Abstract
Background
The potential harm of medicinal herbs has been recently observed following herbal toxicity studies after ingestion of polyherbal remedies. This was the rationale for the food and drug regulatory agency decision for thorough safety evaluation of herbal medicines. Androgenic, antipyretic, analgesic and anti-inflammatory potentials as well as chemical compositions of extracts of massularia acuminata, terminalia ivorensis, anogeissus leiocarpus and macuna pruriens respectively have been documented. Thus, Bon-santé cleanser® (BSC) is formulated from these medicinal plants with the intention to boost body hormones and energizes the body. Considering the wide usage of BSC, we investigated on its safety in male Wistar rats.
Methods
Thirty-two male Wistar rats weighing 201.9 ± 7.5 g were grouped into four treatment groups of eight per group. Group I, (control) received distilled water (10 ml/kg). Groups II-IV received 250 mg/kg, 500 mg/kg and 1000 mg/kg of BSC per oral respectively. Each group was treated for sixty days.
Results
Acute toxicity test, in male Wistar albino mice, showed that LD50 was 600 mg/kg via i.p. while 4 g/kg was nonlethal after oral administration in mice. Hepatic and renal biomarker enzymes were unaltered in all rats. Increased in PCV (p <0.05) was observed at 500 mg/kg. BSC modulates antioxidants biomarkers following sub-chronic administration and increased serum Na+ (p >0.05). BSC at 1000 mg/kg caused mild inflammation of the liver and heart but not kidneys histologically.
Conclusions
BSC has been found to be relatively safe in Wistar rats. Although, our findings indicate that herbal therapy with BSC should be done with caution as a mild alteration in the liver and heart architectures were observed.
Similar content being viewed by others
Background
Up to now, Africa still provides a non-negligible contribution to overall complementary and alternative medicines despite the fact that traditional medical practitioners have been using crude drug extracts for several years [1–4]. Thus, there is rationale in investigating herbal toxicity within the African health care system [5, 6], given that a sizable part of our population regularly use them. Since the majority of these products have not been rigorously screened, scientific studies on their uses and toxicological profile will open up new vistas of rational usage of these herbal preparations [5, 7, 8].
Bon-santé cleanser® (BSC) is a marketed polyherbal formula manufactured by Dabiron Natural Life Care in Nigeria. BSC comprises of different constituents as follows: anogeissus leiocarpus (DC., family Combretaceae), terminalia ivorensis (A. Chev.), massularia acuminata (G.Don,) Bullock ex Hoyle and macuna pruriens (L.,) DC (fabaceae). formulated into capsule. It is interesting to note that the androgenic, antipyretic, analgesic and anti-inflammatory potentials of the extracts of the aforementioned medicinal plants have been documented [9–16]. And recently, Oriola et al. [17] reported a new bioactive thiophenolic glycoside from the leaf of M. acuminata in favour of the antibacterial activities [18] and androgenic potentials [19] as well as pro-sexual effects [16]. Ponou et al. [20, 21] demonstrated the present of a dimeric antioxidant and cytotoxic triterpenoid saponins from T. ivorensis and have also synthesized a novel 3‐Oxo‐and 3, 24‐Dinor‐2, 4‐secooleanane‐Type triterpenes from this plant. Olugbami et al. [22] using an in vitro study have also unveiled the antioxidant potential, phenolic and flavonoid contents of extract of A. leiocarpus while Amadu et al. [23] reported the cytotoxic activity of the same against Ehrlich Ascites Carcinoma cells. Study by Hamzaoui et al. [24] has successfully shown efficient fractionated yields that contained triterpenes, ellagic acid derivatives, flavonoids and phenolic compounds from A. leiocarpus. Another recognition strategy based on 13C NMR used by Hubert et al. [25] achieved seven constituents natural metabolites in a crude A. leiocarpus. In addition, Josephine and Janardhanan [26] and Perumal et al. [27] have dissected separately the proximate composition, seed protein fractions, amino acid composition, minerals and anti-nutritional factors in M. pruriens with high contents of crude protein and crude lipids. Interestingly, aside L-3,4-dihydroxyphenylalanine, M. pruriens was found to be rich in minerals such as K, Mg and P. [26, 28].
The androgenic potential of aqueous extract of massularia acuminata was reported in male rats [10, 16]. Similarly, Shuklaet al. [11] demonstrated improved male fertility of macuna pruriens by its action on the hypothalamus-pituitary-gonadal axis. More so, anogeissus leiocarpus and T. ivorensis were explored and verified for their local uses as antipyretics, analgesics and anti-inflammatory effects in rodents [9, 12–14]. A. leiocarpus also showed endothelium-independent and endothelium-dependent vasorelaxation action [15]. The pharmacological activities of M. acuminata, T. ivorensis, A. leiocarpus and M. pruriens have been adduced to be due to the presence of glycosides [16], dimeric antioxidants [20, 21], phenolics [22] and flavones respectively [26, 27]. Considering the wide usage of BSC, coupled with the warning by the National Agency for Food and Drug Administration and Control (NAFDAC) that it has not been evaluated, we investigated on its safety in male Wistar rats.
