Abstract
Background
Observational studies have found a correlation between the levels of blood lipids and the development and progression of endometriosis (EM). However, the causality and direction of this correlation is unclear. This study aimed to examine the bidirectional connection between lipid profiles and the risk of EM using publicly available genome-wide association study (GWAS) summary statistics.
Methods
Eligible exposure variables such as levels of triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were selected using a two-sample Mendelian randomization (MR) analysis method following a series of quality control procedures. Data on EM were obtained from the publicly available Finnish database of European patients. Inverse variance weighted (IVW), MR Egger, weighted median, and weighted mode methods were used to analyze the causal relationship between lipid exposure and EM, exclude confounders, perform sensitivity analyses, and assess the stability of the results. Reverse MR analyses were performed with EM as exposure and lipid results as study outcomes.
Results
IVW analysis results identified HDL as a protective factor for EM, while TG was shown to be a risk factor for EM. Subgroup analyses based on the site of the EM lesion identified HDL as a protective factor for EM of the uterus, while TG was identified a risk factor for the EM of the fallopian tube, ovary, and pelvic peritoneum. Reverse analysis did not reveal any effect of EM on the levels of lipids.
Conclusion
Blood lipids, such as HDL and TG, may play an important role in the development and progression of EM. However, EM does not lead to dyslipidemia.
Similar content being viewed by others
Introduction
Endometriosis (EM) is a chronic condition that is characterized by the presence of ectopic endometrial tissue outside of the uterine cavity, ectopic endometrial tissue cyclic bleeding, fibrosis [1]. The disease affects 6-10% of the fertile female population and seriously impacts the reproductive health and quality of life of women [2].
Typical symptoms of EM include dysmenorrhea, chronic pelvic pain, menstrual abnormalities, and even infertility. Studies also show that over 40% of women with EM present with central sensitization (CS) [3] that alters pain perception, exacerbates pain symptoms, predisposes women with EM to the development of other chronic conditions, and could lead to worse response to treatments [4].
Pathophysiology of EM is complex and involves chronic inflammation, hormonal changes, genetic and epigenetic factors, altered metabolism, local immune dysregulation [5]. Current studies suggest that abnormal lipid metabolism may also contribute to the development, severity and progression of EM. A prospective observational cohort study that pooled data of 1,299,349 females with up to 20 years of follow-up, found that women with EM had lower body mass index and a peripheral body fat distribution (waist-to-hip ratios below 0.60) [6]. This suggests that abnormalities in lipid metabolism that are manifested by changes in the lipid levels in the peripheral blood [7] may be related to the progression of EM. A retrospective study also found a positive correlation between the levels of TG and the severity of EM [8]. Furthermore, the risk of atherosclerosis was higher in the population of patients with EM, possibly due to long-term chronic inflammation that exacerbates this process [9]. These findings seem to suggest that dyslipidemia is an adverse outcome of the progression of EM. Additionally, dyslipidemia may affect the efficacy of EM treatment. In the rat EM model, antilipidemic treatment decreased the size of endometrial lesions [10]. By using a mouse model, Heard, ME et al. [11] demonstrated that increased fat intake significant affected the size of EM lesions. Studies also showed that dyslipidemia persisted after the pharmacological treatment of EM which was effective in controlling the symptoms or shrinking the lesions [12, 13]. Therefore, understanding the role of lipids in the pathophysiology of EM, and the causal relationship between the two may provide a theoretical basis for the long-term management of EM by adjusting the dietary structure or the use of lipid-lowering drugs.
Mendelian randomization (MR) uses specific single nucleotide polymorphisms (SNPs) as instrumental variables to identify potential causal associations between exposures and outcomes [14]. MR can be considered a natural randomized clinical trial based on the genetic law of “random assignment of parental alleles to offspring”, which allows to reliably infer causality by avoiding potential confounders or reverse causality in prospective or retrospective observational studies.
In this study, we used a two-way MR approach to investigate whether there is a causal relationship between the levels of triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL) and EM.
Methods
Objective
To explore the bidirectional causal associations between blood levels of the lipid quartet (HDL, TG, TC, and LDL) and EM by two-way MR analysis using GWAS data.
