Abstract
Background
Polycystic ovary syndrome (PCOS) is a disorder characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphologic features, and PCOS is associated with infertility. PH domain Leucine-rich repeat Protein Phosphatase 1 (PHLPP1) has been shown to regulate AKT. The aim of present study is to investigate the role of PHLPP1 in PCOS.
Methods
The expression levels of PHLPP1 in dihydrotestosterone (DHT)-treated human ovarian granular KGN cells were determined by qRT-PCR and Western blot. PHLPP1 was silenced or overexpressed using lentivirus. Cell proliferation was detected by CCK-8. Apoptosis and ROS generation were analyzed by flow cytometry. Glycolysis was analyzed by measuring extracellular acidification rate (ECAR).
Results
DHT treatment suppressed proliferation, promoted apoptosis, enhanced ROS, and inhibited glycolysis in KGN cells. PHLPP1 silencing alleviated the DHT-induced suppression of proliferation and glycolysis, and promotion of apoptosis and ROS in KGN cells. PHLPP1 regulated cell proliferation and glycolysis in human KGN cells via the AKT signaling pathway.
Conclusions
Our results showed that PHLPP1 mediates the proliferation and aerobic glycolysis activity of human ovarian granular cells through regulating AKT signaling.
Similar content being viewed by others
Background
Infertility is the inability to get pregnant after 1 year of regular intercourse [1]. Infertility has a 9 to 18% prevalence [2]. In USA, approximately 13% of reproductive age women seek treatment for infertility every year [3]. About 85% of cases are because of ovulatory dysfunction, male factor infertility, and tubal disease. The other 15% of cases have “unexplained infertility” [3]. Polycystic ovary syndrome (PCOS), a common metabolic disorder in premenopausal women, is characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphologic features [4, 5]. PCOS is a major cause of hyperandrogenism and infertility [6]. The prevalence of infertility in women with PCOS ranges from 70 to 80% [7]. PCOS affects 6–20% of reproductive aged women [8]. The etiology is still unclear, but data support that it may be a multigenic disorder with strong epigenetic influences [4]. Risk factors include genetics, neuroendocrine, lifestyle/environment, obesity [9]. Treatment of PCOS is individualized accordingly. For example, weight control should be considered for overweight patients, clomiphene is recommended for infertility [10]. Regardless of the advances in treating PCOS, more studies are needed for better understanding the underlying mechanisms in order to provide more effective and precise treatment.
Glycolysis metabolizes glucose to pyruvate and generates ATP in cytoplasm [11]. The 3 key enzymes for glycolysis are hexokinase, phosphofructokinase, and pyruvate kinase [12]. Glycolysis plays a very important role in various pathological processes. For instance, Lim et al. have reported that inhibiting glycolysis and EGFR effectively suppressed mammary cancer growth [13]. Xiang et al. demonstrated that enhanced glycolysis in skeletal muscle coordinates with adipose tissue in systemic metabolic homeostasis [14]. Glycolysis is also essential for sperm motility and male fertility because sperm require ATP that is produced by mitochondrial oxidative phosphorylation and glycolysis to provide energy for motility [15]. Aberrant expression of glycolytic enzymes has been shown to be associated with infertility [16]. Glycolysis also involves in PCOS. For example, Zhao et al. have demonstrated that elevated glycolysis and decreased tricarboxylic acid cycle were presented in women with PCOS [17]. Dysregulation of glycolysis has also been associated with PCOS patients with hyperplasia [18]. Despite the remarkable success of the study of glycolysis, the exact role of glycolysis in PCOS and how glycolysis is involved remain unclear and need to be elucidated.
PH domain Leucine-rich repeat Protein Phosphatase 1 (PHLPP1) is a new member of the phosphatome [19]. PHLPP1 was first discovered by Gao et al. for its inhibiting growth factor signaling through dephosphorylation of AKT (protein kinase B, PKB) [20]. Dysregulation of PHLPP involves in different pathophysiology, from cancer to metabolic diseases [21]. Moc et al. have reported that physiological activation of AKT signaling by PHLPP1 deletion protects against pathological hypertrophy [22]. In contrast, overexpressing PHLPP1 decreases Akt and suppresses melanoma cell growth [23]. It is known that the phosphoinositide 3-kinase-AKT pathway regulates glycolysis [24]. AKT activation leads to promotion of aerobic glycolysis and overexpressing AKT leads to increased glycolytic rate [25, 26]. As mentioned above, dysregulated glycolysis plays a crucial role in PCOS. However, the role of PHLPP1/AKT/glycolysis in infertility and PCOS is not clear and remains to be elucidated.
