Abstract
Background
The aim of this study was to investigate the association between fasting blood glucose and osteoporosis in women with diabetes, anemia, and renal function.
Methods
The medical records of women who underwent a general health examination at a regional hospital in southern Taiwan were retrospectively reviewed. Logistic regression analysis was performed to assess the association between osteoporosis and fasting blood glucose separately for the eight subgroups (diabetes or non-diabetes, anemia or non-anemia, normal or decreased renal function), adjusting for other clinical characteristics and laboratory findings.
Results
A total of 11,872 women were included in the study. Among women with diabetes, anemia, and decreased renal function, an increment of 10 mg/dL in fasting blood glucose was associated with an increased risk of osteoporosis (adjusted odds ratio [aOR] = 1.57, p = 0.004). Among women without diabetes, fasting blood glucose was significantly associated with an increased risk of osteoporosis in those with anemia and normal renal function (OR = 1.14, p = 0.023) and those without anemia and normal renal function (OR = 1.04, p = 0.015), but these associations were not significant after adjusting for other covariates.
Conclusions
Higher fasting blood glucose levels in women with diabetes, anemia, and decreased renal function were associated with an increased risk of osteoporosis. Clinicians should be vigilant about glucose control in patients with diabetes to reduce the risk of fracture.
Similar content being viewed by others
Introduction
Diabetes and osteoporosis are prevalent diseases with significant associated morbidity and mortality worldwide [1]. Well-known complications of diabetes include microvascular disease (nephropathy, retinopathy, and neuropathy), macrovascular disease (acute coronary syndrome), anemia, and stroke [2]. Both type 1 and 2 diabetes are associated with inferior bone quality and strength, leading to an increased fracture risk [3,4,5].
Diabetic kidney disease affects approximately 40% of people with diabetes and is the leading cause of chronic kidney disease worldwide [6]. It is also one of the main causes of death in these patients [7]. The cross-sectional association between renal function and bone mineral density (BMD) is strongest at higher stages of chronic kidney disease [8]. Despite decreased BMD in type 1 diabetes, BMD in type 2 diabetes is often normal or even slightly elevated compared with age-matched controls [9]. However, a cohort study of 1690 men and 1641 women in Taiwan found that type 2 diabetes was significantly associated with an increased risk of osteoporosis, especially among younger participants [10].
Patients with poorly controlled diabetes and those with diabetes and renal insufficiency are more likely to develop anemia [11]. A retrospective observational study of 19,059 adult patients with type 2 diabetes showed a higher prevalence of anemia (38.5%) in women with diabetes [12]. In another cross-sectional study of 249 patients with type 2 diabetes, poor glycemic control, decreased estimated glomerular filtration rate (eGFR), presence of diabetes-related complications, duration of diabetes > 10 years, and age > 60 years were significantly associated with the development of anemia in these patients [13]. Low hemoglobin levels are also associated with a high risk of osteoporosis in adults [14].
Given the existing ambiguity surrounding the relationship between blood glucose levels and osteoporosis in women, particularly those with coexisting conditions with diabetes, there is a need for more comprehensive research. Understanding how these conditions affect bone metabolism is crucial, as this knowledge can aid clinicians in effectively managing patients with diabetes, given the vital role of bone health in overall patient quality of life. Therefore, the present study aimed to explore the association between elevated fasting blood glucose levels and the risk of osteoporosis in women, focusing on the associations in those having various combinations of diabetes, anemia, and renal insufficiency.
Materials and methods
Study participants and variables
In this retrospective study, medical records of women who had undergone a general health examination at a regional teaching hospital in southern Taiwan between June 2014 and July 2020 were identified and reviewed. The study protocol was approved by the institutional review board of Dalin Tzu Chi Hospital (IRB No. B11001010), and the requirement for obtaining informed consent from patients was waived.
The medical records of the participants were reviewed to obtain information on the following: (1) anthropometric characteristics, including age and body mass index; (2) comorbidities, including hypertension, diabetes mellitus, hyperlipidemia, and chronic kidney disease; (4) laboratory findings, including hemoglobin, systolic blood pressure, low-density lipoprotein cholesterol (LDL-C), fasting blood glucose, estimated glomerular filtration rate (eGFR), and alkaline phosphatase (ALP). Participants with metal materials in the measured areas of bone mineral density were excluded from the study. In addition, those who smoked or consumed alcohol were excluded to eliminate the potential confounding effects of these risk factors for osteoporosis.
Measurements
BMD at the lumbar spine and bilateral hips, including the total and femoral neck regions, was determined using dual-energy X-ray absorptiometry (DXA) with a DiscoveryWi DXA system (Hologic Inc., Marlborough, MA, USA). Participants were categorized into those with osteoporosis (T-score ≤ − 2.5 standard deviation [SD]) or non-osteoporosis (T-score > − 2.5 SD) based on the World Health Organization classification, as determined by their lowest T-score among the three measured sites [15].
Subgroup categorization
Subgroups for stratified analysis were created based on information obtained from questionnaires and medical records. In accordance with the World Health Organization recommendations [16], anemia was defined as a hemoglobin level < 12.0 g/dL in women. Anemia levels were also classified as mild (hemoglobin level 11.0–11.9 g/dL), moderate (hemoglobin level 8.0–10.9 g/dL), and severe (hemoglobin level < 8.0 g/dL) in non-pregnant women [17].