Data source
Data on blood lipid levels were obtained from the Global Lipid Consortium database [15, 16], which included a total population of 1,654,960 patients. EM data were obtained from the Finnish database version R.9 (ICD-10: N14), with a sample size of 15,088, a control group of 107,564, and a number of SNPs of 20,141,087 (https://r9.risteys.finngen.fi/endpoints/N14_ENDOMETRIOSIS). Analysis of EM subtypes at different sites was performed. To avoid bias caused by confounding factors such as ethnicity, we selected only the genetic background of people with European ancestry for the analysis.
The composition of the population included in the analyses for the different sites was as follows: deep EM (n = 2856), EM of the fallopian tube (n = 213), EM of intestine (n = 436), unspecified EM (n = 2982), EM of ovary (n = 5867), EM of pelvic peritoneum (n = 5628), EM of rectovaginal septum and vagina (n = 2456), and EM of uterus (n = 4267).
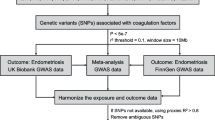
A flowchart of the study design is summarized in Fig. 1.
Since the data source that was used in this study obtained informed consent from all participants, separate institutional review board approval was not required.
Instrumental variable
Screening criteria were as follows: (i) the instrumental variable strongly correlated with the exposure factor, (ii) confounders associated with the outcome were excluded, and (iii) the outcome could be influenced only by the exposure and not by the instrumental variable itself [17, 18]. Conditions required for significant correlation of the instrumental genetic variant were as follows: p < 5 × 10 − 8, r2 < 0.001, genetic distance = 10 000 kb, and all F-test values > 10. Using the website phenoscanner database (http://www.phenoscanner.medschl.cam.ac.uk) [19, 20], all phenotypes associated with the instrumental variables were searched, and SNPs associated with outcome and confounders (P < 5 × 10 − 8) were excluded from the instrumental variables for the multiplicity of validity. The confounders of EM were based on the results of the reference article [21]. For forward univariate MR analyses, exposure factors were as follows: HDL, TG (logarithmic), TC, and LDL. EM was selected as an outcome of interest. Reverse MR analyses were performed with EM set as an exposure factor and the lipid levels as the outcome. Setup parameters were equivalent to those of the forward univariate MR analyses.
Statistic analysis
Univariate MR analysis used inverse variance weighted (IVW) to calculate odds ratio (OR) and 95% confidence interval (CI), and MR Egger, weighted median, and weighted mode [22] were used as supplementary methods to verify the stability of the results. Sensitivity analyses included a heterogeneity test and horizontal multiplicity test to assess the presence of potential bias in the results. P < 0.05 in Cochran’s Q-test indicated the presence of heterogeneity [23]. Multiplicity of SNPs was measured by MR Egger regression, and the intercept term p < 0.05 indicated the presence of horizontal multiplicity in the results [24]. “Leave-one-out” gradually eliminated each SNP, calculated the meta-effects of the remaining SNPs, and observed whether the results changed after the elimination of each SNP. When the results matched the total effect size of the MR analysis, the analysis was considered robust [14].
Two Sample MR packages (version 0.5. 6), and Radial MR package (version 1.0) in R software (4.2.2) were used for analysis, with a test level of α = 0.05.
Results
Positive mendelian analysis of lipid quartiles and EM
Instrumental variables for screening
A total of 323 SNPHDL, 295 SNPlogTG, 315 SNPTC, and 279 SNPLDL were obtained after the removal of linkage disequilibrium (P < 10− 8). Of them, 256 SNPHDL, 241 SNPlogTG, 249 SNPTC, and 235 SNPLD, were strongly associated with EM after removing the unavailability of matches or palindromes. Finally, 206 SNPHDL, 189 SNPlogTG, 221 SNPTC, and 210 SNPLDL were included in the MR analyses after removing confounders associated with EM. The F statistics were greater than 10, indicating that the selected SNPs did not have weak instrumental variable bias. We used IVW, MR Egger, weighted median, and weighted mode to assess the causal relationship between the lipid quartet and EM (Table 1). Scatter plots showed that the analyses had consistency (Fig. 2).
As shown in Table 1, the inverse variance weighted (IVW) model identified HDL as a protective factor for EM, with OR = 0.913, CI = 0.843–0.989, P = 0.025. On the other hand, logTG was a risk factor for EM (OR = 1.156, CI = 1.022–1.308, P = 0.022). TC and LDL had no significant causal relationship with EM (P > 0.1 for all four model results).