Methods
Chemicals and drugs
The study was carried out in the Department of Pharmacology, University of Lagos, Lagos Nigeria. Bon-santé cleanser® was obtained from Dabiron Natural Life Care, Nigeria. Thiobarbituric acid (TBA), Ellman’s reagent (DTNB) and 1-Chloro-2, 4,- dinitrobenzene (CDBN) were purchased from Sigma Chemical Company (USA). Reduced glutathione (GSH), Metaphosphoric acid and Trichloroacetic acid (TCA) were purchased from J.I. Baker (USA). Bovine serum albumin fraction V (BSA) was purchased from SRL, India. All other chemicals and reagents used were of analytical grade.
Method of extraction and preparation of the final formulation
BSC was obtained directly from the Dabiron Natural Life Care in Nigeria. It was assigned Batch number 002 and listed with number A7-5321 L by the National Agency for Food and Drug Administration and Control (NAFDAC). BSC contains Anogeissus leiocarpus (DC., family Combretaceae) Guill & Perr., Terminalia ivorensis (A. Chev., family Combretaceae), Massularia acuminata (G.Don, family Rubiaceae) Bullock ex Hoyle and Macuna pruriens (L., family leguminosae) DC. in the ratio 4:2:1:1 respectively. The extraction and formulation procedures complied with the regulatory manual of NAFDAC. In this context, a single capsule of BSC (total content 442 mg) was dissolved in distilled water (80 mg/ml) which was administered via oral gavage according to standard toxicological guidelines.
Animals
Albino rats of the Wistar strain weighing between 150–300 g were purchased from the animal house of the Redeemers University, Ogun State, Nigeria. The rats were housed under controlled conditions in the experimental animal handling facility of the College of Medicine, University of Lagos, Nigeria. The experimental animal room had a 12 h light/12 h dark schedule and maintained at a temperature of 22 ± 3 °C throughout the study. Animals were fed with commercially available rat pelleted diet (Ladoke Akintola Growers Mash, Nigeria) and were allowed access to water ad libitum throughout the period of the experiment. The experimental protocols were approved by the Institutional Animal Care and Use Committee, Department of Pharmacology, Therapeutic and Toxicology, College of Medicine, University of Lagos. Animals were certified fit for the experiment by the Institution’s Animal Health Officers before the commencement of the study. Beddings were changed on alternate days and the animals were sacrificed in a humane manner at the end of the experiment by cervical dislocation. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the U. S. National Institutes of Health (NIH Publication No. 85-23, revised 1996)” for studies involving experimental animals and the procedures as documented by Kilkenny et al. [29] for reporting animal research.
Experimental design
Acute oral toxicity test
Thirty (30) male Wistar mice (average weight, 20 g) in separate cages were randomly divided into six groups and allowed to acclimatize for two weeks. Graded doses of BSC of 250, 500, 1000, 2000 and 4000 mg/kg were administered to the animals orally and were allowed access to water ad libitum. The control group was administered 0.1 ml distilled water orally. The Mice were observed for 24 h post-treatment for mortality, behavioral changes including hyperactivity, attempting circular movement, leaning on hind limbs, increased feeding habit, scratching of lower jaw immediately after treatment and 2 h after treatment, writings, and agitation. All the animals were further monitored for 14 days post administration.
Acute intraperitoneal toxicity test
Thirty (30) male mice (average weight, 20 g) placed in separate cages were randomly divided into five groups. Graded doses of BSC of 200, 400, 800 and 1600 mg/kg were administered intraperitoneally. The control group was administered 0.1 ml of distilled water. The Mice were observed 24 h post-treatment for mortality, behavioural changes, and signs of toxicity. At 200 mg/kg (i.p.) of BSC, animals showed agitation, climbing, gripping, hyperphagia, leaning on hind limbs and circular movement. Animals that received doses of 400 mg/kg and above showed signs of restlessness, weakness, flattened abdomen, and tears after 2 h of administration. It was observed that at about 6th–10th hour, body weight reduced and most animals shivered. Mortality was observed and recorded for each group of animals following 24 h of administration. All animals were further monitored for 14 days post administration. The Median Lethal Dose was estimated according to the method of Finney [30].
Subchronic study
Thirty-Two (32) adult male Wistar rats weighing between 150–300 g were divided into 4 groups of 8 rats per group. Group I, the control group, received 10 ml/kg of distilled water daily. The treated animals received only BSC. Groups II, III and IV were administered 250 mg/kg, 500 mg/kg and 1000 mg/kg of BSC respectively. The rats were weighed weekly throughout the course of the experiment. The animals were closely observed for behavioural changes as aforementioned.
Collection of blood samples and tissues for assays
Sub-chronic administration of BSC in experimental rats was for 60 days. Twenty-four (24) hours following the last administration, blood samples were obtained by ocular puncture into bottles treated with anticoagulants which were either lithium heparin or ethylenediaminetetraacetic acid (EDTA). The animals were sacrificed by cervical dislocation. The anticoagulated blood samples were centrifuged at 4200 rpm for 5 min to separate out the plasma from which all biochemical assays were carried out. The liver, kidney, heart and brain were all harvested, weighed and were homogenized in four volumes of buffer solution (0.1 M, pH 7.4). A portion of each organ was taken out for histological studies. The remaining was weighed and homogenized for biochemical assays.