Kidney function was evaluated by eGFR, which was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation [18]: 186 × (serum creatinine in mg/dL)−1.154 × (age)−0.203 × 0.742 (if female). Participants were divided into two groups based on their eGFR: decreased renal function if eGFR < 90 mL/min/1.73m2 and normal renal function if eGFR ≥ 90 mL/min/1.73m2. Eight subgroups were created based on the three factors of diabetes, anemia, and renal function. These subgroups were then analyzed separately in the regression analyses.
Statistical analysis
Summary statistics were presented as means with SDs or as numbers with percentages, as appropriate. Differences in means or frequencies of characteristics and variables between the eight subgroups were evaluated using the Chi-square test or the independent t-test, as applicable.
Univariate logistic regression analysis was conducted using the osteoporotic state (osteoporosis or non-osteoporosis) as the dependent variable and fasting blood glucose, as well as other clinical characteristics and laboratory findings, as independent variables. Due to the presence of interactions between diabetes and anemia as well as anemia and renal function, stratified analyses were conducted based on the eight defined groups, which combined the statuses of diabetes, anemia, and renal impairment. Variables exhibiting a p-value less than 0.20 in the univariate analysis were subsequently included in the multiple logistic regression analysis to further assess their independent associations with osteoporosis. In addition, multiple logistic regression analyses were performed to explore the independent association between fasting blood glucose and osteoporosis, adjusting for clinical and laboratory variables that were significantly associated with osteoporosis in the univariate analysis.
All statistical analyses were performed using PASW Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered to indicate statistical significance.
Results
Characteristics of the study participants
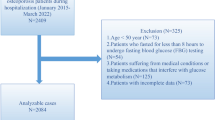
A total of 11,872 women were included in the study, comprising 838 with diabetes, 11,034 without diabetes, 1,653 with anemia, 10,219 without anemia, 2,427 with an eGFR < 90 mL/min/1.73 m², and 9,445 with an eGFR ≥ 90 mL/min/1.73 m². According to their T-score for BMD, 3312 of the women were classified as having osteoporosis, representing 27.9% of the study participants. Figure 1 presents a flowchart of the study participants.
Table 1 shows the demographic and clinical characteristics of women, stratified by renal function, diabetes, and anemia status. First, women with normal renal function and anemia who also had diabetes were significantly older, had higher body mass index, higher proportions of hypertension and hyperlipidemia, higher systolic blood pressure, lower LDL-C, higher fasting blood glucose, and higher proportions of osteoporosis, compared to those without diabetes. Second, women with normal renal function but without anemia who had diabetes were significantly older, had higher body mass index, higher hemoglobin, higher proportions of hypertension and hyperlipidemia, higher systolic blood pressure, a lower LDL-C, and higher fasting blood glucose, compared to those without diabetes. Third, women with decreased renal function and anemia who had diabetes were significantly older, had higher body mass index, higher proportions of hypertension and hyperlipidemia, higher systolic blood pressure, lower LDL-C, higher fasting blood glucose, and lower eGFR, compared to those without diabetes. Finally, women with decreased renal function and without anemia who had diabetes were significantly older, had higher body mass index, lower hemoglobin, higher proportions of hypertension and hyperlipidemia, lower LDL-C, higher fasting blood glucose, and lower eGFR, compared to those without diabetes. In addition, the level of anemia was not significant different between diabetes and non-diabetes in normal renal function and decreased renal function.
Table 2 presents the BMD and T-score of women, stratified by renal function, diabetes, and anemia. In women with anemia, BMD and T-scores at the three measured sites showed no significant differences between those with and without diabetes, regardless of renal function status. Conversely, in women without anemia, the bilateral hip total BMD and T-scores were higher in those with diabetes, irrespective of renal function. In addition, women with decreased renal function, diabetes also had significantly higher lumbar spine BMD and T-score, as well as right hip femoral neck BMD, and left hip femoral neck BMD and T-score.
Univariate logistic regression analysis of osteoporosis
Among women without diabetes, 3068 of them (27.8%) were diagnosed with osteoporosis. Logistic regression analysis (Table 3) showed that several factors were significantly associated with an increased risk of osteoporosis in this group, including older age, lower body mass index, higher hemoglobin, non-anemia, hypertension, hyperlipidemia, higher systolic blood pressure, higher LDL-C, higher fasting blood glucose, lower eGFR, and higher ALP levels.
Among women with diabetes, 244 (29.1%) were diagnosed with osteoporosis. The results of the logistic regression analysis (Table 4) revealed that increased risk of osteoporosis was significantly associated with older age, lower body mass index, lower hemoglobin, and higher ALP levels.
Table 5 shows the results of univariate logistic regression analysis assessing the association between fasting blood glucose and osteoporosis, stratified by diabetes, anemia, and renal function status. In women without diabetes and with normal renal function, an increase of 10 mg/dL in fasting blood glucose was significantly associated with an increased risk of osteoporosis in those with anemia (OR = 1.14, p = 0.023) and those without anemia (OR = 1.04, p = 0.015). Among women with diabetes, an increase of 10 mg/dL in fasting blood glucose was significantly associated with an increased risk of osteoporosis only in those with anemia and decreased renal function (OR = 1.25, p = 0.010).
Multiple logistic regression analysis of osteoporosis and fasting blood glucose
Table 6 presents the results of the multiple logistic regression analysis after adjusting for age, body mass index, hemoglobin, systolic blood pressure, LDL-C, eGFR, and ALP. Among women with diabetes, an increase of 10 mg/dL in fasting blood glucose was independently and significantly associated with an increased risk of osteoporosis in those with concurrent anemia and decreased renal function (adjusted OR = 1.57, p = 0.004). Conversely, for women without diabetes, fasting blood glucose was not significantly associated with osteoporosis after adjusting for other covariates, irrespective of their anemia or renal function status.