Sensitivity analysis
To test for the presence of bias in the MR analysis, further sensitivity analyses were performed. Cochran Q test results revealed heterogeneity in SNPs (P < 0.001). Therefore, we next focused on the results of the IVW random effects model (Table 2).
The results of the funnel plot (Fig. 3) showed a largely symmetrical distribution of SNPs included in the analyses, suggesting that the inferred causal effect was less affected. As shown in Table 3, the pleiotropy test showed no horizontal multiplicity of SNPs (P > 0.05). In addition, we used the leave-one-out method to confirm the effect of HDL, logTG, TC, and LDL on EM potential outliers in the instrumental variables for causal effects. The removal of any individual SNP did not have a large impact on the results (Supplementary Figs. 1–4), indicating that the results of the MR analysis were robust and reliable.
Inverse mendelian analysis of blood lipid profile and EM
A total of 27 SNPs with a genome-wide threshold of significance (P < 5 × 10− 8), associated with EM, were identified and included in the analyses after removing sequences that could not be matched to lipids or had palindromic sequences (21 SNPHDL, 19 SNPlogTG, 22 SNPTC, 22 SNPLDL. No significant evidence of a causal effect of EM on lipids was found on IVW, weighted median and weighted mode analyses (Fig. 4, Supplementary 5). The results of the multiple validity test showed that the intercepts of the MR Egger regressions were 0.002 (HDL, LDL, TC) and 0.004 (logTG), respectively, with PHDL = 0.567, PHDL = 0.527, PHDL = 0.179, and PHDL = 0.451, suggesting that there was no potential horizontal versatility. Leave-one-out method showed similar results (Supplementary Fig. 6).
Subgroup analyses
Subgroup analyses were then performed based on the site of endometriosis lesions. Exposure factors included HDL, LDL, logTG, and TC, and the following outcomes were selected: deep EM, EM of fallopian tube, intestine, unspecified EM, EM of ovary, EM of pelvic peritoneum, rectovaginal septum and vagina, and uterus.
As shown in Table 4, HDL was identified as a protective factor for EM of uterus, IVW (OR = 0.837, CI = 0.731–0.959, P = 0.01), while logTG was a risk factor for the EM of fallopian tube (OR = 1.946, CI = 1.093–3.464, P = 0.02), EM of ovary (OR = 1.149, CI = 1.027–1.286, P = 0.02), and EM of pelvic peritoneum (OR = 1.186, CI = 1.035–1.360, P = 0.01).
LDL and TC had no genetic factor effects on the various subtypes of EM. There was no evidence of horizontal pleiotropy of SNPs (p > 0.05).
MR results of four blood lipids based on the location of lesions, and Egger intercept test results are shown in Supplementary Tables 1 and 2.
Discussion
In this study, we explored the bidirectional causal associations between blood levels of the lipid quartet (HDL, TG, TC, and LDL) and EM by two-way MR analysis using GWAS data to provide a theoretical basis for the adjustment of lipid metabolism and dietary interventions in the long-term management of EM.
Bidirectional MR analyses showed that HDL reduced the risk of developing EM, whereas TG was a risk factor for EM. On the other hand, EM did not affect the values of HDL, TG, TC, and LDL. Our results suggest that HDL and TG may play a key role in the pathophysiological process of EM. Subgroup analyses based on the site of the EM lesion identified HDL as a protective factor for endometriosis of the uterus, while TG was identified as a risk factor for the EM of the fallopian tube, ovary, and pelvic peritoneum. Several previous cross-sectional studies have shown increased levels of lipid metabolites in the blood of women suffering from EM [25,26,27,28]. In a prospective cohort study, Naoko Sasamoto et al. found that disorders of lipid metabolism were strongly associated with chronic pelvic pain due to EM by comparing lipid metabolites of preoperative and postoperative EM patients [29]. Another prospective cohort study reported that women with EM had a higher risk of hypercholesterolemia and hypertension compared to women without the condition [30]. Moreover, EM patients had higher arterial stiffness [9] and incidence of cerebrovascular-related headaches [31]. In addition, Poeta do Couto, C et al. [32] found that patients diagnosed with EM had a significantly higher risk of cardiovascular disease. Considering the complexity of lipid metabolism and EM, the causal relationship between the two has not been completely clarified. Our results suggest that clinicians should pay special attention to the HDL and TG profiles of patients in the long-term management of EM, which may help to control or delay the progression of this disease.
