorial drug-resistant cancer cells. Phytomedicine. 2015;22(12):1096–102.
Kuete V, Sandjo LP, Djeussi DE, Zeino M, Kwamou GM, Ngadjui B, et al. Cytotoxic flavonoids and isoflavonoids from Erythrina sigmoidea towards multi-factorial drug resistant cancer cells. Investig New Drugs. 2014;32:1053–62.
Kuete V, Sandjo LP, Kwamou GM, Wiench B, Nkengfack AE, Efferth T. Activity of three cytotoxic isoflavonoids from Erythrina Excelsa and Erythrina Senegalensis (neobavaisoflavone, sigmoidin H and isoneorautenol) toward multi-factorial drug resistant cancer cells. Phytomedicine. 2014;21(5):682–8.
Okoye NN, Ajaghaku DL, Okeke HN, Ilodigwe EE, Nworu CS, Okoye FB. Beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm Biol. 2014;52(11):1478–86.
Wandji J, Tillequin F, Mulholland DA, Wansi JD, Fomum TZ, Fuendjiep V, et al. Fatty acid esters of triterpenoids and steroid glycosides from Gambeya africana. Planta Med. 2002;68(9):822–6.
Shen JC, Chien-Kuang C, Shoei-Sheng L. Polar constituents from Sageretiathea leaf characterized by HPLC-SPE-NMR assisted approaches. J Chin Chem Soc. 2009;56:1002–9.
Rasoanaivo LH, Wadouachi A, Andriamampianina TT, Andriamalala SG, Razafindrakoto EJB, Raharisololalao A, et al. Triterpenes and steroids from the stem bark of Gambey aboiviniana Pierre. J Pharmacogn Phytochem. 2014;3:68–72.
Borenfreund E, Babich H, Martin-Alguacil N. Comparisons of two in vitro cytotoxicity assays-the neutral red (NR) and tetrazolium MTT tests. Toxicol in Vitro. 1988;2(1):1–6.
Sivas H, Tomsuk Ö. Antiproliferative and apoptotic effects of the essential oil of Origanum Onites and carvacrol on Hep-G2 cells. Anadolu Univ J Scie Technol-C Life Sci Biotechnol. 2011;1(2):171–80.
Kuete V, Omosa LK, Tala VR, Midiwo JO, Mbaveng AT, Swaleh S, et al. Cytotoxicity of plumbagin, rapanone and 12 other naturally occurring quinones from Kenyan flora towards human carcinoma cells. BMC Pharmacol Toxicol. 2016;17(1):60.
Kuete V, Dongmo Mafodong FL, Celik I, Fobofou SAT, Ndontsa BL, Karaosmanoğlu O, et al. In vitro cytotoxicity of compounds isolated from Desbordesia glaucescens against human carcinoma cell lines. S Afr J Bot. 2017;111:37–43.
Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3(7):1125–31.
Kuete V, Krusche B, Youns M, Voukeng I, Fankam AG, Tankeo S, Lacmata S, Efferth T. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J Ethnopharmacol. 2011;134(3):803–12.
Kuete V, Sandjo L, Nantchouang Ouete J, Fouotsa H, Wiench B, Efferth T. Cytotoxicity and modes of action of three naturally occuring xanthones (8-hydroxycudraxanthone G, morusignin I and cudraxanthone I) against sensitive and multidrug-resistant cancer cell lines. Phytomedicine. 2013;21(3):315–22.
Kuete V, Fankam AG, Wiench B, Efferth T. Cytotoxicity and modes of action of the methanol extracts of six Cameroonian medicinal plants against multidrug-mesistant tumor cells. Evid Based Complement Alternat Med. 2013;2013:285903.
Kuete V, Tankeo SB, Saeed ME, Wiench B, Tane P, Efferth T. Cytotoxicity and modes of action of five Cameroonian medicinal plants against multi-factorial drug resistance of tumor cells. J Ethnopharmacol. 2014;153(1):207–19.