Discussion
Osteoporosis, bone mineral density, and fasting blood glucose
Osteoporosis is a silent disease that is associated with substantial morbidity, mortality, and economic burden [19]. Both type 1 and type 2 diabetes have been linked to an increased risk for osteoporosis-related fractures. However, the relationship between diabetes and BMD is still inconclusive. Table 7 presents studies that examine the relationships between diabetes, anemia, and fasting blood glucose levels in connection with osteoporosis, bone mineral density, and the risk of fractures. A systematic review of 47 articles published between January 1950 and October 2010 revealed that 26 articles showed increased BMD in patients with diabetes, 13 articles showed decreased BMD, and eight articles reported no difference in bone mass [20]. In patients with type 2 diabetes, factors such as obesity, increased bone load, and insulin resistance can contribute to hyperinsulinemia, leading to increased bone formation [21]. Yet, despite having higher BMD, these patients are still at an increased risk of non-vertebral fractures [22]. In this study, increased BMD was observed among women with diabetes and without anemia only in specific measured sites, regardless of renal function. Older age and female sex were associated with an increased risk of osteoporosis [23], and the association was also observed among those with type 2 diabetes [24]. Being overweight or obese and having a disease duration over five years have also been implicated in the development of osteoporosis. Uncontrolled blood glucose levels are a major contributor to osteoporosis [24].
Some studies, but not all, have shown that higher fasting blood glucose was associated with increased BMD and a reduced risk of osteoporosis in patients both with and without diabetes [21, 25, 26]. Considering the temporal trajectories of fasting plasma glucose, the relationship between fasting blood glucose and osteoporosis risk appears bidirectional. In individuals with diabetes, those who maintained high levels of fasting blood glucose over time had the highest risk of osteoporosis (OR = 3.09; 95% [confidence interval] CI 1.16–8.22), and those with moderate-high fasting blood glucose levels had the second-highest risk (OR = 2.49; 95% CI 1.02–6.12) in men. Among those without diabetes, an elevated-increasing trajectory of fasting blood glucose was inversely associated with osteoporosis risk in women (OR = 0.62; 95% CI 0.43–0.88) [25].
Diabetes and osteoporosis
Fasting blood glucose is a key indicator for diagnosing diabetes and evaluating glucose homeostasis. It is influenced by various cytokines secreted by bone and regulates the differentiation and maturation of osteoblasts [27]. Elevated blood glucose levels can stimulate collagen synthesis and bone formation by osteoblasts [28, 29]. However, a high glucose state with insulin resistance could impair bone formation, leading to diabetic bone disease [27, 30]. A higher degree of insulin resistance was associated with an increased risk of osteoporosis [31]. Several candidate loci affecting fasting glucose and BMD have been identified, such as rs6867040 on ITGA1 [32]. It is essential to consider not only the temporal trajectory of fasting blood glucose but also comorbidities in its influence on osteoporosis. To date, no studies have examined the potential impact of combining hemoglobin and eGFR, which are affected by diabetes, on the relationship between fasting blood glucose and osteoporosis.
The main finding of the current study was that fasting blood glucose was independently and significantly associated with an increased risk of osteoporosis in women with diabetes, anemia, and decreased renal function. Anemia and renal insufficiency, common complications of diabetes, can contribute to osteoporosis development. Elevated blood glucose levels may adversely impact bone matrix properties [33], directly or indirectly through sarcopenia [34]. Hyperglycemia and the accumulation of advanced glycation end products (AGEs) in collagen can lead to decreased bone mass and strength, increasing fracture risk [35, 36]. Persistent elevated blood glucose levels can result in the formation of AGEs, impairing osteoblast function and leading to osteoporosis [37]. Bone formation markers osteocalcin and amino-terminal propeptide of procollagen type 1 (PINP) were reduced in patients with diabetes [38, 39], and their levels were inversely correlated with glucose levels [40]. This finding supports the notion that patients with type 2 diabetes have lower biochemical indices of bone formation than controls. Recent reports indicated that patients with poor glycemic control had lower 25-hydroxy vitamin D levels [41], which may be a biological cause of osteoporosis.
Anemia and osteoporosis
Anemia, particularly when caused by nutritional deficiencies, can compromise bone health due to inadequate nutrient supply necessary for bone formation [14]. Iron deficiency anemia, a common complication of chronic kidney disease [42], is an independent risk factor for osteoporosis [43]. Among patients with comorbidities, a consistent association between low hemoglobin levels and osteoporosis has been observed [14]. Chronic iron deficiency anemia is thought to induce bone resorption, increasing the risk of osteoporosis [44]. Reduced blood volume promotes the proliferation of hematopoietic cells, including osteoclasts, leading to increased bone resorption. While blood loss can stimulate osteoblast formation, heightened bone resorption may disrupt bone remodeling cycles, potentially inducing osteoblast fatigue [45]. In addition, inflammation may also mediate the association between anemia and osteoporosis. Studies have shown that pro-inflammatory cytokines can affect hematopoiesis [46], and interleukin 6 (IL-6) may promote osteoclast differentiation and activation [47]. Anemia, characterized by decreased red blood cells or hemoglobin concentration, leads to reduced oxygen delivery to tissues, including bone. Insufficient oxygen supply can negatively impact bone cells and remodeling [48]. Chronic hypoxia may increase oxidative stress levels, and acidification of the extracellular matrix can impair bone metabolism [49]. Hypoxia has been demonstrated to decrease BMD in both humans and rats [50]. The coexistence of chronic diseases could amplify the effect of an anemic condition on osteoporosis [49]. Hypoxia-inducible factors (HIFs), crucial in the cellular response to hypoxia [51], with HIF1-α and HIF2-α may impact osteoblast and osteoclast functions in bone homeostasis [52, 53]. Iron deficiency directly or indirectly impedes osteoblastic differentiation and promotes osteoclastic differentiation by leading to hypoxia-induced increases in HIF-1/2α expression and a reduction in active vitamin D levels [54]. Adequate nutrient intake and normal red blood cell production generally support bone health in non-anemic individuals, but factors such as hormonal balance and physical activity can also play significant roles.