Numerous studies showed that the lesions of EM patients are caused by ectopic endothelial cell implantation outside the uterine cavity and that the local inflammatory response promotes angiogenesis, cyclic bleeding, hemostasis, and accelerated tissue fibrosis formation [33]. Angiogenesis, cyclic hemorrhage, and local inflammatory response are all important parts of the disease progression in EM. Coagulation plays a key role in the inflammatory response and angiogenesis. Li, Yan, et al. used MR to explore the causal association between coagulation factors and EM and found that the cascade of local coagulation and anticoagulation mediated by coagulation factors is an important cause for the development of EM. They also showed that the local aggregation of platelets triggered by the Von Willebrand factor (vWF) is a protective factor against EM [34]. vWF, which can serve as a reflection of the degree of vascular endothelial cell damage, is mainly synthesized by vascular endothelial cells, and is an indispensable bridging factor in the process of inducing platelet adhesion and aggregation [35]. Previous studies have demonstrated that HDL has anti-vascular endothelial oxidation, inflammation, and platelet aggregation functions [36]. HDL ameliorates and repairs endothelial cell damage by decreasing the level of inflammatory response and inhibiting LDL oxidation [37]. In addition, low levels of HDL and impaired vascular endothelial function result in elevated vWF levels in peripheral blood [38]. We hypothesize that HDL reduces the development of EM possibly by stimulating or working in synergy with vWF. Reduction of vascular proliferation and inhibition of ectopic lesion formation by HDL is one of the potential pathways for its role as a protective factor against EM.
Studies show that HDL reduces peripheral blood cholesterol levels by reverse transporting cholesterol to the liver and metabolizing it [39]. Various dietary and environmental factors can influence blood cholesterol levels [40]. For instance, dietary cholesterol increases serum total cholesterol and HDL [41]. Cholesterol in peripheral blood is an important source of steroid hormone synthesis in the body, and same abnormalities of cholesterol metabolism are present in EM patients [30], suggesting an potential role of cholesterol synthesis regulation in EM. We, therefore, may hypothesize that lowering estrogen synthesis by elevating peripheral blood levels of HDL through dietary management may delay EM progression. Previous studies have found high levels of aromatase and estrogen receptors in ectopic lesions [42]. High estrogen was shown to increase the release of cytokines and chemokines from macrophages, exacerbating the local inflammatory response and promoting the growth and invasion of ectopic lesions [43]. Estrogen precursor, a homeobox protein HOXA10, is highly expressed in endometrial mesenchymal stromal cells and acts through the steroid hormone-cholesterol synthesis pathway [44]. Levels of HOXA10 positively correlate with HDL and negatively correlate with TG in peripheral blood [45]. Cirillo, M et al. [46] found that the Mediterranean Diet lowered peripheral blood cholesterol and improved metabolic and oxidative status, as well as improved overall quality of life of EM patients. Therefore, modifying HDL through dietary modification to slow down the progression of EM is a feasible future direction.
Studies by Crook, D [47] and Melo, AS [48] found higher TG levels in EM patients. The results of these studies showed that patients with EM stages I-II had significantly higher levels of TG [49], while patients in stages III-IV showed a similar trend with worse lipid profiles and significant correlation with c-reactive (CRP) levels [48], suggesting that TG may be exacerbating the course of EM through the inflammatory response pathway. A cross-sectional study Li, Baijia et al. [7] found a high rate of metabolic syndrome in EM patients and associated it with high levels of peripheral blood TG which is involved in the atherosclerotic process, and increases the risk of cardiovascular disease [9] and metabolic syndrome [7].
