Abstract
Pseudo-Meigs syndrome is a rare syndrome characterized by hydrothorax and ascites associated with pelvic masses, and patients occasionally present with elevated serum cancer antigen-125 (CA125) levels. Hydropic leiomyoma (HLM) is an uncommon subtype of uterine leiomyoma characterized by hydropic degeneration and secondary cystic changes. Rapidly enlarging HLMs accompanied by hydrothorax, ascites, and elevated CA125 levels may be misdiagnosed as malignant tumors. Here, we report a case of HLM in a 45-year-old Chinese woman who presented with ascites and hydrothorax. Preoperative abdominopelvic CT revealed a giant solid mass in the fundus uteri measuring 20 × 15 × 12 cm. Her serum CA125 level was elevated to 247.7 U/ml, while her hydrothorax CA125 level was 304.60 U/ml. The patient was initially diagnosed with uterine malignancy and underwent total abdominal hysterectomy and adhesiolysis. Pathological examination confirmed the presence of a uterine hydropic leiomyoma with cystic changes. After tumor removal, the ascites and hydrothorax subsided quickly, with no evidence of recurrence. The patient’s serum CA125 level decreased to 116.90 U/mL on Day 7 and 5.6 U/mL on Day 40 postsurgery. Follow-up data were obtained at 6 months, 1 year, and 2 years after surgery, and no recurrence of ascites or hydrothorax was observed. This case highlights the importance of accurate diagnosis and appropriate management of HLM to achieve successful outcomes.
Similar content being viewed by others
Introduction
Pseudo-Meigs syndrome is a rare condition characterized by ascites and hydrothorax, along with masses in the abdomen and pelvis, similar to Meigs syndrome, which is usually associated with ovarian fibromas or fibroid tumors. Surgical removal of the tumor leads to the rapid resolution of symptoms and a good outcome [1]. Tumors linked to pseudo-Meigs syndrome include uterine cancer [2], metastatic cancer [3], ovarian struma ovarii [4], and uterine leiomyoma [5]. Uterine leiomyoma is the most common benign tumor of the female reproductive system, and the diagnostic methods for these tumors include ultrasound examination, transvaginal ultrasound examination, computed tomography (CT), and magnetic resonance imaging (MRI). The clinical behavior and imaging features of leiomyomas can vary, especially in those with degenerative changes, which make it difficult to distinguish them from malignant tumors in the uterus or ovaries. Pseudo-Meigs syndrome caused by uterine leiomyoma in patients with elevated CA125 levels is extremely rare, with only a few cases reported in the literature, and this syndrome be mistaken for ovarian cancer or uterine sarcoma. Accurate diagnosis is crucial for appropriate treatment. We present the case of a 45-year-old Chinese woman with a rapidly growing pelvic mass, ascites, hydrothorax, and elevated CA125 levels who was initially misdiagnosed with a malignancy. Pathological examination revealed a large uterine leiomyoma with hydropic degeneration and cystic changes. This case highlights the importance of recognizing pseudo-Meigs syndrome in patients with elevated CA125 levels caused by uterine leiomyoma and emphasizes the need for accurate diagnosis, appropriate treatment, and an improved understanding of patient prognosis.
Case report
A 45-year-old Chinese woman presented with complaints of abdominal distension and intermittent abdominal pain. She was admitted to the gynecology department of our hospital. Her medical and family history was unremarkable. Menarche occurred at the age of 13 years, and her menstrual cycles were regular. An abdominopelvic MRI revealed a solid mass measuring 7.3 × 6.1 × 6.9 cm in the fundus uteri. The patient was discharged without intervention. Eight months later, the patient returned to the gynecology department with complaints of abdominal pain, discomfort in the lower-abdominal region, and vaginal bleeding.