Renal function and osteoporosis
A Korean study supported the association between eGFR and BMD, suggesting that osteoporosis might be highly prevalent in patients with moderate to severe chronic kidney disease [55]. Long-term complications of diabetes, particularly micro- or macroalbuminuria and decreased renal function, have been found to be associated with osteoporosis [23]. Calcium, phosphate, vitamin D, and parathyroid hormone (PTH) are vital for bone formation and mineralization, maintaining normal bone homeostasis. This process relies on the interplay of various factors such as PTH, vitamin D, calcitonin, fibroblast growth factor-23, and klotho, which regulate calcium and phosphate levels. The parathyroid gland, kidney, and intestine are involved in this system [56]. Serum levels of vitamin D and PTH play pivotal roles as mediators in the link between renal function and bone health [8]. Reduced renal function can lead to decreased activation of vitamin D and retention of phosphorus, potentially lowering serum calcium levels and leading to increased PTH secretion, then secondary hyperparathyroidism. Elevated PTH levels can stimulate bone resorption, leading to bone loss and decreased bone mineral density, which can increase bone turnover and osteoporosis risk [57]. Metabolic acidosis, commonly seen in advanced chronic kidney disease, can lead to bone buffering, where bone minerals are used to buffer the excess acid, resulting in bone loss [58, 59]. With normal renal function, the body can effectively regulate mineral balance, thereby supporting optimal bone health, provided other risk factors for osteoporosis are controlled.
Interactions between diabetes, anemia, and renal function
Diabetes is one of the leading causes of chronic kidney disease and can result in diabetic nephropathy, which is characterized by progressive kidney damage [60]. The decline in eGFR is nearly twice as rapid as in patients with type 2 diabetes mellitus compared to those without diabetes [61]. The interplay between diabetes and chronic kidney disease involves complex mechanisms, including inflammation, oxidative stress, and alterations in the renin-angiotensin-aldosterone system, all of which can accelerate renal dysfunction in patients with diabetes [62]. Diabetic nephropathy is associated with a higher risk of anemia in patients with diabetes. Moreover, the production of erythropoietin decreases as eGFR declines [63]. Chronic kidney disease can result in reduced erythropoietin production by the kidneys, leading to anemia due to inadequate stimulation of red blood cell production in the bone marrow [64]. In diabetes, chronic hyperglycemia and insulin resistance can impair erythropoietin production, contributing to anemia [13]. Approximately 90% of erythropoietin is produced by the kidneys [65]. With the decline of functional renal tissue in patients with chronic kidney disease, the body’s ability to produce sufficient erythropoietin in response to hypoxia in the kidney diminishes [66]. The prevalence of anemia gradually increases with the progressive stages of chronic kidney disease, being higher in patients with diabetes compared to non-diabetes counterparts, with diabetes being an independent predictor of anemia occurrence [67].
Schematic representation of biological interactions among diabetes, anemia, and renal function in the pathogenesis of osteoporosis. The diagram illustrates potential pathways through which diabetes mellitus (DM), anemia, and chronic kidney disease (CKD) may influence bone mineral density (BMD) and contribute to the development of osteoporosis (OS). Key molecular and physiological factors include advanced glycation end products (AGEs), erythropoietin (EPO), hypoxia-inducible factors (HIF), and parathyroid hormone (PTH), among others. The figure also denotes the roles of fasting blood glucose (FBS), insulin resistance (IR), and body mass index (BMI) in the context of bone metabolism, highlighting the complex interplay of systemic factors in bone health. (Ca: calcium; P: phosphorus; OC: osteocalcin; PINP: amino-terminal propeptide of type I procollagen)
Multifactorial influences on osteoporosis
Diabetes, obesity, and higher muscle strength in older men were associated with a lower prevalence of osteoporosis and anemia [68]. Age, sex, body weight, body mass index, high-density lipoprotein cholesterol (HDL-C), and diabetes are key predictors of osteoporosis [69]. Moreover, plasma levels of LDL-C and HDL-C have opposite effects on BMD in males and females. [70, 71]. Obesity, a significant risk factor for type 2 diabetes, might offer some protection against osteoporosis due to the positive correlation between body mass index and BMD [24].
The impact of diabetes with varying renal functions and anemic statuses on osteoporosis in women has not been thoroughly studied. However, higher levels of hemoglobin have been shown to be protective against osteoporosis in older men with type 2 diabetes [72]. Although patients with diabetes have higher BMD, they are still at increased risk of fracture due to poor bone quality [1]. BMD measurements alone do not fully reflect bone fragility in diabetes, as deterioration in bone quality, rather than loss of bone mass, is a critical factor.