In contrast to previous observational studies, our study accounted for confounding factors to a greater extent, provided bidirectional causal associations, and identified key risk and protective factors for EM from a genetic perspective. Our study may have clinical implications that may change the approach to the diagnosis and treatment of EM. Combined assessment of HDL and TG lipid profiles may be potentially used for screening patients and establishing early diagnosis and staging of EM. This, in turn, may contribute to a more multimodal approach to the treatment, since abnormal lipid profiles are significant risk factors of systemic comorbidities such as cardiovascular diseases [50]. Moreover, oral contraceptives are often used in the treatment of EM, as they are considered safe and efficient in the reduction of ovarian endometrioma size [51, 52]. However, the use of oral contraceptives was reported to be associated with significantly higher concentrations of high-density-lipoprotein cholesterol, and increased incidence of stroke and myocardial infarction [53]. The results of this study further emphasize the importance of a more informed approach to contraceptive prescription in EM patients due to potentially lipid higher levels in this population of patients. In addition, our findings provide a potential therapeutic approach to management and prevention of progression and postoperative recurrence of EM through modulating HDL and TG levels by diet or medication.
There are some limitations of this study. The results of the MR analysis were based on the European population, introducing a potential ethnic bias and limiting the extrapolation of causality. SNPs that were used for the analysis may correlate with other traits due to genetic polymorphisms, creating a confounding bias that may have impacted the accuracy of the causal inference. Our data were not stratified according to the different stages of the EM, and age, which may have resulted in some bias. We also acknowledge that further clinical studies are needed to investigate the effect of modifying lipid profiles of patients on endometriosis. Since this study was based on the data that were obtained from publicly available databases, we were unable to address these points in the scope of this paper. Further studies are needed to perform independent validation of our results.
Conclusion
Bidirectional MR analysis found that hereditary HDL and TG levels were closely associated with the risk of developing EM. Our results suggest the need to focus on lipid levels in the long-term management of EM. Adjustment of dietary structure or use of lipid-lowering drugs instead of hormonal therapy are feasible directions for managing EM.
References
Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, et al. Endometr Endocr Rev. 2019;40:1048–79.
Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–75.
Raimondo D, Raffone A, Renzulli F, Sanna G, Raspollini A, Bertoldo L, et al. Prevalence and risk factors of Central Sensitization in women with endometriosis. J Minim Invasive Gynecol. 2023;30:73–e801.
Quintas-Marquès L, Martínez-Zamora M-Á, Camacho M, Gràcia M, Rius M, Ros C, et al. Central sensitization in patients with deep endometriosis. Pain Med. 2023;24:1005–7.
Saunders PTK, Horne AW, Endometriosis. Etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807–24.
Shah DK, Correia KF, Vitonis AF, Missmer SA. Body size and endometriosis: results from 20 years of follow-up within the nurses’ Health Study II prospective cohort. Hum Reprod. 2013;28:1783–92.
Li B, Zhang Y, Zhang L, Zhang L. Association between endometriosis and metabolic syndrome: a cross-sectional study based on the National Health and Nutrition Examination Survey data. Gynecol Endocrinol. 2023;39:2254844.
Zheng R, Du X, Lei Y. Correlations between endometriosis, lipid profile, and estrogen levels. Med (Baltim). 2023;102:e34348.
Kilic D, Guler T, Sevgican CI, Kabukcu C, Buber I, Kilinc M, et al. Association between endometriosis and increased arterial stiffness. Kardiol Pol. 2021;79:58–65.
Tapdıgova R, Bayrak G, Yılmaz BC, Aytan H. Antilipidemic ezetimibe induces regression of endometriotic explants in a rat model of endometriosis with its anti-inflammatory and anti-angiogenic effects. Naunyn Schmiedebergs Arch Pharmacol. 2022;395:673–80.
Heard ME, Melnyk SB, Simmen FA, Yang Y, Pabona JMP, Simmen RCM. High-Fat Diet Promotion of endometriosis in an Immunocompetent Mouse Model is Associated with altered peripheral and Ectopic Lesion Redox and inflammatory status. Endocrinology. 2016;157:2870–82.
Anastasilakis AD, Polyzos SA, Vorkas PA, Gkiomisi A, Yavropoulou MP, Rauner M, et al. Lipid Profile after Pharmacologic Discontinuation and Restoration of Menstruation in Women with endometriosis: a 12-Month Observational prospective study. J Clin Med. 2023;12:5430.
Sakurada T, Matsushita H, Noguchi Y, Shinohara K, Watanabe K, Wakatsuki A. Effects of androgenic properties of progestin combined with ethinyl estradiol on vascular endothelial reactivity, plasma lipids and free radical production in women with endometriosis. J Obstet Gynaecol Res. 2021;47:941–8.