Biochemical assays
Liver and renal function tests
Plasma levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined following the principle described by Reitman and Frankel [31] while the alkaline phosphates (ALP) was carried out according to the method described by Roy [32] to assess liver function. Renal function was assessed by measuring plasma creatinine (CREA) levels and blood urea nitrogen (BUN) was assayed following the method of Fossati et al. [33] and Skegg’s [34].
Proteins and uric acid
In order to assess the synthetic function of the liver, total serum protein (TP) and albumin (ALB) concentrations were determined according to the principles based on the Biuret reaction [35] and bromocresol green reaction [36] respectively. Uric acid (UA) levels were determined according to the methods of Fossati et al. [37].
Lipid profiles
Total Serum cholesterol (TC) and Triglyceride (TG) concentrations were estimated following the method described by Trinder [38] by using commercial kits obtained from Randox Laboratories Ltd (Crumlin, UK). High-Density Lipoprotein (HDL) was estimated according to Warnick and Albers [39] while serum low-density lipoprotein (LDL) was calculated using Freidewald formula [40].
Antioxidants and oxidative stress
The method of Beutler et al. [41] was followed in estimating the level of reduced glutathione (GSH). Glutathione-S-transferase (GST) activity was determined according to Habig et al. [42]. The level of superoxide dismutase (SOD) activity was determined by the method of Misra and Fridovich [43]. Catalase activity was determined according to the method of Sinha [44]. Lipid peroxidation was determined by measuring the formation of thiobarbituric acid reactive substances (TBARS) according to the method of Varshney and Kale [45].
Hematological assessments
Packed Cells Volume (PCV), Hemoglobin (Hb), Platelet (PLT), Total White Blood Cell Count (TWBC) were determined using a fully automated haematology analyzer (Pentra-XL 80, Horiba ABX, USA).
Serum electrolytes
Sodium ion (Na+), Potassium ion (K+), Chloride ion (Cl−), total Calcium ion (tCa2+) and intracellular Calcium ion (iCa2+) were determined in serum using a Flame Photometer (Sherwood, Model 410).
Histological assessment
The tissue samples from the liver, kidney and heart for histological examination were passed through the process of fixation, dehydration, clearing, infiltration, embedding, sectioning and staining. To ensure good fixation, the tissues were trimmed to about 5 mm thickness, so as to obtain good fixation. The tissues were then fixed in 10 % formol saline and were then transferred to 50 % alcohol (70 %, 80 %, 85 %, 95 and 100 %), for two hours. Alcohol was removed from the treated tissues by titrating them through first an equal mixture 100 % (absolute) alcohol and xylene for one hour each in that order. Infiltration was carried out twice by passing each tissue through molten paraffin wax in an oven at a temperature of 30 °C for one and a half hours each. The tissues so embedded in molten paraffin wax were later placed on a wooden block and trimmed to size. Serial sections 10μm thick were made using a rotatory microtome. The cut sections were then floated in a warm water bath at a temperature of 30 - 40 °C and were placed slides. Eight sections were obtained from each treated organ from each animal. Four samples were placed on each slide. Microscopic examination was done by using varying magnifications of 10, 40, 100 and 400 to determine if the samples were properly fixed on the slides. Following staining, mounting of sections was carried out using dimethyl paraffinate xylene (DPX) as a mounting agent, after which microscopic examination was done.
Statistical analysis of data
All data were expressed as mean ± standard error of mean (SEM). Significant differences among the group were determined by one-way analysis of variance (ANOVA) and Post hoc testing was performed for inter-group comparisons for least significant difference (LSD) [46] using the statistical analysis programme for social sciences (SPSS). Graphs were plotted using GraphPad Prism 6. Results were considered to be significant at p ≤ 0.05.
Results
Acute oral toxicity test
Table 1 shows the results of acute oral toxicity testing of BSC administered at graded doses of 250, 500, 1000, 2000 and 4000 mg/kg respectively. The animals showed no mortality at the doses given. There were also no toxic changes observed with 250 mg/kg while doses of 500 mg/kg and above manifested reduced locomotion, mild scratching of the lower jaw and initial slight hyperactivity followed by weakness in treated mice when compared with control group in first two hours post treatment.
Acute intraperitoneal toxicity test
Table 2 shows the acute i.p. toxicity testing of BSC. Here, we observed mortality or toxic changes in mice administered with BSC at doses of 200, 400, 800 and 1600 mg/kg respectively. At 200 mg/kg (i.p.) of BSC, animals showed signs of agitation, climbing, gripping, hyperphagia, leaning on hind limbs, and circular movements. Doses of 400 mg/kg and above showed signs of restlessness, weakness, flattened the abdomen, tears, and increased shallow breathing after 2 h of administration. And at about 6th–10th hour, most animals shivered. We obtained a mean lethal dose (LD50) of 600 mg/kg.
Liver function
Table 3 shows that the levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) did not change significantly (p >0.05) when compared with the control group. BSC at lowest dose (250 mg/kg) reduced ALT and ALP levels respectively. Similarly, there was statistically insignificant (p >0.05) dose dependent increase in AST levels when administration BSC was administered at 250 mg/kg (32.4 %), 500 mg/kg (39.2 %) and 1000 mg/kg (51.4 %). ALT levels were insignificantly (p >0.05) elevated at doses of 500 mg/kg (41.4 %) and 1000 mg/kg (29.3 %) respectively.