Physical examination revealed the presence of a large, solid mass on the right side of the lower abdomen, with no other abnormalities detected. Palpation revealed tenderness in the area of the mass, which filled the pelvic cavity. An abdominopelvic CT scan revealed a cystic solid mass located in the posterior wall of the uterus. The mass measured 14.7 × 11.3 × 12.7 cm in size and was accompanied by a significant amount of ascites (Figs. 1 and 2). The solid component demonstrated clear and uniform enhancement on enhanced scanning, in contrast to the low-density area. A chest CT scan revealed the presence of a right hydrothorax. The initial diagnosis favored an ovarian tumor or leiomyoma with degeneration. Prior to surgery, 6 L of hydrothorax fluid and 6 L of ascites fluid were collected for laboratory examination and cytology. The results revealed elevated levels of CA125 in both the serum and hydrothorax, with values of 304.60 U/mL and 247.7 U/mL, respectively (normal values < 35 U/mL). The levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, progesterone, prolactin, testosterone II, total protein, albumin, lactate dehydrogenase (LDH), and carcinoembryonic antigen (CEA) were within the normal ranges in all the samples. Cytology was negative for malignant cells or Mycobacterium tuberculosis.
Contrast-enhanced CT (coronal view) revealed a giant mass measuring 14 × 12 × 10 cm in size in the pelvis, with heterogeneous and intense enhancement, a scattered low-density edema area, and a cyst in the center (blue arrow), and massive ascites and a right-sided hydrothorax were observed (red arrow)
According to imaging tests and laboratory analyses, the patient was diagnosed with either ovarian cancer or leiomyosarcoma before surgery. Total abdominal hysterectomy was performed, along with excision of the mass and adhesiolysis. Intraoperative observations revealed a distinct subserosal solid tumor arising from the uterine fundus and extending into the pelvic cavity, accompanied by a moderate quantity of light-red ascites.
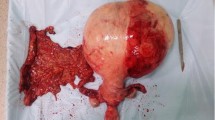
The tumor measured 20 × 15 × 12 cm on gross examination. The cut surface of the tumor exhibited a pale and fleshy appearance, with evidence of hydropic and cystic degeneration. No evidence of hemorrhage or necrosis was observed (Fig. 3). Microscopic examination revealed that the tumor demonstrated the typical characteristics of a leiomyoma, with no evidence of myometrial or vascular invasion. The tumor cells demonstrated mild cytologic atypia, with no mitoses or necrosis present, and were surrounded by edema or a watery matrix (Figs. 4 and 5). Immunohistochemical staining revealed diffuse positivity for h-Caldesmon (Fig. 6) and desmin but negativity for HMB45, Melan-A, CD10, P16, and P53. The Ki67 index was less than 1%. A pathological diagnosis of hydropic leiomyoma was established on the basis of these findings.
After tumor removal, the hydrothorax and ascites subsided rapidly. A follow-up was conducted at 6 months, 1 year, and 2 years postsurgery. The patient recovered uneventfully. A final diagnosis of pseudo-Meigs syndrome was made.
The patient provided written informed consent for the publication of this case report and accompanying images.
Discussion
In 1937, ovarian fibroma accompanied by ascites and hydrothorax was first described by Meigs and Cass. In 1954, Meigs reviewed the cases of 84 patients who presented with rapid resolution of ascites and/or hydrothorax following the removal of ovarian fibromas or fibroma-like tumors. These patients were subsequently diagnosed with Meigs syndrome [6]. Pseudo-Meigs syndrome, similar to Meigs syndrome, is frequently associated with ovarian cancer, metastatic tumors, mature teratoma, struma ovarii, borderline tumors, and uterine leiomyoma [7, 8]. The clinical features of pseudo-Meigs syndrome include the presence of a large pelvic mass, recurrent ascites and hydrothorax. The presence of elevated CA125 levels in patient with pseudo-Meigs syndrome can result in the misdiagnosis of malignancy with pelvic and pleural metastases, resulting in unnecessary treatment. Patients with pseudo-Meigs syndrome who have elevated CA125 levels caused by uterine leiomyoma are exceedingly rare and are likely to be misdiagnosed [1]. This report describes a case of pseudo-Meigs syndrome caused by a rapidly enlarging uterine hydropic leiomyoma in a patient with elevated CA125 levels, and a review of the relevant literature is provided.