Carnegie R, Zheng J, Sallis HM, Jones HJ, Wade KH, Evans J, et al. Mendelian randomisation for nutritional psychiatry. Lancet Psychiatry. 2020;7:208–16.
Graham SE, Clarke SL, Wu K-HH, Kanoni S, Zajac GJM, Ramdas S, et al. The power of genetic diversity in genome-wide association studies of lipids. Nature. 2021;600:675–9.
Ramdas S, Judd J, Graham SE, Kanoni S, Wang Y, Surakka I, et al. A multi-layer functional genomic analysis to understand noncoding genetic variation in lipids. Am J Hum Genet. 2022;109:1366–87.
Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16:309–30.
Evans DM, Davey Smith G. Mendelian randomization: New Applications in the coming age of hypothesis-free causality. Annu Rev Genomics Hum Genet. 2015;16:327–50.
Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–9.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35:4851–3.
Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1–15.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Letsiou S, Peterse DP, Fassbender A, Hendriks MM, van den Broek NJ, Berger R, et al. Endometriosis is associated with aberrant metabolite profiles in plasma. Fertil Steril. 2017;107:699–e7066.
Vouk K, Ribič-Pucelj M, Adamski J, Rižner TL. Altered levels of acylcarnitines, phosphatidylcholines, and sphingomyelins in peritoneal fluid from ovarian endometriosis patients. J Steroid Biochem Mol Biol. 2016;159:60–9.
Maignien C, Santulli P, Kateb F, Caradeuc C, Marcellin L, Pocate-Cheriet K, et al. Endometriosis phenotypes are associated with specific serum metabolic profiles determined by proton-nuclear magnetic resonance. Reprod Biomed Online. 2020;41:640–52.
Murgia F, Angioni S, D’Alterio MN, Pirarba S, Noto A, Santoru ML, et al. Metabolic Profile of patients with severe endometriosis: a prospective experimental study. Reprod Sci. 2021;28:728–35.
Sasamoto N, Zeleznik OA, Vitonis AF, Missmer SA, Laufer MR, Avila-Pacheco J, et al. Presurgical blood metabolites and risk of postsurgical pelvic pain in young patients with endometriosis. Fertil Steril. 2022;117:1235–45.
Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Forman JP, Missmer SA. Association between endometriosis and Hypercholesterolemia or Hypertension. Hypertension. 2017;70:59–65.
Cirillo M, Coccia ME, Petraglia F, Fatini C. Role of endometriosis in defining cardiovascular risk: a gender medicine approach for women’s health. Hum Fertil (Camb). 2022;25:745–53.
Poeta do Couto C, Policiano C, Pinto FJ, Brito D, Caldeira D. Endometriosis and cardiovascular disease: a systematic review and meta-analysis. Maturitas. 2023;171:45–52.
Zondervan KT, Becker CM, Missmer SA, Endometriosis. N Engl J Med. 2020;382:1244–56.
Li Y, Liu H, Ye S, Zhang B, Li X, Yuan J, et al. The effects of coagulation factors on the risk of endometriosis: a mendelian randomization study. BMC Med. 2023;21:195.
Moore JC, Hayward CP, Warkentin TE, Kelton JG. Decreased Von Willebrand factor protease activity associated with thrombocytopenic disorders. Blood. 2001;98:1842–6.
Allard-Ratick MP, Kindya BR, Khambhati J, Engels MC, Sandesara PB, Rosenson RS, et al. HDL: Fact, fiction, or function? HDL cholesterol and cardiovascular risk. Eur J Prev Cardiol. 2021;28:166–73.
Kumboyono K, Chomsy IN, Firdaus DH, Setiawan M, Wihastuti TA. Protective cardiovascular benefits of exercise training as measured by circulating endothelial cells and high-density lipoprotein in adults. J Taibah Univ Med Sci. 2022;17:701–6.
Chung DW, Platten K, Ozawa K, Adili R, Pamir N, Nussdorfer F, et al. Low-density lipoprotein promotes microvascular thrombosis by enhancing Von Willebrand factor self-association. Blood. 2023;142:1156–66.
Mahdy Ali K, Wonnerth A, Huber K, Wojta J. Cardiovascular disease risk reduction by raising HDL cholesterol–current therapies and future opportunities. Br J Pharmacol. 2012;167:1177–94.