A meta-analysis suggested fasting blood glucose might be a causal risk factor for a reduced hip bone area and increased BMD [73]. Poor glycemic control in type 2 diabetes was associated with increased fracture risk, despite higher BMD and thicker femoral cortex in these individuals [74]. As low BMD is linked with a greater risk of falls, maintaining normal blood glucose levels is vital for preventing osteoporosis. The detrimental effects of chronic high blood glucose levels on bone health should be acknowledged alongside other well-recognized complications of diabetes [75].
Chronically high fasting blood glucose levels can damage osteoblasts by glycosylating hemoglobin. This damage, combined with hyperinsulinemia, leads to harmful deposits in bone collagen. This issue, along with reduced serum levels of insulin-like growth factor, hypercalciuria, inflammation, microangiopathy, and progressive renal failure in diabetes, leads to poor bone health [76, 77]. In non-diabetic older women, higher fasting blood glucose level was associated with BMD. Conversely, low fasting blood glucose may increase the risk of osteoporosis [78].
Both diabetics and non-diabetics can exhibit insulin resistance and elevated insulin levels. Physiological concentrations of insulin can inhibit osteoclast activity while promoting the proliferation of bone-forming osteoblasts, along with the synthesis of collagen, ALP production, and glucose absorption. Insulin deficiency was associated with reduced bone mineralized surface area, osteoid surface, the rate of mineral deposition, as well as osteoblast activity [79]. Studies in mouse models have shown that, in the absence of hyperglycemia, increased insulin levels and insulin resistance might lead to lower bone turnover and higher areal BMD [80].
An inverse relationship exists between insulin resistance and BMD, suggesting that in type 2 diabetes mellitus, insulin resistance might diminish the beneficial effects of insulin on bones [31]. Our results from multiple logistic regression analysis suggested that while normal fasting blood glucose levels are essential for overall health, their influence on bone health in non-diabetic individuals might not be as significant as in those with diabetes. This is especially true when other risk factors for osteoporosis, such as decreased renal function and anemia, are not present.
Limitations and future directions
This study has several limitations that warrant mention. First, it is based on data from a relatively healthy population, comprised of individuals who participated in health examinations. This may affect the generalizability of our findings. Second, while menopausal status may affect the risk of bone fracture [81], we lacked information on the menopausal status of our participants. Nevertheless, the potential impact of menopause was indirectly adjusted by including age in all our multiple regression models. Third, our questionnaire did not include questions about gravidity or parity [82], history of thyroid surgery [83], or thyroid-related diseases [84]. As a result, we were unable to adjust for their effects on osteoporosis risk in our multiple regression models. Fourth, our dataset did not contain information on the type and duration of diabetes. Nevertheless, we can reasonably infer that the majority of cases were likely Type II diabetes, given its considerably higher prevalence compared to Type I diabetes. This inference is supported by a 2019 study, which reported that the standardized prevalence of Type II diabetes in women was 9% across all ages, and over 30% in those aged 60–79 years in 2014, compared to a mere 0.05% prevalence of Type I diabetes in women during the same period [85]. Therefore, we anticipate that the proportion of Type I diabetes in our study sample is relatively small and unlikely to impact our conclusions significantly. Fifth, information on medication usage was not available. It is known that medications for hypertension and diabetes can influence bone status. Some medications can increase fracture risk, while others may increase BMD [86]. Sixth, while we have data on areal BMD via DXA, bone microarchitecture and extensive laboratory data, including hormone profiles, bone markers, and vitamin D levels, were unavailable from the health examination records. Previous research has shown that postmenopausal women with type 2 diabetes had significantly lower BMD, serum osteocalcin, and osteopontin levels, which could be vital in osteoporosis screening for patients with diabetes [87]. A cohort study of patients with diabetes found that volumetric BMD measured by quantitative computed tomography was more reliable for diagnosing osteoporosis and assessing fracture risk compared with areal BMD measured by DXA [88]. Future research should incorporate more diverse measurements such as trabecular bone score and glycated hemoglobin HbA1c.
Despite the aforementioned limitations, this study possesses several strengths contributing to its scientific value. One of the key strengths is the large sample size. Moreover, the simultaneous consideration of various combinations of diabetes, anemia, and renal function in examining the association between fasting blood glucose and osteoporosis risk represents a comprehensive approach rarely seen in previous research. This detailed analysis allows for a more thorough understanding of how these co-existing conditions might influence the risk of osteoporosis, offering valuable insights for clinical practice and future research. Furthermore, the interplay between osteoporosis and the trio of diabetes, anemia, and chronic renal disease is intricate and multifaceted, involving various physiological pathways and mechanisms, as illustrated in Fig. 2. These conditions may interact and potentially worsen each other’s progression and complications. This complexity highlights the need for prospective and animal studies to confirm the associations and further investigate the underlying mechanisms.
Conclusions
The findings from this retrospective medical review study showed that fasting blood glucose levels were independently and significantly associated with an increased risk of osteoporosis in women with coexistent conditions of diabetes, anemia, and chronic kidney disease beyond stage II. The results highlight the importance of diligent monitoring of both blood glucose and bone mineral density in these women, coupled with the initiation of early interventions. Maintaining long-term glycemic control is important to prevent diabetic complications, such as chronic kidney disease and anemia. Our findings offer further evidence of the benefits of stringent management in lowering osteoporosis risk. Moreover, the complexity of metabolic and renal dysfunctions in increasing osteoporosis risk necessitates a multidisciplinary approach. This should encompass nutritional status assessments, rigorous control of inflammation, maintaining hormonal balance, and meticulous medication management, all aimed at lessening the compounded risks associated with osteoporosis in patients with these complex health challenges.