The etiology of ascites and hydrothorax remains unclear, with several hypotheses proposed for the development of ascites: (1) Mechanical irritation of the peritoneum by a large tumor may lead to pelvic inflammation and fluid accumulation [6, 9, 10]; (2) fluid secretion due to lymphatic obstruction, increased permeability of the neovasculature from tumor pedicle torsion, and low serum protein levels may occur [11,12,13]; and (3) cytokines and inflammatory mediators may promote the production of ascites, with studies demonstrating that elevated serum vascular endothelial growth factor (VEGF) is associated with ascites and hydrothorax [14]. Furthermore, cytokines such as VEGF, fibroblast growth factor (FGF), and interleukin (IL)-6 have been demonstrated to stimulate mesothelial cells and promote ascites and hydrothorax in patients with Meigs’ syndrome or pseudo-Meigs syndrome, with levels returning to normal following tumor removal [15]. Further research has revealed the pivotal role of VEGF, FGF, IL-1β, IL-6, and IL-8 in the development of ascites and hydrothorax [16]. In patients with pseudo-Meigs syndrome, right-sided hydrothorax is more common due to developmental defects in the right hemidiaphragm, typically occurring approximately 3 h after ascites formation and potentially originating from pelvic effusions [11].
The occurrence of Meigs syndrome or pseudo-Meigs syndrome in conjunction with elevated CA125 levels is relatively uncommon, and the underlying mechanism remains unclear [8, 17]. CA125, also known as MUC16, is a glycoprotein that was first identified in 1981 [18]. CA125 is not considered a reliable marker for the diagnosis of malignancy because of its low sensitivity and specificity. Elevated CA125 levels can be observed in individuals with various benign and malignant conditions, such as pregnancy, pelvic inflammatory disease, endometriosis, tuberculosis, cirrhosis, and autoimmune diseases [18, 19]. The CA125 present in ascites may be produced by activated mesothelial cells, whose expression is potentially upregulated by proinflammatory cytokines [20].
Pseudo-Meigs syndrome, caused by leiomyomas in patients with elevated CA125, is a rarer condition. A total of 24 cases have been documented in the English literature (Table 1). Uterine leiomyomas are the most common benign tumors of the female reproductive system, with an incidence rate of approximately 70% [21]. Approximately 50% of uterine leiomyomas undergo degenerative changes, including myxoid degeneration, hyalinosis, calcification, steatosis, red degeneration, watery degeneration, and cystic degeneration. These changes can be precipitated by pregnancy or hormonal therapy. Although degeneration of uterine leiomyomas can lead to rapid tumor enlargement, infiltration or metastasis is not observed [22]. Edema leiomyoma or hydropic leiomyoma (HLM) is a rare subtype characterized by diffuse watery degeneration and secondary cystic changes [23]. Such lesions may undergo rapid enlargement and may be misdiagnosed as leiomyosarcomas or ovarian malignancies. HLMs display characteristics distinct from those of typical leiomyomas, including differences in tumor size, edema area, extracellular matrix composition, vascular proliferation, and molecular genetic changes. Studies have demonstrated that t(4;8)(p16;q22) is a unique abnormality found in individuals with HLMs [24]. Additionally, overexpression of the HMGA2 gene, HMGA2 gene rearrangement, and increased pAKT activity have been identified in HLM patients, which strongly suggests a distinct association with HMGA2 overexpression [25].
As previously stated, uterine leiomyoma is a rare cause of pseudo-Meigs syndrome. Accurate diagnosis prior to surgical intervention can be challenging, particularly when the tumor exhibits rapid enlargement with water degeneration and elevated levels of CA125. A review of reported cases in the English literature revealed that the majority of patients present with symptoms such as abdominal swelling or distension and dyspnea. Imaging modalities such as CT, MRI, and ultrasound lack the capacity to differentiate between benign and malignant tumors, particularly in cases of degeneration. Consequently, there is a high likelihood of misdiagnosis as an ovarian or uterine malignancy, despite the tumor having a favorable prognosis. In patients with pseudo-Meigs syndrome and elevated CA125 levels, an accurate diagnosis is crucial for determining appropriate treatment. To achieve an accurate diagnosis, it is essential to perform a preoperative cytological analysis of ascites or hydrothorax, as well as a pathological examination via frozen section during surgery, particularly in patients with rapidly enlarging tumors and elevated CA125 levels.