Lüscher TF, Landmesser U, von Eckardstein A, Fogelman AM. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res. 2014;114:171–82.
Kanter MM, Kris-Etherton PM, Fernandez ML, Vickers KC, Katz DL. Exploring the factors that affect blood cholesterol and heart disease risk: is dietary cholesterol as bad for you as history leads us to believe? Adv Nutr. 2012;3:711–7.
Marla S, Mortlock S, Houshdaran S, Fung J, McKinnon B, Holdsworth-Carson SJ, et al. Genetic risk factors for endometriosis near estrogen receptor 1 and coexpression of genes in this region in endometrium. Mol Hum Reprod. 2021;27:gaaa082.
Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C. Pathophysiology and Immune Dysfunction in Endometriosis. Biomed Res Int. 2015;2015:795976.
Yu M, Tang J, Huang Y, Guo C, Du P, Li N, et al. HOXA10 regulates the synthesis of cholesterol in endometrial stromal cells. Front Endocrinol (Lausanne). 2022;13:852671.
Wang Y, Hu S, Yao G, Sun Y. Identification of HOXA10 target genes in human endometrial stromal cells by RNA-seq analysis. Acta Biochim Biophys Sin (Shanghai). 2021;53:365–71.
Cirillo M, Argento FR, Attanasio M, Becatti M, Ladisa I, Fiorillo C, et al. Atherosclerosis and endometriosis: the role of Diet and oxidative stress in a gender-specific disorder. Biomedicines. 2023;11:450.
Crook D, Howell R, Sidhu M, Edmonds DK, Stevenson JC. Elevated serum lipoprotein(a) levels in young women with endometriosis. Metabolism. 1997;46:735–9.
Melo AS, Rosa-e-Silva JC, Rosa-e-Silva ACJ, de Poli-Neto S, Ferriani OB, Vieira RA. Unfavorable lipid profile in women with endometriosis. Fertil Steril. 2010;93:2433–6.
Verit FF, Erel O, Celik N. Serum paraoxonase-1 activity in women with endometriosis and its relationship with the stage of the disease. Hum Reprod. 2008;23:100–4.
Martire FG, Giorgi M, D’Abate C, Colombi I, Ginetti A, Cannoni A, et al. Deep infiltrating endometriosis in adolescence: early diagnosis and possible Prevention of Disease Progression. J Clin Med. 2024;13:550.
Del Forno S, Mabrouk M, Arena A, Mattioli G, Giaquinto I, Paradisi R, et al. Dienogest or Norethindrone acetate for the treatment of ovarian endometriomas: can we avoid surgery? Eur J Obstet Gynecol Reprod Biol. 2019;238:120–4.
Ferrero S, Remorgida V, Venturini PL, Leone Roberti Maggiore U. Norethisterone acetate versus norethisterone acetate combined with letrozole for the treatment of ovarian endometriotic cysts: a patient preference study. Eur J Obstet Gynecol Reprod Biol. 2014;174:117–22.
Wahl P, Walden C, Knopp R, Hoover J, Wallace R, Heiss G, et al. Effect of estrogen/progestin potency on lipid/lipoprotein cholesterol. N Engl J Med. 1983;308:862–7.
Acknowledgements
We want to acknowledge the participants and investigators of the FinnGen study. The authors would like to thank all the genetics consortiums for making the GWAS summary data publicly available.
Funding
This work was supported by the Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2021Y9187), Nature Foundation of Fujian Province (No. 2021J01421 ), and Fujian Provincial Health Commission Young and Middle-aged Backbone Personnel Training Project (No. 2022GGB004 ).
Author information
Authors and Affiliations
Contributions
ZW, CZ, SL, and SY conceived and designed the study. SL and SY supervised the research and data analysis. LL and YL performed the data analysis with help from ZW and CZ. ZW and CZ wrote the manuscript. SL and SY substantially revised the manuscript. All authors confirmed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The analyses were based on publicly available data that has been approved by relevant review boards.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Z., Zhan, C., Liao, L. et al. Bidirectional causality between the levels of blood lipids and endometriosis: a two-sample mendelian randomization study. BMC Women's Health 24, 387 (2024). https://doi.org/10.1186/s12905-024-03213-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-024-03213-w