Lipid profiles
BSC shows a slight reduction (p >0.05) in serum TG levels by 11.6 %, 9.8 % and 1.5 % respectively, although, the lowest dose of BSC (250 mg/kg) slightly elevated serum TC levels (Table 4). Serum low density lipoprotein (LDL) was insignificantly (p >0.05) lowered by 14.7 % and 4.6 % at doses of 250 mg/kg and 500 mg/kg. BSC (1000 mg/kg) slightly elevated LDL by 28.5 % (p >0.05).
Renal function, protein synthesis and uric acid
Blood Urea Nitrogen levels was increased by 11.8 %(p >0.05) and 13.7 %(p >0.05) at 500 mg/kg and 1000 mg/kg respectively as shown in Table 5. BSC (250 mg/kg) also elevated Creatinine levels by 7.1 % (p >0.05), but, did not significant alter serum albumin (ALB), total protein (TP) and UA respectively in all subjects. But, BSC doses of 500 mg/kg and 1000 mg/kg, produced insignificantly (p >0.05) elevated levels of ALB, TP, and UA in the treated rats.
Haematological assessments
BSC shows elevated packed cell volumes at 250 mg/kg (p >0.05) and 500 mg/kg (p <0.05) respectively (Table 6). Similarly, heamoglobin levels were insignificantly (p >0.05) when compared with control as well. BSC increased TWBC at 250 mg/kg by 62.6 % (p <0.05) and platelets count by 50.6 % (p >0.05) when administered at 500 mg/kg.
Serum electrolytes
BSC causes a dose related insignificantly increased Na+ following oral administration from 250 mg/kg through 1000 mg/kg (Table 7). iCa2+ levels was decreased at 250 mg/kg(p >0.05) while it increased(p >0.05) at the middle and highest doses respectively. The serum K+, Cl− and tCa2+ levels were unaltered in animals.
Tissue proteins
Figure 1, BSC produces an increased in the levels of hepatic TP, although, insignificantly (p >0.05). No difference was observed in renal, brain and heart TP levels respectively in all subjects when compared with control group (p >0.05).
Lipid peroxidation
BSC at doses of 250 mg/kg, 500 mg/kg and 1000 mg/kg shows reduced (P >0.05) hepatic and brain LPO (Malondialdehyde, MDA) (Fig. 2). Similarly, low (250 mg/kg) and highest (1000 mg/kg) doses also reduced renal MDA (P >0.05). BSC (500 mg/kg) shows slight elevation (P >0.05) in MDA levels. Both 250 mg/kg and 500 mg/kg doses elevated MDA levels in heart, although, insignificantly when compared with control (p >0.05).
Tissue anti-oxidants
Hepatic antioxidants
BSC shows at low (250 mg/kg) and highest (1000 mg/kg) doses an increased (p <0.05) hepatic GSH levels by 68 % and 49.2 % respectively (Fig. 3). No observable statistical difference in GST, superoxide dismutase and the control. But, a 1000 mg/kg of BSC indicated elevated hepatic CAT levels by 54.1 % (p <0.05).
Effects of Bon-santé cleanser® (BSC) on hepatic antioxidants in normal male rats. Result expressed as mean ± SEM. DW: Distilled water (10 ml/kg); GSH: Reduced Glutathione (μmol/mg protein); CAT: Catalase (μmol/ml/mg protein); SOD: Superoxide Dismutase Activity (μmol/min/mg protein); GST: Glutathione-S-Transferase (μmol/ml/min)
Renal antioxidants
There were increased GSH levels in the kidneys at highest BSC dose (1000 mg/kg) used in this present study (Fig. 4). However, renal GST levels decreased given all administered doses of BSC. Only the lowest dose (250 mg/kg) shows increased CAT levels. On the other, both 250 mg/kg and 500 mg/kg elevated renal SOD levels but insignificant (p >0.05).
Effects of Bon-santé cleanser® (BSC) on renal antioxidants in normal male rats. Result expressed as mean ± SEM. * p <0.05 when compared with control group. DW: Distilled water (10 ml/kg); GSH: Reduced Glutathione (μmol/mg protein); CAT: Catalase (μmol/ml/mg protein); SOD: Superoxide Dismutase Activity (μmol/min/mg protein); GST: Glutathione-S-Transferase (μmol/ml/min)
Heart antioxidants
BSC highest dose (1000 mg/kg) produces elevated heart GSH levels by 9.7 % and decreases (p <0.05) heart GST levels in treated rats by 54 % (Fig. 5). In addition, CAT levels increased in the heart at 500 mg/kg and 1000 mg/kg (p >0.05). There were decreased (p <0.05) SOD levels in the heart of treated rats, in a dose related manner by 30.1 % (250 mg/kg), 40.5 % (500 mg/kg) and 39.1 % (1000 mg/kg) respectively.