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Abbreviations
- ALP:
-
Alkaline phosphatase
- BMD:
-
Bone mineral density
- CI:
-
Confidence interval
- DXA:
-
Dual-energy X-ray absorptiometry
- eGFR:
-
Estimated glomerular filtration rate
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- OR:
-
Odds ratio
References
Jackuliak P, Payer J. Osteoporosis, fractures, and diabetes. Int J Endocrinol. 2014;2014:820615.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 2):64–71.
Räkel A, Sheehy O, Rahme E, LeLorier J. Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes Metab. 2008;34(3):193–205.
Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495–505.
Romero-Díaz C, Duarte-Montero D, Gutiérrez-Romero SA, Mendivil CO. Diabetes and bone fragility. Diabetes Ther. 2021;12(1):71–86.
Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45.
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–76.
Jassal SK, von Muhlen D, Barrett-Connor E. Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res. 2007;22(2):203–10.
Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208–19.
Lin HH, Hsu HY, Tsai MC, Hsu LY, Chien KL, Yeh TL. Association between type 2 diabetes and osteoporosis risk: a representative cohort study in Taiwan. PLoS ONE. 2021;16(7):e0254451.
Aljohani AH, Alrubyyi MA, Alharbi AB, Alomair AM, Alomair AA, Aldossari NA, et al. The relation between diabetes type II and anemia. Egypt J Hosp Med. 2018;70(4):526–31.
AlDallal SM, Jena N. Prevalence of anemia in type 2 diabetic patients. J Hematol. 2018;7(2):57–61.
Taderegew MM, Gebremariam T, Tareke AA, Woldeamanuel GG. Anemia and its associated factors among type 2 diabetes mellitus patients attending Debre Berhan Referral Hospital, North-East Ethiopia: a cross-sectional study. J Blood Med. 2020;11:47–58.
Kim SY, Yoo DM, Min C, Choi HG. Association between osteoporosis and low hemoglobin levels: a nested case-control study using a national health screening cohort. Int J Environ Res Public Health. 2021;18(16):8598.
Kanis JA, Devogelaer JP, Gennari C. Practical guide for the use of bone mineral measurements in the assessment of treatment of osteoporosis: a position paper of the European foundation for osteoporosis and bone disease. The Scientific Advisory Board and the Board of National Societies. Osteoporos Int. 1996;6(3):256–61.
Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37.
World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. World Health Organization. 2011. Avaiable from: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1 [Accessed on 2023-11-26].
Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11(Suppl 2):155A.
Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C. The epidemiology of osteoporosis. Br Med Bull. 2020;133(1):105–17.
Abdulameer SA, Sulaiman SA, Hassali MA, Subramaniam K, Sahib MN. Osteoporosis and type 2 diabetes mellitus: what do we know, and what we can do? Patient Prefer Adherence. 2012;6:435–48.
Li KH, Liu YT, Yang YW, Lin YL, Hung ML, Lin IC. A positive correlation between blood glucose level and bone mineral density in Taiwan. Arch Osteoporos. 2018;13(1):78.
de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam study. Osteoporos Int. 2005;16(12):1713–20.
Gad S, Elagrody A. Are diabetes mellitus and diabetic nephropathy good predictors of osteoporosis. The Egyptian. J Hosp Med. 2021;82(3):497–501.
Quang TH, Hoai TP, Duy QD, Thi MH, Anh TN, Ngoc CN, et al. Associated osteoporosis factors in patients with type 2 diabetes. Nat Volatiles & Essent Oils. 2021;8(4):11110–20.
Wang P, Zhang Y, Shan R, Wu J, Man S, Deng Y, et al. Association between trajectories of fasting plasma glucose and risk of osteoporosis in non-diabetic and diabetic populations. Front Public Health. 2022;10:960928.
Park SK, Jung JY, Oh CM, Choi JM, Kim MH, Ha E, et al. Fasting glucose level and the risk of incident osteoporosis in the koreans. Bone. 2021;142:115690.
Liu JM, Rosen CJ, Ducy P, Kousteni S, Karsenty G. Regulation of glucose handling by the skeleton: insights from mouse and human studies. Diabetes. 2016;65(11):3225–32.
Wei J, Shimazu J, Makinistoglu MP, et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;161(7):1576–91.
Groesbeck DK, Bluml RM, Kossoff EH. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol. 2006;48(12):978–81.
Shanbhogue VV, Mitchell DM, Rosen CJ, Bouxsein ML. Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet Diabetes Endocrinol. 2016;4(2):159–73.
Wang X, Jiang L, Shao X. Association analysis of insulin resistance and osteoporosis risk in Chinese patients with T2DM. Ther Clin Risk Manag. 2 021;17:909–16.
Billings LK, Hsu YH, Ackerman RJ, Dupuis J, Voight BF, Rasmussen-Torvik LJ, et al. Impact of common variation in bone-related genes on type 2 diabetes and related traits. Diabetes. 2012;61(8):2176–86.
Yamaguchi T, Sugimoto T. Bone metabolism and fracture risk in type 2 diabetes mellitus. Endocr J. 2011;58(8):613–24.
Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123(2 Suppl):465–8.