Therefore, how PHLPP1 regulates glycolysis and how this PHLPP1-regulated-glycolysis affects human ovarian granular cell proliferation were investigated in this study to provide scientific basis for developing new treatments for PCOS.
Methods
Cell culture and treatment
The human ovarian granular KGN cells from Shanghai Biology Institute were cultured in DMEM with 10% FBS, 2 mM l-glutamine (Biyuntian, Suzhou) and antibiotics (100 IU/mL penicillin, 100 µg/mL streptomycin) (Sigma, Saint Louis, MO, USA) under 37 °C. Cells were treated with 25 nM DHT (Abmole Bioscience Inc., Houston, TX, USA) with or without PHLPP1 knockdown, followed by 5 µM MK-2206 (an AKT inhibitor; Selleck, Shanghai, China) or not. Otherwise, cells with PHLPP1 overexpression were treated with 5 µg/mL SC79 (an AKT activator; Selleck).
qRT-PCR
Total RNA was isolated by TRIzol, reverse-transcribed to synthesize cDNA utilizing PrimeScript RT Reagent Kit (Takara Biomedical Technology (Beijing) Co., Ltd.). qRT-PCR was performed using Maxima SYBR Green/ROX qPCR (Thermo Fisher Scientific). Fold change was calculated by 2−ΔΔCt. Primers (5’-3’) are as follows: PHLPP1 (NM_194449.4): F: TGTGCCTGAGTGGGTATGTG, R: CATCAGAAGGTTAGGTGGGAG; PHLPP2 (NM_015020.3): F: TACCTGCCTTATCGTTTC, R: GAGTATTGCCGTCGCTTC; ACTB (NM_001101.5): F: GTCACCAACTGGGACGACAT, R: TAGCAACGTACATGGCTGGG. mRNA expression levels were quantified with ACTB as the reference gene. The PCR cycling conditions were as follows: 95 °C for 10 min; followed by 40 cycles at 95 °C for 15 s and 60 °C for 45 s; and a final extension step of 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s and 60 °C for 15 s.
Immunoblotting
Cells were lysed on ice with lysis buffer, and quantified using a Bicinchoninic Acid Protein Assay Kit (Beyotime Biotechnology). Equivalent quantities (25 µg) of protein lysates were separated by sodium dodecyl sulfate-polyacrylamide gel and then transferred electrophoretically to polyvinylidene fluoride membranes (Millipore). Once the transfer of the proteins was completed, the membranes were blocked with 5% skimmed-milk solution and incubated overnight at 4 °C with the primary antibody, PHLPP1 (PA5-34434, Invitrogen), PHLPP2 (ab227673, Abcam), β-actin (4970s, CST), AKT (4691s, CST), p-AKT (4060s, CST). Secondary antibodies were labeled with horseradish Peroxidase. Visualization was detected using an ECL detection system (Bio-Rad, CA, USA).
Knockdown or overexpression of PHLPP1
Short hairpin interfering RNAs (shRNA) targeting human PHLPP1 (shPHLPP1-1: 5’-GGAAGACGCTGCTTCTGAA-3’; shPHLPP1-2, 5’-GAATGTATAATGTCCGTAA-3’; and shPHLPP1-3, 5’-GGCTGCGACAAGTCTCCAA-3’), and negative control (shNC, 5’-TTCTCCGAACGTGTCACGTTT-3’) were synthesized and constructed into lentiviral plasmids (pLKO.1). For overexpression, pLVX-puro lentiviral plasmid containing PHLPP1 (NM_194449.4) cDNA was used along with an empty vector as a control (oeNC). Transfection was performed using the Lipofectamine 2000 system. Subsequently, 48 h after transfection, recombinant vector in the cell supernatant was collected and transduced into KGN cells.