Effects of Bon-santé cleanser® (BSC) on heart antioxidants in normal male rats. Result expressed as mean ± SEM. # p <0.05 and * p <0.001 when compared with control group. DW: Distilled water (10 ml/kg); GSH: Reduced Glutathione (μmol/mg protein); CAT: Catalase (μmol/ml/mg protein); SOD: Superoxide Dismutase Activity (μmol/min/mg protein); GST: Glutathione-S-Transferase (μmol/ml/min)
Brain antioxidants
An administration of BSC at doses used in this study produces a dose dependent increased (p <0.05) in brain GSH levels by 18.8 %, 96.4 % and 212.3 % respectively (Fig. 6). More so, BSC at 250 mg/kg shows a decreased brain GST levels in the treated animals (p <0.05). Brain CAT levels was also elevated (p <0.05) given 500 mg/kg and 1000 mg/kg respectively. However, there was a decrease (p >0.05) in brain SOD levels in the treated rats.
Effects of Bon-santé cleanser® (BSC) on brain antioxidants in normal male rats. Result expressed as mean ± SEM. * p <0.05 when compared with control group. DW: Distilled water (10 ml/kg); GSH: Reduced Glutathione (μmol/mg protein); CAT: Catalase (μmol/ml/mg protein); SOD: Superoxide Dismutase Activity (μmol/min/mg protein); GST: Glutathione-S-Transferase (μmol/ml/min)
Body and organ system weights
The effects of sub-chronic administration of BSC on body weights in male rats administered with 250 mg/kg and 1000 mg/kg were not significantly (p >0.05) different from those of control groups (Fig. 7). However, only 500 mg/kg of BSC ably increased (p <0.05) body weights. And similarly, a 1000 mg/kg reduced (p <0.05) heart as well.
Histological assessments
Control group (Distilled Water, 10 ml/kg)
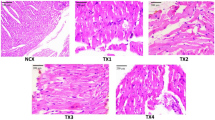
Figure 8: Control (Distilled Water, 10 ml/kg, A) and BSC (250 mg/kg, B; 500 mg/kg, C) showed normal heart. However, BSC (1000 mg/kg, D) shows dense aggregates inflammatory heart (see arrow). (H & E stain, Mag. X400). (BSC = Bon-santé cleanser®).
BSC (250 mg/kg)
Figure 9: Control (Distilled Water, 10 ml/kg, E) and BSC (250 mg/kg, F; 500 mg/kg, G; 1000 mg/kg, H) showed normal kidney (H & E stain, Mag. X400). (BSC = Bon-santé cleanser®).
BSC (500 mg/kg)
Figure 10: Control (Distilled Water, 10 ml/kg, I). BSC (250 mg/kg, J; 500 mg/kg, K) showed normal liver. But, BSC (1000 mg/kg, L) shows congestion of the hepatic sinusoids and the central vein (see arrow). (H & E stain, Mag. X400). (BSC = Bon-santé cleanser®).
Discussions
There is a new global interest in the use of alternative means of maintaining and healing sick people [47]. The WHO fact sheet (No.134) estimates that about 80 % of the populations in African and Asian countries rely on traditional medicine for their primary healthcare [48]. It also recognizes traditional medicine as ‘an accessible, affordable and culturally acceptable form of healthcare trusted by large numbers of people, which stands out as a way of coping with the relentless rise of diseases in the midst of soaring health-care costs and nearly universal austerity [47]. There are many reasons for this new phenomenon. The first is that many of the producers describe multifaceted great healing properties to these alternative means [5, 6, 49]. The second is that they propose that these therapies are natural and so have less or little side effects [5, 50]. Finally because of poverty and the inability to pay for some of the western medicines that have verifiable efficacies many patients turn to herbal medicines. Unfortunately, there are evidence to show that the majority of these herbal medicines have not been as well screened for efficacy and toxicity when compared to their western counterparts [1, 51]. As toxicity studies and scientific investigations into these herbal remedies are often expensive [52], it is now left to the scientific community to investigate some of the claims of the manufacturers of these herbal preparations to verify or refute their claims. This is an important public health service that must not be overlooked if it is really true that academics must contribute to safeguarding the health of our people.
In view of the foregoing, scientific investigations of alternative means to healthcare are now focusing on safety and toxicological evaluation of herbal formulation [53–55]. Our group decided to study BSC for the following reasons. BSC contains A. lerocarroa, T. accularia, M. accuminata and M. pruiens formulated into a capsule. Several authors at different times have contributed to the elucidation of the chemical compositions of the individual component of the popular BSC. Also, The chemical compositions and hypotheses on folklore medicine uses of these plants have been well documented [17–21, 23–28, 56]. However, some investigators have shown that there is potential to augment the individual toxicities in herbal preparations when mixed [5, 6, 8, 55, 57–59]. To the best of our knowledge, not much is known in the literature with respect to the toxicity of the components of the herbs contained in the BSC capsule. Thus, there is rational in carrying out toxicological studies on this herbal preparation as it is on the open market, and the manufacturers claim they have many clients. Therefore, we investigated the Safety evaluation of BSC when ingested orally in male Wistar rats.
The determination of acute toxicity testing is usually a step ab inicio in the assessment and evaluation of the toxic characteristics of a substance [60]. Our results show that when BSC is given orally, the herbal preparation was relatively safe as there was no mortality during the acute toxicity tests. This is an important finding as the major route of administration of herbal medicines is oral. The acute oral and intraperitoneal LD50 in mice were >4000 mg/kg and 600 mg/kg respectively.