Yamagishi S, Nakamura K, Inoue H. Possible participation of advanced glycation end products in the pathogenesis of osteoporosis in diabetic patients. Med Hypotheses. 2005;65(6):1013–5.
Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28(2):195–201.
Sanguineti R, Puddu A, Mach F, Montecucco F, Viviani GL. Advanced glycation end products play adverse proinflammatory activities in osteoporosis. Mediators Inflamm. 2014;2014:975872.
Rubin MR. Bone cells and bone turnover in diabetes mellitus. Curr Osteoporos Rep. 2015;13(3):186–91.
Rosen CJ, Chesnut CH 3rd, Mallinak NJ. The predictive value of biochemical markers of bone turnover for bone mineral density in early postmenopausal women treated with hormone replacement or calcium supplementation. J Clin Endocrinol Metab. 1997;82(6):1904–10.
Shu A, Yin MT, Stein E, Cremers S, Dworakowski E, Ives R, et al. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int. 2012;23(2):635–41.
Perez-Diaz I, Sebastian-Barajas G, Hernandez-Flores ZG, Rivera-Moscoso R, Osorio-Landa HK, Flores-Rebollar A. The impact of vitamin D levels on glycemic control and bone mineral density in postmenopausal women with type 2 diabetes. J Endocrinol Invest. 2015;38(12):1365–72.
Kim SY, Yoo DM, Min C, Choi HG. Association between osteoporosis and low hemoglobin levels: A nested case-control study using a national health screening cohort. Int J Environ Res Public Health. 2021;18(16):8598
Gafter-Gvili A, Schechter A, Rozen-Zvi B. Iron deficiency anemia in chronic kidney disease. Acta Haematol. 2019;142(1):44–50.
Pan ML, Chen LR, Tsao HM, Chen KH. Iron deficiency anemia as a risk factor for osteoporosis in Taiwan: a nationwide population-based study. Nutrients. 2017;9(6):616.
Toxqui L, Vaquero MP. Chronic iron deficiency as an emerging risk factor for osteoporosis: a hypothesis. Nutrients. 2015;7(4):2324–44.
Gurevitch O, Slavin S. The hematological etiology of osteoporosis. Med Hypotheses. 2006;67(4):729–35.
Baraldi-Junkins CA, Beck AC, Rothstein G. Hematopoiesis and cytokines. Relevance to cancer and aging. Hematol Oncol Clin North Am. 2000;14(1):45–61. viii.
Shahen VA, Gerbaix M, Koeppenkastrop S, Lim SF, McFarlane KE, Nguyen ANL, et al. Multifactorial effects of hyperglycaemia, hyperinsulinemia and inflammation on bone remodelling in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 2020;55:109–18.
Oh YH, Moon JH, Cho B. Association between hemoglobin level and bone mineral fensity in Korean adults. J Bone Metab. 2017;24(3):161–73.
Fujimoto H, Fujimoto K, Ueda A, Ohata M. Hypoxemia is a risk factor for bone mass loss. J Bone Miner Metab. 1999;17(3):211–6.
Upala S, Sanguankeo A, Congrete S. Association between obstructive sleep apnea and osteoporosis: a systematic review and meta-analysis. Int J Endocrinol Metab. 2016;14(3):e36317.
Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408.
Qin Q, Liu Y, Yang Z, Aimaijiang M, Ma R, Yang Y, et al. Hypoxia-inducible factors signaling in osteogenesis and skeletal repair. Int J Mol Sci. 2022;23(19):11201.
Mendoza SV, Genetos DC, Yellowley CE. Hypoxia-inducible factor-2α signaling in the skeletal system. JBMR Plus. 2023;7(4):e10733.
Yang J, Li Q, Feng Y, Zeng Y. Iron deficiency and iron deficiency anemia: potential risk factors in bone loss. Int J Mol Sci. 2023;24(8):6891.
Myong JP, Kim HR, Koo JW, Park CY. Relationship between bone mineral density and moderate to severe chronic kidney disease among general population in Korea. J Korean Med Sci. 2013;28(4):569–74.
Tasnim N, Dutta P, Nayeem J, Masud P, Ferdousi A, Ghosh AS, et al. Osteoporosis, an inevitable circumstance of chronic kidney disease: a systematic review. Cureus. 2021;13(10):e18488.
Brandenburg V, Ketteler M. Vitamin D and secondary hyperparathyroidism in chronic kidney disease: a critical appraisal of the past, present, and the future. Nutrients. 2022;14(15):3009.
Bushinsky DA. Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am J Physiol. 1996;271(1 Pt 2):F216–22.
Kraut JA, Madias NE. Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol. 2010;6(5):274–85.
Dabla PK. Renal function in diabetic nephropathy. World J Diabetes. 2010;1(2):48–56.
Warren B, Rebholz CM, Sang Y, Lee AK, Coresh J, Selvin E, et al. Diabetes and trajectories of estimated glomerular filtration rate: a prospective cohort analysis of the atherosclerosis risk in communities study. Diabetes Care. 2018;41(8):1646–53.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–25.
Mojiminiyi OA, Abdella NA, Zaki MY, El Gebely SA, Mohamedi HM, Aldhahi WA. Prevalence and associations of low plasma erythropoietin in patients with type 2 diabetes mellitus. Diabet Med. 2006;23(8):839–44.
Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316(2):73–8.
O’Mara NB. Anemia in patients with chronic kidney disease. Diabetes Spectr. 2008;21(1):12–20.
Hodges VM, Rainey S, Lappin TR, Maxwell AP. Pathophysiology of anemia and erythrocytosis. Crit Rev Oncol Hematol. 2007;64(2):139–58.