Cell proliferation
Cell Counting Kit-8 (CCK-8; Dojindo, Japan) was used to detect cell proliferation. Briefly, KGN cells were planted into 96-well culture plate with a primary density of 3 × 103 cells per well. At the specified time points, CCK-8 solution was added into the cells at 37 °C for 2 h incubation, and the absorbance was scanned by a microplate reader.
Flow cytometry assay
The staining procedures followed two steps: (1) 20 min of incubation with Annexin-V-FITC at 4 °C and (2) another 20 min of incubation with propidium iodide (PI). A flow cytometer (BD Biosciences) was used to measure apoptosis.
ROS production
Intracellular ROS accumulation was measured via 2’,7’-diclorodihydrofluorescein diacetate (DCFDA) staining. Briefly, KGN cells were incubated with 10 µM DCFDA in PBS for 25 min at 37 °C, and nuclei were stained with DAPI for 10 min. The cells were collected and analysed using a flow cytometer.
Glycolysis assay
Extracellular acidification rate (ECAR) was measured with a XF24 Extracellular Flux Analyzer [27]. Briefly, cells digested to a density of 1 × 104/well, were seeded in XF-24 culture plates (Agilent Technologies, Santa Clara, CA, USA), and were then placed in an incubator of 37 °C and 5% CO2 for 24 h. Around 1 h before detection, culture medium was replaced by XF Base Medium (Agilent Technologies). Subsequently, the cells were treated sequentially with 1 µM of glucose, 1 µM of oligomycin (ATP synthase inhibitor) and 0.5 µM of 2-DG (the glycolytic inhibitor) at time points for measurement of ECAR.
Sample collections
This study was approved by the Ethics Committee of Affiliated Hospital of Inner Mongolia Minzu University. Informed consents were obtained from women ages 25 to 38 years old. Granulosa cells were collected from 30 PCOS women [15 hyperandrogenic PCOS (HA-PCOS), 15 normo-androgenic PCOS (NA-PCOS)] and 15 controls who had normal endocrine as previously described [28]. Demographic and clinical parameters of participants were listed in Table 1.
Statistical analysis
GraphPad Prism8.4.2 was used for data analysis. Data was expressed as mean ± SD. Comparisons between 2 groups were performed by T-test, comparisons among multiple groups were performed by one-way ANOVA. p-values < 0.05 indicate statistical significance.
Results
DHT treatment inhibited the proliferation and glycolysis activity in human KGN cells
DHT was used to detect the action of high levels of androgen on proliferation and glycolysis activity in KGN cells. DHT treatment significantly suppressed KGN cell proliferation (Fig. 1A), and promoted the apoptosis of human KGN cells (Fig. 1B). DHT treatment also significantly inhibited glycolysis (Fig. 1C), and elevated ROS generation (Fig. 1D) in KGN cells. These data suggest that DHT treatment inhibited the proliferation and glycolysis of KGN cells.
DHT treatment inhibited the proliferation and glycolysis activity in KGN cells. A Proliferation of KGN cells was deeply decreased after DHT treatment. B DHT treatment significantly increased apoptosis of KGN cells. C The glycolysis activity of KGN cells was suppressed by DHT treatment. D DHT treatment significantly promoted the production of ROS in human KGN cells. * p < 0.05, *** p < 0.001 vs. Vehicle
Knockdown of PHLPP1 ameliorated the DHT-induced injury in human KGN cells
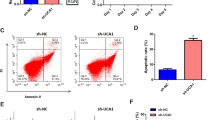
As mentioned above, PHLPPs are important regulars of glycolysis and AKT. To figure out whether PHLPPs play a role in DHT regulation of cell proliferation and glycolysis, the expression of PHLPPs was examined after DHT treatment. Results showed that DHT treatment time-dependently up-regulated PHLPP1 at both mRNA and protein levels, but did not affect PHLPP2 (Fig. 2A and B). Similarly, it was found that the mRNA and protein levels of PHLPP1 were also increased significantly in the HA-PCOS subjects compared with the controls or NA-PCOS subjects (Fig. 2C and D). Therefore, PHLPP1 was focused in the subsequent study. To study the role of PHLPP1 in DHT regulation of cell proliferation and glycolysis, lentivirus was used to silence PHLPP1. Results showed that PHLPP1 was successfully silenced (Fig. 3A and B). More importantly, silencing PHLPP1 significantly ameliorated DHT-suppressed proliferation of KGN cells (Fig. 3C). DHT treatment promoted apoptosis of KGN cells, which was abolished by silencing of PHLPP1 (Fig. 3D and E). The inhibitory effect of DHT on glycolysis was significantly suppressed by PHLPP1 knockdown (Fig. 3F). ROS levels were significantly elevated by DHT treatment, but silencing of PHLPP1 remarkably decreased ROS levels in KGN cells (Fig. 3G). These results demonstrate that knockdown of PHLPP1 ameliorated the DHT-induced injury in human KGN cells.