Nevertheless, there were small but statistically insignificant changes in serum liver and renal enzymes as well as serum electrolytes (Tables 3, 5 and 7). Whether this will later translate into outright hepatic and renal damage with the chronic administration is very hard to tell. One important outcome of this study is the increase in PCV (Table 6) and decrease in LDL (Table 4) with oral administration. While these results may look exciting, it is too early to determine whether BSC could be deployed as a lipid modulating remedy either on its own or as an adjunct to well-known compounds that carry out this role in the health system. No histological changes following a subchronic oral administration were seen on the kidneys unlike mild to moderate alterations that were observed in the liver and heart when the highest dose (1000 mg/kg) was administered (Figs. 8, 9 and 10). However, the limitation of our study lies in the duration since a chronic toxicity evaluation of BSC would be necessary in order to comment on the long-term outcomes.
Since there have been suggestions that botanicals, when used alone or in combination with other medications, could precipitate severe organ system toxicity [61]. Our observation is that treatment with different doses of BSC did not result in predictable changes with graded doses (Tables 3, 4, 5, 6, and 7 & Figs. 1, 2, 3, 4, 5, and 6). Thus, there is a lot of cautions to be expressed in humans as our study indicate altered levels of hepatic biomarkers and some antioxidants, though, it does not pinpoint the part of departure between dose and biochemical outcomes. More so, there was a small improvement in lipid profile at low doses. Thus, at this stage, it could be suggested that further observations be made in humans who have consumed the medicine in the past to verify its usefulness and comments on side effects.
Conclusion
BSC has been found to be relatively safe in Wistar rats. Although, our findings indicate that herbal therapy with BSC should be done with caution as a mild alteration in the liver and heart architectures were observed.
Abbreviations
BSC, Bon-santé cleanser®; ED50, Mean Effective Dose; LD50, Mean Lethal Dose
References
Lindamulage IK, Soysa P. Evaluation of anticancer properties of a decoction containing Adenanthera pavonina L. and Thespesia populnea L. BMC Complem and Altern Med. 2016;16:1:1.
Press I. Problems in the definition and classification of medical systems. Soc Sci Med B. 1980;1;14(1):45–57.
Sahoo N, Manchikanti P, Dey S. Herbal drugs: Standards and regulation. Fitoterapia. 2010;81:462–71.
Still J. Use of animal products in traditional Chinese medicine: environmental impact and health hazards. Complem Therapy in Med. 2003;11:118–22.
Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontier Pharmacol. 2014;4:177.
Chan K. Understanding interactions between Chinese medicines and pharmaceutical drugs in integrative healthcare. Chinese J of Integrative Med. 2015;21(2):83–9.
Wickramasinghe MB. Modern Phytomedicine. In: Ahmad I, Aqil F, Owais M, editors. Turning Medicinal Plants into Drugs, vol. 22. 2006. p. 2.
Zhan S, Guo W, Shao Q, Fan X, Li Z, Cheng Y. A pharmacokinetic and pharmacodynamic study of drug-drug interaction between ginsenoside Rg1, ginsenoside Rb1 and schizandrin after intravenous administration to rats. J Ethnopharmacol. 2014;152(2):333–9.
Iwu MM, Anyanwu BN. Phytotherapeutic profile of Nigerian herbs. I: Anti-inflammatory and anti-arthritic agents. J Ethnopharmacol. 1982;6(3):263–74.
Yakubu MT, Akanji MA, Oladiji AT, Adesokan AA. Androgenic potentials of aqueous extract of Massularia acuminata (G. Don) Bullock ex Hoyl. stem in male Wistar rats. J Ethnopharmacol. 2008;118(3):508–13.
Shukla KK, Mahdi AA, Ahmad MK, Shankhwar SN, Rajender S, Jaiswar SP. Mucuna pruriens improves male fertility by its action on the hypothalamus-pituitary-gonadal axis. Fertil Steril. 2009;92(6):1934–40.
Akanbi OM, Omonkhua AA, Cyril-Olutayo CM, Fasimoye RY. The antiplasmodial activity of Anogeissus leiocarpus and its effect on oxidative stress and lipid profile in mice infected with Plasmodium bergheii. Parasitol Res. 2012;110(1):219–26.
Obogwu MB, Akindele AJ, Adeyemi OO. Hepatoprotective and in vivo antioxidant activities of the hydroethanolic leaf extract of Mucuna pruriens (Fabaceae) in antitubercular drugs and alcohol models. Chin J Nat Med. 2014;12(4):273–83.
Adeoluwa OA, Aderibigbe AO, Agu GO, Adewole FA, Eduviere AT. Neurobehavioural and Analgesic Properties of Ethanol Bark Extract of Terminalia ivorensis A Chev. (Combrataceae) in Mice. Drug research. 2015;65(10):545–51.
Belemnaba L, Ouedraogo S, Auger C, Chataigneau T, Traore A, Guissou IP, Lugnier, C, Schini-Kerth, VB, Bucher B. Endothelium-independent and endothelium-dependent vasorelaxation by a dichloromethane fraction from Anogeissus Leiocarpus (DC) Guill. Et Perr. (Combretaceae): possible involvement of cyclic nucleotide phosphodiesterase inhibition. Afr J Tradit Complement Altern Med. 2012;10(2):173–9.