Boik J. Natural compounds in cancer therapy. Minnesota USA: Oregon Medical Press; 2001.
Brahemi G, Kona FR, Fiasella A, Buac D, Soukupova J, Brancale A, Burger AM, Westwell AD. Exploring the structural requirements for inhibition of the ubiquitin E3 ligase breast cancer associated protein 2 (BCA2) as a treatment for breast cancer. J Med Chem. 2010;53(7):2757–65.
Zhang L, Zhang Y, Yang X, Lv Z. Lupeol, a dietary triterpene, inhibited growth, and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Investig. 2009;27(2):163–70.
He Y, Liu F, Zhang L, Wu Y, Hu B, Zhang Y, Li Y, Liu H. Growth inhibition and apoptosis induced by lupeol, a dietary triterpene, in human hepatocellular carcinoma cells. Biol Pharm Bull. 2011;34(4):517–22.
Tarapore RS, Siddiqui IA, Adhami VM, Spiegelman VS, Mukhtar H. The dietary terpene lupeol targets colorectal cancer cells with constitutively active Wnt/beta-catenin signaling. Mol Nutr Food Res. 2013;57(11):1950–8.
Wu XT, Liu JQ, Lu XT, Chen FX, Zhou ZH, Wang T, Zhu SP, Fei SJ. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int Immunopharmacol. 2013;16(2):332–40.
Galluzzi L, Lopez-Soto A, Kumar S, Kroemer G. Caspases connect cell-death signaling to organismal homeostasis. Immunity. 2016;44(2):221–31.
Lee HJ, Lee EO, Ko SG, Bae HS, Kim CH, Ahn KS, Lu J, Kim SH. Mitochondria-cytochrome C-caspase-9 cascade mediates isorhamnetin-induced apoptosis. Cancer Lett. 2008;270(2):342–53.
Jaramillo S, Lopez S, Varela LM, Rodriguez-Arcos R, Jimenez A, Abia R, Guillen R, Muriana FJ. The flavonol isorhamnetin exhibits cytotoxic effects on human colon cancer cells. J Agric Food Chem. 2010;58(20):10869–75.
Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171.
Fulda S, Kroemer G. Mitochondria as therapeutic targets for the treatment of malignant disease. Antioxid Redox Signal. 2011;15:2937–49.
Kuete V, Efferth T. Cameroonian medicinal plants: pharmacology and derived natural products. Front Pharmacol. 2010;1:123.
Acknowledgments
Authors are grateful to Şennur Görgülü for flow cytometry measurements.
Funding
The study was funded by the Scientific and Technological Research Counsel of Turkey (TÜBİTAK) and to Scientific Research Projects Commission of Anadolu University, Eskisehir, Turkey (funding grant 1507F563). A grant for part of this work was also provided by International Science Programme, Uppsala University, Sweden (ISP)-KEN-02 project through the Network for Analytical and Bioassay Services in Africa (NABSA) at the University of Botswana.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
VK, DN, GWF and OK carried out the experiments; VK wrote the manuscript. VK, BTN, FK, KA-M and HS designed the experiments; HS and SOY supervised the work, provided the facilities for the study. All authors read the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
VK, DN, GWF and OK carried out the experiments; VK wrote the manuscript. VK, BTN, FK, KA-M and HS designed the experiments; HS and SOY supervised the work, provided the facilities for the study. All authors read the manuscript and approved the final version.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
S1. Details on extraction and isolation of compounds (1, 4, 6, 7, 8) from Synsepalum zenkeri; S2. Further details on tested compounds. (DOCX 24 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kuete, V., Ngnintedo, D., Fotso, G.W. et al. Cytotoxicity of seputhecarpan D, thonningiol and 12 other phytochemicals from African flora towards human carcinoma cells. BMC Complement Altern Med 18, 36 (2018). https://doi.org/10.1186/s12906-018-2109-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-018-2109-9