Loutradis C, Skodra A, Georgianos P, Tolika P, Alexandrou D, Avdelidou A, et al. Diabetes mellitus increases the prevalence of anemia in patients with chronic kidney disease: a nested case-control study. World J Nephrol. 2016;5(4):358–66.
Heidari B, Muhammadi A, Javadian Y, Bijani A, Hosseini R, Babaei M. Associated factors of bone mineral density and osteoporosis in elderly males. Int J Endocrinol Metab. 2017;15(1):e39662.
Cui R, Zhou L, Li Z, Li Q, Qi Z, Zhang J. Assessment risk of osteoporosis in Chinese people: relationship among body mass index, serum lipid profiles, blood glucose, and bone mineral density. Clin Interv Aging. 2016;11:887–95.
Adami S, Braga V, Zamboni M, Gatti D, Rossini M, Bakri J, et al. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcif Tissue Int. 2004;74(2):136–42.
Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, et al. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002;49(2):211–7.
Xiu S, Mu Z, Sun L, Zhao L, Fu J. Hemoglobin level and osteoporosis in Chinese elders with type 2 diabetes mellitus. Nutr Diabetes. 2022;12(1):19.
Mitchell A, Larsson SC, Fall T, Melhus H, Michaëlsson K, Byberg L. Fasting glucose, bone area and bone mineral density: a mendelian randomisation study. Diabetologia. 2021;64(6):1348–57.
Oei L, Zillikens MC, Dehghan A, Buitendijk GH, Castaño-Betancourt MC, Estrada K, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam study. Diabetes Care. 2013;36(6):1619–28.
Oei L, Rivadeneira F, Zillikens MC, Oei EH. Diabetes, diabetic complications, and fracture risk. Curr Osteoporos Rep. 2015;13(2):106–15.
Boonen S, Mohan S, Dequeker J, Aerssens J, Vanderschueren D, Verbeke G, et al. Down-regulation of the serum stimulatory components of the insulin‐like growth factor (IGF) system (IGF‐I, IGF‐II, IGF binding protein [BP]‐3, and IGFBP‐5) in age‐related (type II) femoral neck osteoporosis. J Bone Miner Res. 1999;14(12):2150–8.
Eastell R, O’Neill TW, Hofbauer LC, Langdahl B, Reid IR, Gold DT, et al. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2:16069.
Lu YH, Gu L, Jiang Y. Positive association of fasting plasma glucose with bone mineral density in non-diabetic elderly females. J Bone Miner Metab. 2022;40(5):755–62.
Thrailkill KM, Lumpkin CK Jr., Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289(5):E735–45.
From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO), Sacks D, Baxter B et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J Stroke. 2018;13(6):612–32.
Bhatnagar A, Kekatpure AL. Postmenopausal osteoporosis: a literature review. Cureus. 2022;14(9):e29367.
Yang Y, Wang S, Cong H. Association between parity and bone mineral density in postmenopausal women. BMC Womens Health. 2022;22(1):87.
Wang TS. Thyroidectomy increases the risk for osteoporosis. Clin Thyroidol. 2018;30(12):563–5.
Delitala AP, Scuteri A, Doria C. Thyroid hormone diseases and osteoporosis. J Clin Med. 2020;9(4):1034.
Sheen YJ, Hsu CC, Jiang YD, Huang CN, Liu JS, Sheu WH. Trends in prevalence and incidence of diabetes mellitus from 2005 to 2014 in Taiwan. J Formos Med Assoc. 2019;118(Suppl 2):66–S73.
Zhang YS, Zheng YD, Yuan Y, Chen SC, Xie BC. Effects of anti-diabetic drugs on fracture risk: a systematic review and network meta-analysis. Front Endocrinol (Lausanne). 2021;12:735824.
Roomi AB, Mahdi Salih AH, Noori SD, Nori W, Tariq S. Evaluation of bone mineral density, serum osteocalcin, and osteopontin levels in postmenopausal women with type 2 diabetes mellitus, with/without osteoporosis. J Osteoporos. 2022;2022:1437061.
Wang L, Zhao K, Zha X, Ran L, Su H, Yang Y, et al. Hyperglycemia is not associated with higher volumetric BMD in a Chinese health check-up cohort. Front Endocrinol (Lausanne). 2022;12:794066.
Shanbhogue VV, Finkelstein JS, Bouxsein ML, Yu EW. Association between insulin resistance and bone structure in nondiabetic postmenopausal women. J Clin Endocrinol Metab. 2016;101(8):3114–22.
Funding
This study was funded by TCMMP 109-03-02(111), Buddhist Tzu Chi Medical Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.L.C. and M.K.; funding acquisition, T.L.C., M.K. and Y.F.W.; project administration, T.L.C.; methodology, T.L.C.; formal analysis, T.L.C. and M.K.; investigation, T.L.C.; writing—original draft preparation, T.L.C. and M.K.; writing—review and editing, T.L.C., M.K. and Y.F.W. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of the Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taiwan, approved the protocol of this retrospective medical record review study and waived the requirement for patient informed consent (No. B11001010, approval date: February 8, 2021).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chuang, TL., Koo, M. & Wang, YF. The impact of diabetes, anemia, and renal function in the relationship between osteoporosis and fasting blood glucose among Taiwanese women: a cross-sectional study. BMC Women's Health 24, 23 (2024). https://doi.org/10.1186/s12905-023-02851-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02851-w