Yakubu MT, Akanji MA. Effect of aqueous extract of Massularia acuminata stem on sexual behaviour of male Wistar rats. Evidence-Based Compl Altern Med. 2011;2:2011.
Oriola AO, Aladesanmi AJ, Idowu TO, Akinkunmi EO, Obuotor EM, Ogunsina MO. A new bioactive thiophenolic glycoside from the leaf of Massularia acuminata (g. Don Bullock) ex Hoyle (Rubiaceae). African J Trad and Compl Alternative Med. 2014;11(2):319–23.
Taiwo O, Xu HX, Lee SF. Antibacterial activities of extracts from Nigerian chewing sticks. Phytotherapy Res. 1999;13(8):675–9.
Yakubu MT, Awotunde OS, Ajiboye TO, Oladiji AT, Akanji MA. Pro-sexual effects of aqueous extracts of Massularia acuminata root in male Wistar rats. Andrologia. 2011;43(5):334–40.
Ponou BK, Teponno, RB, Ricciutelli M, Quassinti L, Bramucci M, Lupidi G, Barboni, L, Tapondjou LA. Dimeric antioxidant and cytotoxic triterpenoid saponins from Terminalia ivorensis A. Chev. Phys Chem Chem Phys. 2010;71(17–18):2108–15.
Ponou BK, Teponno RB, Ricciutelli M, Nguelefack TB, Quassinti L, Bramucci M, Lupidi G, Barboni L, Tapondjou LA. Novel 3‐Oxo‐and 3, 24‐Dinor‐2, 4‐secooleanane‐Type Triterpenes from Terminalia ivorensis A. Chev. Chem & biodiversity. 2011;8(7):1301–9.
Olugbami JO, Gbadegesin MA, Odunola OA. In vitro evaluation of the antioxidant potential, phenolic and flavonoid contents of the stem bark ethanol extract of Anogeissus leiocarpus. African J Med and Medical Sc. 2014;43 Suppl 1:101.
Amadu KS, Musa TY, Adenike TO. Cytotoxic activity of aqueous extracts of Anogeissus leiocarpusand Terminalia avicennioides root barks against Ehrlich Ascites Carcinoma cells. Indian J Pharmacol. 2013;45(4):381–5.
Hamzaoui M, Renault JH, Nuzillard JM, Reynaud R, Hubert J. Stepwise Elution of a Three‐phase Solvent System in Centrifugal Partition Extraction: A New Strategy for the Fractionation and Phytochemical Screening of a Crude Bark Extract. Phytochem Analysis. 2013;24(4):367–73.
Hubert J, Nuzillard JM, Purson S, Hamzaoui M, Borie N, Reynaud R, Renault JH. Identification of natural metabolites in mixture: a pattern recognition strategy based on 13C NMR. Analyt chemistry. 2014;86(6):2955–62.
Josephine RM, Janardhanan K. Studies on chemical composition and antinutritional factors in three germplasm seed materials of the tribal pulse, Mucuna pruriens (L.) DC. Food Chem. 1992;43(1):13–8.
Siddhuraju P, Vijayakumari K, Janardhanan K. Chemical Composition and Protein Quality of the Little-Knon Legume, Velvet Bean (Mucuna pruriens (L.) DC.). J Agric Food Chem. 1996;44(9):2636–41.
Ramya KB, Thaakur S. Herbs containing L-Dopa: an update. Anc Sci Life. 2007;27(1):50.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010;8(6):e1000412.
Finney DJ. The Median Lethal Dose and Its Estimation. Archives of Toxicol. 1985;56:215–8.
Reitman S, Frankel SA. Colorimetric method for the determination of serum glutamate-oxaloacetate and pyruvate transaminases. Am J Clin Pathol. 1970;28:56–63.
Roy AV. Rapid method for determining alkaline phosphatase activity in serum with thymolphthalein monophosphate. Clin. Chem. 1970;16(5):431–6.
Fossati P, Prencipe L, Bert G. Use of 3,5-dichloro- 2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in indirect enzymatic assay of uric acid in serum and urine. Clin Chem. 1980;26:227–31.
Skeggs LT. An automatic method for colorimetric analysis. American J Clin. Pathol. 1957;28(3):311–22.
Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the Biuret reaction. J Biol Chem. 1949;177:751.
Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromocresol green reaction. Clin Chem. 1971;22:616–22.
Fossati P, Prencipe L, Berti G. Enzymic creatinine assay: a new colorimetric method based on hydrogen peroxide measurement. Clin. Chem. 1983;29(8):1494–6.
Trinder P. Quantitative determination of triglyceride using GPO-PAP method. Annals Clin Biochem. 1969;6:24–7.
Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8.
Habig WJ, Pabst MJ, Jacoby WB. Glutathione-S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9.
Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5.
Sinha A. Colorimetric assay of catalase. Analytical Biochem. 1971;47:389–95.
Varshney R, Kale RK. Effect of calmodulin antagonist on radiation induced lipid peroxidation in microsomes. Inter J Radiation Biol. 1990;58(5):733–43.