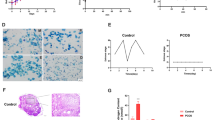
PHLPP1 is upregulated in DHT-treated KGN cells and patients with PCOS. A, B mRNA and protein levels of PHLPP1 and PHLPP2 in KGN cells after 6, 12 and 24 h of DHT treatment. C, D mRNA and protein levels of PHLPP1 in patients of the control, HA-PCOS, and NA-PCOS groups. ** p < 0.01,*** p < 0.001 vs. Vehicle or control. !!! p < 0.001 vs. NA-PCOS
Knockdown of PHLPP1 ameliorated the DHT-induced damage of KGN cells. A, B PHLPP1 was successfully silenced. C Proliferation of DHT-treated cells was significantly promoted by knockdown of PHLPP1. D, E Silencing PHLPP1 inhibited apoptosis of DHT-treated KGN cells. F Silencing PHLPP1 promoted glycolysis in DHT-treated KGN cells. G ROS production in DHT-treated cells was suppressed by knockdown of PHLPP1. * p < 0.05, *** p < 0.001 vs. Vehicle or shNC. !!! p < 0.001 vs. DHT + shNC
Inhibiting AKT rescued the effect of PHLPP1 silencing on cell proliferation and glycolysis in DHT-treated KGN cells
To figure out whether AKT signaling pathway play a role in DHT regulation of cell proliferation and glycolysis, the activation of AKT was examined after DHT treatment. Results showed that DHT treatment time-dependently down-regulated activation of AKT signaling pathway (Fig. 4A). To further investigate the role of AKT signaling, AKT inhibitor, MK-2206 was introduced. Administration of MK-2206 abolished DHT-suppressed proliferation of KGN cells with PHLPP1 silencing (Fig. 4B). MK-2206 also inhibited the glycolysis activity in DHT-treated PHLPP1-silencing KGN cells (Fig. 4C). Western blotting results indicated that MK-2206 significantly decreased levels of p-AKT induced by DHT and PHLPP1 silencing (Fig. 4D). Together, these results suggest that inhibiting AKT rescued the effect of PHLPP1 silencing on cell proliferation and glycolysis in DHT-treated KGN cells.
Inhibiting AKT rescued the function of PHLPP1 silencing in DHT-treated KGN cells. A, B Protein levels of AKT and p-AKT. B. AKT inhibitor MK-2206 abolished DHT-induced proliferation suppression in shPHLPP1-silencing KGN cells. C MK-2206 inhibited the glycolysis activity in DHT-treated PHLPP1-silencing KGN cells. D Levels of AKT and p-AKT. ** p < 0.01, *** p < 0.001 vs. Vehicle; !! p < 0.01, !!! p < 0.001 vs. DHT + shNC + Vehicle; ### p < 0.001 vs. DHT + shPHLPP1-1 + Vehicle
AKT activator SC79 disrupted the effect of PHLPP1 overexpression on cell proliferation and glycolysis in KGN cells
To further study PHLPP1 and AKT, PHLPP1 was successfully overexpressed (Fig. 5A and B), and an AKT activator, SC79, was introduced. Results demonstrated that administration of AKT activator, SC79, significantly increased proliferation of KGN cells with PHLPP1 overexpression (Fig. 5C). Administration of SC79 also remarkably increased glycolysis activity of KGN cells with PHLPP1 overexpression (Fig. 5D). Western blotting results indicated that SC79 significantly increased level of p-AKT in KGN cells with PHLPP1 overexpression (Fig. 5E). These data suggest that the AKT activator, SC79, disrupted the effect of PHLPP1 overexpression on cell proliferation and glycolysis in KGN cells.