Conclusion
In this case report, we describe a rare case of pseudo-Meigs syndrome in a patient with elevated CA125 levels caused by a rapidly enlarging hydropic leiomyoma. This condition is frequently misdiagnosed as ovarian cancer or uterine malignancy, and a comprehensive differential diagnosis encompassing both benign and malignant tumors is essential. A definitive diagnosis may be made intraoperatively through pathological frozen section analysis. In cases of pseudo-Meigs syndrome, patients generally have a favorable prognosis following resection of the pelvic mass. The available literature indicates that the majority of patients do not exhibit any signs of hydrothorax or ascites during follow-up assessments, which range from 10 days to 8 months postoperatively. At the two-year follow-up, the patient in our case remained free of hydrothorax and ascites.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Dunn JS, Anderson CD, Method MW, Brost BC. Hydropic degenerating leiomyoma presenting as Pseudo-meigs syndrome with elevated CA 125. Obstet Gynecol. 1998;92(4 Pt 2):648–9.
Okazaki A, Nishi K, Kasahara K. Pseudo-meigs syndrome caused by cancer of the uterine corpus. Am J Obstet Gynecol. 2019;221:71–2.
Feldman ED, Hughes MS, Stratton P, Schrump DS, Alexander HR. Pseudo-meigs’ syndrome secondary to isolated colorectal metastasis to ovary: a case report and review of the literature. Gynecol Oncol. 2004;93:248–51.
Loizzi V, Cormio G, Resta L, Fattizzi N, Vicino M, Selvaggi L. Pseudo-meigs syndrome and elevated CA125 associated with struma ovarii. Gynecol Oncol. 2005;97:282–4.
Yaguchi A, Ban K, Koshida Y, Fujikami Y, Ogura E, Terada A, et al. Pseudo-meigs Syndrome caused by a giant uterine leiomyoma with cystic degeneration: a Case Report. J Nippon Med Sch Nippon Ika Daigaku Zasshi. 2020;87:80–6.
Meigs JV. Fibroma of the ovary with ascites and hydrothorax; Meigs’ syndrome. Am J Obstet Gynecol. 1954;67:962–85.
Meigs JV. Pelvic tumors other than fibromas of the ovary with ascites and hydrothorax. Obstet Gynecol. 1954;3:471–86.
Domingo P, Montiel JA, Monill JM, Prat J. Pseudo-meigs syndrome with elevated CA 125 levels. Arch Intern Med. 1998;158:1378–9.
Kazanov L, Ander DS, Enriquez E, Jaggi FM. Pseudo-meigs’ syndrome. Am J Emerg Med. 1998;16:404–5.
Saha S, Robertson M. Meigs’ and pseudo-meigs’ syndrome. Australas J Ultrasound Med. 2012;15:29–31.
Amant F, Gabriel C, Timmerman D, Vergote I. Pseudo-meigs’ syndrome caused by a hydropic degenerating uterine leiomyoma with elevated CA 125. Gynecol Oncol. 2001;83:153–7.
Khan J, McClennan BL, Qureshi S, Martell M, Iyer A, Bokhari SJ. Meigs syndrome and gliomatosis peritonei: a case report and review of literature. Gynecol Oncol. 2005;98:313–7.
Krenke R, Maskey-Warzechowska M, Korczynski P, Zielinska-Krawczyk M, Klimiuk J, Chazan R, et al. Pleural effusion in Meigs’ syndrome-transudate or exudate? Systematic review of the literature. Med (Baltim). 2015;94:e2114.
Ishiko O, Yoshida H, Sumi T, Hirai K, Ogita S. Vascular endothelial growth factor levels in pleural and peritoneal fluid in Meigs’ syndrome. Eur J Obstet Gynecol Reprod Biol. 2001;98:129–30.