Levine G. A guide to SPSS for analysis of variance. Hillsdale, NJ: Lawrence Erlbaum Associates Inc., Publishers; 1991. p. 65–7.
Mosihuzzaman M, Choudhary MI. Protocols on safety, efficacy, standardization, and documentation of herbal medicine (IUPAC Technical Report). Pure and App Chem. 2008;80(10):2195–230.
Feijen-de Jong EI, Jansen DE, Baarveld F, Spelten E, Schellevis F, Reijneveld SA. Determinants of use of care provided by complementary and alternative health care practitioners to pregnant women in primary midwifery care: a prospective cohort study. BMC Pregnancy Childbirth. 2015;15(1):1.
Van AT, Mitchell S, Volpato G, Vandebroek I, Swier J, Ruysschaert S, Renteria JC, Raes N. In search of the perfect aphrodisiac: parallel use of bitter tonics in West Africa and the Caribbean. J Ethnopharmaco. 2012;143(3):840–50.
Gilbert N. Regulations: Herbal medicine rule book. Nature. 2011;480(7378):S98–9.
Govindaraghavan S, Sucher NJ. Quality assessment of medicinal herbs and their extracts: Criteria and prerequisites for consistent safety and efficacy of herbal medicines. Epilepsy and Behav. 2015;S1525–5050(15):00109–2.
Musthaba M, Baboota S, Athar TM, Thajudeen KY, Ahmed S, Ali J. Patented herbal formulations and their therapeutic applications. Recent Pat Drug Deliv Form. 2010;4(3):231–44.
Lee MY, Shin IS, Seo CS, Kim JH, Han SR, Shin HK. Sub chronic oral toxicity studies of the traditional herbal formular Bangpungtongseong-san in Crl: CD (SD) rats. J Ethnopharmaco. 2012;144:720–5.
Edorh MS, Agbere S, Osei-Safo D, Adam Z, Agbonon A, Karou DS, Agbere RA, Gbeassor M. Toxicological screening of Daouri, a polyherbal formulation used in children in the Central Region of Togo. J Ethnopharmaco. 2015;S0378-8741(15):00060–4.
Zainulabedin MS, Krishanu S, Alluri VK, Golakoti T, Francis CL, James PL. Safety and toxicological evaluation of Meratri®: An herbal formulation for weight management. Food Chem Toxicol. 2015;78:122–9.
Nwafor PA, Bassey AI. Evaluation of anti-diarrhoeal and anti-ulcerogenic potential of ethanol extract of Carpolobia lutea leaves in rodents. J Ethnopharmacol. 2007;111(3):619–24.
Sahoo HB, Nandy S, Senapati AK, Sarangi SP, Sahoo SK. Aphrodisiac activity of polyherbal formulation in experimental models on male rats. Pharmacog Res. 2014;6(2):120–6.
Yang Y, Zhang Z, Li S, Ye X, Li X, He K. Synergy effects of herb extracts: pharmacokinetics and pharmacodynamic basis. Fitoterapia. 2014;92:133–47.
Creton S, Dewhurst IC, Earl LK, Gehen SC, Guest RL, Hotchkiss JA, Indans I, Woolhiser MR, Billington R. Acute toxicity testing of chemicals-opportunities to avoid redundant testing and use alternative approaches. Crit Rev Toxicol. 2010;1;40(1):50–83.
Ernst E. Risks of herbal medicinal products. Pharmacoepidem drug safety. 2004;13:767–71.
Steinsbekk A, Rise MB, Johnsen R. Changes among male and female visitors to practitioners of complementary and alternative medicine in a large adult Norwegian population from 1997 to 2008 (The HUNT studies). BMC Compl Altern Med. 2011;11:1:1.
Acknowledgements
The authors’ acknowledged Prof. Adefule-Ositelu A.O. of the Guinness Eye Center, Lagos University Teaching Hospital, Idi-Araba Lagos, Nigeria for her enthusiasm in herbal medicines research and suggestion towards investigating this product. We also acknowledged the manufacturer of Bon-Sante Cleanser®, Dabiron Natural Life Care Nigeria for permission to investigate the safety profile. We also acknowledged Professor Oladapo Walker for the expert review and English editing of this manuscript.
Funding
This research was solely funded by AO and KOE.
Availability of data and materials
The results reported in this study were those directly obtained from this study.
Authors’ contributions
AO designed and coordinated all laboratory experiments. KOE conducted all experiments, statistical analysis, drafted the manuscript and both authors interpreted the results. AO and KOE read and approved the manuscript.
Competing interests
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare that they have no competing interests.
Consent for publication
Authors gave their consent to the publication of this manuscript.
Ethics approval and consent to participate
This study was approved by the University of Lagos, Nigeria ethical committee on the use of animals, and conformed to the Guide for the Care and Use of Laboratory Animals published by the U. S. National Institutes of Health (NIH Publication No. 85-23, revised 1996)” for studies involving experimental animals.
Declarations
The research was self-funded and a full-waiver for publication was granted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kale, O.E., Awodele, O. Safety evaluation of Bon-santé cleanser® polyherbal in male Wistar rats. BMC Complement Altern Med 16, 188 (2016). https://doi.org/10.1186/s12906-016-1188-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-016-1188-8