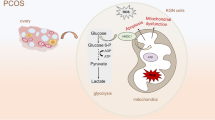
AKT activator SC79 disrupted the function of PHLPP1 overexpression. A, B PHLPP1 was successfully overexpressed. C SC79 promoted the proliferation of PHLPP1-overexpressing KGN cells. D SC79 promoted glycolysis of PHLPP1-overexpressing KGN cells. E Expression of AKT and p-AKT. F Schematic diagram of the mechanism by which PHLPP1 inhibits the growth and glycolysis of human ovarian granular cells through inactivating AKT pathway. ** p < 0.01, *** p < 0.001 vs. oeNC; !!! p < 0.001 vs. oePHLPP1 + Vehicle
Discussion
We demonstrated that DHT treatment suppressed proliferation, promoted apoptosis and ROS, and inhibited glycolysis of KGN cells, which were ameliorated by silencing PHLPP1. Mechanism study indicated that DHT-caused injury in PHLPP1-silencing cells was abolished by administration of AKT inhibitor. In contrast, administration of AKT activator disrupted the function of PHLPP1. For the first time, our study indicated that PHLPP1 mediates cell proliferation, apoptosis, ROS generation, and glycolysis of KGN cells by regulating AKT signaling pathway (Fig. 5F).
Converting glucose to pyruvate, glycolysis involves in a many bioprocesses [11]. Glycolysis plays a fundamental role in supporting cell growth and proliferation [12]. More importantly, it has been reported that perturbation of the expression of glycolytic enzymes was associated with infertility [16]. Zhao et al. have reported that enhanced glycolysis and inhibited tricarboxylic acid cycle were found in women with PCOS [17]. Here, we found that DHT administration resulted in significant damage of KGN cells through regulating PHLPP1 expression. Data demonstrates, for the first time, that silencing PHLPP1 ameliorated DHT-induced cell injury.
AKT plays a very important role in cell growth, division, and apoptosis [29]. AKT activation increases glycolysis [25]. Hojlund et al. have shown that AKT activation was significantly inhibited in PCOS skeletal muscle [30]. These data suggested that the status of AKT pathway might be tissue or cell-type specific. Our study indicated that inhibition of AKT rescued the role of PHLPP1 silencing in DHT-treated KGN cells, while activation of AKT disrupted the function of PHLPP1 overexpression. The findings suggest that PHLPP1 regulates glycolysis and cell proliferation through regulating AKT, and indicate that targeting AKT/PHLPP1 might provide a new direction for manipulating glycolysis and cell proliferation.
PHLPP1, first discovered by Gao et al., suppresses growth factor signaling through dephosphorylating Akt [20]. Overexpression of PHLPP1 has been shown to inhibit cell proliferation [23], while silencing of PHLPP1 has been shown to contribute to cell growth through regulating of AKT phosphorylation [23]. Studies also supported that AKT activation promoted glucose uptake and aerobic glycolysis [25, 26]. In the current study, we revealed a role of PHLPP1, showing that PHLPP1 suppressed glycolysis and promoted apoptosis of KGN cells through inhibition of AKT signaling pathway. Our study further showed PHLPP1 overexpression-caused cell injury could be abolished by administration of AKT activator, SC79. The results improved the understanding of the possible mechanism by which PHLPP1 regulates the proliferation and glycolysis of KGN cells. Previous studies have demonstrated that glycolytic enzymes are regulated by complex mechanisms, such as AKT, ERK, and NF-κB pathways [31,32,33]. The AKT pathway is responsible for glycolysis by regulating HIF-1α target genes ENO1 and LDHA [34, 35]. These data indicate that PHLPP1 may regulate ENO1 and LDHA expression via the AKT/HIF-1α signaling pathway. Xiong et al. demonstrated that PHLPP1 suppresses glycolysis via inhibiting Akt phosphorylation and mitochondrial localization of HK2, a key enzyme that determines the direction and magnitude of glucose flux [36]. However, whether other glycolytic enzymes and signlaing pathways are regulated by PHLPP1 should be further determined. It is worth to mention that only in vitro experiments and human ovarian granular KGN cells were included. Future in vivo studies and using primary cultured granulosa cells of women with PCOS may show more meaningful results. Although further studies are needed, this study identifies a new molecular mechanism by which PHLPP1/AKT/glycolysis regulates DHT-induced injury of human ovarian granular cells.