Abramov Y, Anteby SO, Fasouliotis SJ, Barak V. Markedly elevated levels of vascular endothelial growth factor, fibroblast growth factor, and interleukin 6 in Meigs syndrome. Am J Obstet Gynecol. 2001;184:354–5.
Abramov Y, Anteby SO, Fasouliotis SJ, Barak V. The role of inflammatory cytokines in Meigs’ syndrome. Obstet Gynecol. 2002;99(5 Pt 2):917–9.
Meden H, Fattahi-Meibodi A. CA 125 in benign gynecological conditions. Int J Biol Markers. 1998;13:231–7.
Felder M, Kapur A, Gonzalez-Bosquet J, Horibata S, Heintz J, Albrecht R, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:129.
Quintero-Muñoz E, Gómez Pineda MA, Araque Parra C, Vallejo Castillo CA, Ortega Marrugo V, Bonilla Jassir J, et al. Is there any relationship between massive ascites and elevated CA-125 in systemic lupus erythematosus? Case report and review of the literature. Mod Rheumatol Case Rep. 2021;5:292–9.
Bottoni P, Scatena R. The role of CA 125 as tumor marker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:229–44.
Kurman RJ. Blaustein’s Pathology of the female genital tract. 7th eds ed. New York: Springer; 2019.
Price N, Nakade K, Kehoe ST. A rapidly growing uterine fibroid postpartum. BJOG Int J Obstet Gynaecol. 2004;111:503–5.
Clement PB, Young RH, Scully RE. Diffuse, perinodular, and other patterns of hydropic degeneration within and adjacent to uterine leiomyomas. Problems in differential diagnosis. Am J Surg Pathol. 1992;16:26–32.
Van den Berghe I, Dal Cin P, Sciot R, Vanvuchelen J, Michielssen P, Hagemeijer A, et al. Translocation (4;8) as a primary chromosome change in a hydropic leiomyoma. Histopathology. 1999;34:378.
Griffin BB, Ban Y, Lu X, Wei J-J. Hydropic leiomyoma: a distinct variant of leiomyoma closely related to HMGA2 overexpression. Hum Pathol. 2019;84:164–72.
Lee MJ, Kazer RR. Massive ascites after leuprolide acetate administration for the treatment of leiomyomata uteri. Fertil Steril. 1992;58:416–8.
Ollendorff AT, Keh P, Hoff F, Lurain JR, Fishman DA. Leiomyoma causing massive ascites, right pleural effusion and respiratory distress. A case report. J Reprod Med. 1997;42:609–12.
Brown RS, Marley JL, Cassoni AM. Pseudo-meigs’ syndrome due to broad ligament leiomyoma: a mimic of metastatic ovarian carcinoma. Clin Oncol R Coll Radiol G B. 1998;10:198–201.
Migishima F, Jobo T, Hata H, Sato R, Ikeda Y, Arai M, et al. Uterine leiomyoma causing massive ascites and left pleural effusion with elevated CA 125: a case report. J Obstet Gynaecol Res. 2000;26:283–7.
Kebapci M, Aslan O, Kaya T, Yalcin OT, Ozalp S. Pedunculated uterine leiomyoma associated with pseudo-meigs’ syndrome and elevated CA-125 level: CT features. Eur Radiol. 2002;12(Suppl 3):S127–129.
Weise M, Westphalen S, Fayyazi A, Emons G, Krauss T. Pseudo-meigs syndrome: uterine leiomyoma with bladder attachment associated with ascites and hydrothorax - a rare case of a rare syndrome. Onkologie. 2002;25:443–6.
Weinrach DM, Wang KL, Keh P, Sambasiva Rao M. Pathologic quiz case: a 40-year-old woman with a large pelvic mass, ascites, massive right hydrothorax, and elevated CA 125. Uterine symplastic leiomyoma associated with pseudo-meigs syndrome and elevated CA 125. Arch Pathol Lab Med. 2004;128:933–4.