Conclusions
Taken together, the results revealed a novel role of PHLPP1/AKT axis, indicating that knockdown of PHLPP1 ameliorated the DHT-induced injury in human KGN cells through regulation of AKT signaling pathway. The data highlighted the significance of PHLPP1/AKT axis which may benefit the treatment of PCOS.
Availability of data and materials
All data generated or analysed during this study are available from the corresponding author on reasonable request.
Abbreviations
- PCOS:
-
Polycystic ovary syndrome
- PHLPP1:
-
PH domain Leucine-rich repeat Protein Phosphatase 1
- DHT:
-
Dihydrotestosterone
- ECAR:
-
Extracellular acidification rate
- AKT:
-
Protein kinase B
- DCFDA:
-
2’,7’-dichlorofluorescein diacetate
References
Lindsay TJ, Vitrikas KR. Evaluation and treatment of infertility. Am Family Phys. 2015;91(5):308–14.
Aghajanova L, Hoffman J, Mok-Lin E, Herndon CN. Obstetrics and gynecology residency and fertility needs. Reproductive Sci. 2017;24(3):428–34.
Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76.
Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Reviews Endocrinol. 2018;14(5):270–84.
McCartney Ch R, Marshall JC. Polycystic ovary syndrome. N Engl J Med. 2016;375(14):1398–9.
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–9.
Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics. 2015;70(11):765–9.
Witchel SF, Oberfield SE, Pena AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc. 2019;3(8):1545–73.
Shaw J, Bulsara C, Cohen PA, Gryta M, Nichols CB, Schofield L, O’Sullivan S, Pachter N, Hardcastle SJ. Investigating barriers to genetic counseling and germline mutation testing in women with suspected hereditary breast and Ovarian cancer syndrome and Lynch syndrome. Patient Educ Couns. 2018;101(5):938–44.
Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Family Phys. 2016;94(2):106–13.
Li XB, Gu JD, Zhou QH. Review of aerobic glycolysis and its key enzymes - new targets for Lung cancer therapy. Thorac cancer. 2015;6(1):17–24.
Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64.
Lim SO, Li CW, Xia W, Lee HH, Chang SS, Shen J, Hsu JL, Raftery D, Djukovic D, Gu H, et al. EGFR Signaling enhances aerobic glycolysis in Triple-negative Breast Cancer cells to promote Tumor Growth and Immune Escape. Cancer Res. 2016;76(5):1284–96.
Xiang C, Zhang Y, Chen Q, Sun A, Peng Y, Zhang G, Zhou D, Xie Y, Hou X, Zheng F, et al. Increased glycolysis in skeletal muscle coordinates with adipose tissue in systemic metabolic homeostasis. J Cell Mol Med. 2021;25(16):7840–54.
Narisawa S, Hecht NB, Goldberg E, Boatright KM, Reed JC, Millan JL. Testis-specific cytochrome c-null mice produce functional sperm but undergo early testicular atrophy. Mol Cell Biol. 2002;22(15):5554–62.
Liu X, Li Q, Wang W, Liu F. Aberrant expression of spermspecific glycolytic enzymes are associated with poor sperm quality. Mol Med Rep. 2019;19(4):2471–8.
Zhao Y, Fu L, Li R, Wang LN, Yang Y, Liu NN, Zhang CM, Wang Y, Liu P, Tu BB, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med. 2012;10:153.
Wang T, Zhang J, Hu M, Zhang Y, Cui P, Li X, Li J, Vestin E, Brannstrom M, Shao LR, et al. Differential expression patterns of Glycolytic Enzymes and Mitochondria-Dependent apoptosis in PCOS patients with Endometrial Hyperplasia, an early Hallmark of Endometrial Cancer, in vivo and the impact of Metformin in Vitro. Int J Biol Sci. 2019;15(3):714–25.
Chen MJ, Dixon JE, Manning G. Genomics and evolution of protein phosphatases. Sci Signal. 2017;10(474):eaag1796.
Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates akt, promotes apoptosis, and suppresses Tumor growth. Mol Cell. 2005;18(1):13–24.
Grzechnik AT, Newton AC. PHLPPing through history: a decade in the life of PHLPP phosphatases. Biochem Soc Trans. 2016;44(6):1675–82.
Moc C, Taylor AE, Chesini GP, Zambrano CM, Barlow MS, Zhang X, Gustafsson AB, Purcell NH. Physiological activation of akt by PHLPP1 deletion protects against pathological hypertrophy. Cardiovascular Res. 2015;105(2):160–70.
Dong L, Jin L, Tseng HY, Wang CY, Wilmott JS, Yosufi B, Yan XG, Jiang CC, Scolyer RA, Zhang XD, et al. Oncogenic suppression of PHLPP1 in human Melanoma. Oncogene. 2014;33(39):4756–66.
Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20(2):74–88.
Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23(20):7315–28.
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64(11):3892–9.
Guo Y, Liang F, Zhao F, Zhao J. Resibufogenin suppresses Tumor growth and Warburg effect through regulating miR-143-3p/HK2 axis in Breast cancer. Mol Cell Biochem. 2020;466(1–2):103–15.
Yan MQ, Wang Y, Wang Z, Liu XH, Yang YM, Duan XY, Sun H, Liu XM. Mitoguardin2 is Associated with Hyperandrogenism and regulates steroidogenesis in human ovarian granulosa cells. J Endocr Soc. 2023;7(5):bvad034.
Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proc Natl Acad Sci USA. 2007;104(29):12105–10.
Hojlund K, Glintborg D, Andersen NR, Birk JB, Treebak JT, Frosig C, Beck-Nielsen H, Wojtaszewski JF. Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes. 2008;57(2):357–66.
Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, Dai W, Guo C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Experimental Clin cancer Research: CR. 2020;39(1):1–19.
Wang Y, Xian H, Qi J, Wei F, Cheng X, Li S, Wang Q, Liu Z, Yu Y, Zhou J, et al. Inhibition of glycolysis ameliorate arthritis in adjuvant arthritis rats by inhibiting synoviocyte activation through AMPK/NF-кB pathway. Inflamm Research: Official J Eur Histamine Res Soc [et al]. 2020;69(6):569–78.
Wen LJ, Hu XL, Li CY, Liu J, Li ZY, Li YZ, Zhou JY. Myosin 1b promotes migration, invasion and glycolysis in Cervical cancer via ERK/HIF-1α pathway. Am J Translational Res. 2021;13(11):12536–48.
Pan T, Sun S, Chen Y, Tian R, Chen E, Tan R, Wang X, Liu Z, Liu J, Qu H. Immune effects of PI3K/Akt/HIF-1α-regulated glycolysis in polymorphonuclear neutrophils during sepsis. Crit Care. 2022;26(1):29.
Reece KM, Richardson ED, Cook KM, Campbell TJ, Pisle ST, Holly AJ, Venzon DJ, Liewehr DJ, Chau CH, Price DK, et al. Epidithiodiketopiperazines (ETPs) exhibit in vitro antiangiogenic and in vivo antitumor activity by disrupting the HIF-1α/p300 complex in a preclinical model of Prostate cancer. Mol Cancer. 2014;13:91.
Xiong X, Wen YA, Mitov MI, Miyamoto MCO, Gao S. PHLPP regulates hexokinase 2-dependent glucose metabolism in colon Cancer cells. Cell Death Discovery. 2017;3:16103.
Acknowledgements
Not applicable.
Funding
Natural Science Foundation of Inner Mongolia (2022MS08073 and 2021LHBS08004).
Author information
Authors and Affiliations
Contributions
AW designed the project and revised the manuscript. XY performed the experiments and drafted the manuscript. MA, TG and BD analyzed the data and edited the figures. YZ provided technical assistance. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by Medical Ethics Committee of Affiliated Hospital of Inner Mongolia Minzu University. Informed consent was signed by all participants in this study. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, X., A, M., Gegen, T. et al. PHLPP1 inhibits the growth and aerobic glycolysis activity of human ovarian granular cells through inactivating AKT pathway. BMC Women's Health 24, 25 (2024). https://doi.org/10.1186/s12905-023-02872-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02872-5