Kurai M, Shiozawa T, Noguchi H, Konishi I. Leiomyoma of the ovary presenting with Meigs’ syndrome. J Obstet Gynaecol Res. 2005;31:257–62.
Munteanu M, Petrescu F, Pleşea E, Stanciu E, Enache SD, Munteanu MC, et al. Pseudo-meigs syndrome, a rare variant. Chir Buchar Rom 1990. 2006;101:205–8.
Landrum LM, Rutledge TL, Osunkoya AO, Mannel R. Uterine leiomyoma with associated pseudo-meigs syndrome mimicking ovarian carcinoma. J Okla State Med Assoc. 2008;101:38–9.
Ricci G, Inglese S, Candiotto A, Maso G, Piccoli M, Alberico S, et al. Ascites in puerperium: a rare case of atypical pseudo-meigs’ syndrome complicating the puerperium. Arch Gynecol Obstet. 2009;280:1033–7.
Chourmouzi D, Papadopoulou E, Drevelegas A. Magnetic resonance imaging findings in Pseudo-meigs’ syndrome associated with a large uterine leiomyoma: a case report. J Med Case Rep. 2010;4:120.
Yip H-K, Huang L-W, Lin Y-H, Hwang J-L. Massive ascites caused by a large pedunculated subserosal uterine leiomyoma that has feeding arteries from peripheral tissues and exhibits elevated CA125: a case report of atypical pseudo-meigs’ syndrome. J Obstet Gynaecol J Inst Obstet Gynaecol. 2014;34:107.
Seo MR, Sung JY, Cho HJ, Ryu HJ, Choi H-J, Park C-Y, et al. Ascites associated with uterine leiomyoma in a 22-year-old woman with systemic lupus erythematosus. Lupus. 2014;23:1207–10.
Dong R, Jin C, Zhang Q, Yang X, Kong B. Cellular leiomyoma with necrosis and mucinous degeneration presenting as pseudo-meigs’ syndrome with elevated CA125. Oncol Rep. 2015;33:3033–7.
Musleh JKM, Almulhem M, Al Qahtani NH. Massive ascites, pelvic mass, elevated CA-125 and weight loss: think outside the ovaries. BMJ Case Rep. 2017;2017:bcr2016217497, bcr-2016–217497.
Kim JH, Baek JH. A challenging case of Intracardiac Leiomyomatosis accompanied by Pseudo-meigs Syndrome originating from Uterine Leiomyoma. Ann Vasc Surg. 2019;55:e3095–8.
Pauls M, MacKenzie H, Ramjeesingh R. Hydropic leiomyoma presenting as a rare condition of Pseudo-meigs syndrome: literature review and a case of a Pseudo-meigs syndrome mimicking ovarian carcinoma with elevated CA125. BMJ Case Rep. 2019;12:bcr–2018.
Wang Y-W, Fan Q, Qian Z-X, Wang J-J, Li Y-H, Wang Y-D. Abdominopelvic leiomyoma with large ascites: a case report and review of the literature. World J Clin Cases. 2021;9:1424–32.
Abdelgawad M, Barghuthi L, Davis T, Omar M, Kamel OM, Gibbons J, et al. Large uterine leiomyoma presenting as pseudo-meigs’ syndrome with an elevated ca125: a case report and literature review. J Surg Case Rep. 2022;2022:rjac253.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LQ.Z. Provided and analyzed the cases. JL.L. and HR.H. organized the Literature database. LQ.Z. wrote the first draft of the manuscript. L.X. analyzed the cases and revised the manuscript. All authors reviewed the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Ethics Committee of Zigong Fourth People’s Hospital.
Consent for publication
The patient provided written informed consent for the publication of this case report and accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zou, L., Lou, J., Huang, H. et al. Pseudo-Meigs syndrome caused by a rapidly enlarging hydropic leiomyoma with elevated CA125 levels mimicking ovarian malignancy: a case report and literature review. BMC Women's Health 24, 445 (2024). https://doi.org/10.1186/s12905-024-03285-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-024-03285-8