Abstract
Background
Childbirth is one of the biggest risk factors for incontinence. Urinary and anal incontinence can cause pain and social limitations that affect social life, cohabitation, and work. There is currently no up-to-date literature study on the effect of pelvic floor muscle training with feedback from a physiotherapist, which involves verbal instructions based on vaginal and anal digital palpation, compared to treatment without feedback (e.g., recommendations for pelvic floor muscle training).
Aim
The objective of this systematic review was to examine the scientific evidence regarding the impact of pelvic floor muscle training (PFMT) with feedback from a physiotherapist and/or biofeedback on urinary and anal incontinence in women during the first six months following vaginal delivery, compared to treatment without feedback.
Methods
The literature search was conducted in the databases PubMed, Cochrane, and CINAHL. In addition, a manual search was conducted. The search terms consisted of MeSH terms and synonyms in the respective search block including population, intervention, and study design, as well as the terms pelvic floor and postpartum. An evaluation of each included study was conducted for methodological quality, evidence value, and clinical relevance.
Results
Eight studies were included, three of which showed a significant difference between groups, in favor of the intervention group that received pelvic floor muscle training with feedback from a physiotherapist and/or biofeedback. Due to the varying results and insufficient quality for the majority of the studies, the scientific basis was considered insufficient.
Conclusion
The scientific evidence for pelvic floor muscle training with feedback from a physiotherapist or biofeedback on postpartum urinary and anal incontinence compared to treatment without feedback is considered insufficient. Further research on the subject is needed. The study is registered in PROSPERO CRD42022361296.
Similar content being viewed by others
Background
Urinary incontinence (UI) is defined as the involuntary leakage of urine through the urethra [1]. Anal incontinence (AI) is defined as the involuntary passage of gas or stool (solid or liquid) through the anal canal [2, 3]. Vaginal delivery is associated with a higher risk for UI, and operative vaginal delivery is associated with AI [4, 5]. The reported prevalence of UI in the postpartum period ranges from 3 to 40% [6,7,8]. The prevalence of AI following vaginal delivery is between 5 and 26% [2, 3]. Although there is a natural history of recovery of pelvic floor structures during postpartum, UI does not always resolve due to this recovery [9].
Moreover, UI and AI are associated with significant reductions in health-related quality of life [10,11,12,13]. Women who experience a new onset of AI after childbirth report persistently negative quality of life as long as two years after delivery [14]. Patients in the general population with UI may have higher rates of depression and anxiety than individuals without UI [15]. UI is also associated with postpartum depression in the first six months after childbirth [16].
Urinary and anal incontinence is often assessed through self-assessment questionnaires [17, 18] containing questions about symptoms, frequency, and when symptoms occur. In addition, self-assessment questionnaires usually include questions about self-reported health and quality of life, as urinary incontinence is a barrier to women’s participation in sports and fitness activities and therefore may be a threat to women’s health, self-esteem, and well-being [19, 20]. There is some evidence to suggest that women with UI and AI may benefit from pelvic floor muscle training (PFMT) and other rehabilitative care, commonly referred to as pelvic floor physiotherapy, during the postpartum period [21, 22].
PFMT has been shown to reduce UI and may reduce AI [23, 24]. It is recommended as the first line treatment for all women with UI and/or AI [25]. Feedback from a physiotherapist or biofeedback may provide benefits in addition to PFMT in women with UI [26]. Biofeedback in combination with PFMT has been shown to reduce UI [26] and to potentially reduce AI [23] in adults. However, there is uncertainty about the effect of PFMT as a treatment for UI in postpartum women [21].
Feedback is the sensory information that becomes accessible following an individual’s conducted activity [27]. Feedback from a physiotherapist, in PFMT consist of verbal instructions based on vaginal and anal digital palpation, to ensure a correct contraction [28]. The main mechanism of action for feedback in training is an increased adherence to the training program [29]. Biofeedback entails utilising an external sensor to provide insight into bodily processes, typically with the aim of modifying the measured quality [30] and could be used both superficially and intravaginally and/or intra-analy [23]. The technique aims to make the patient aware of a usually unconscious bodily function, where the mechanisms of action behind reduced UI and AI could be increased rectal sensitivity, increased strength, and coordination [23].
In recent years, various systematic reviews have been published in the field. Zhu et al. [31] investigated the effect of PFMT with biofeedback and/or electrical stimulation in women after childbirth. The results showed that PFMT and electrical stimulation with or without biofeedback were more effective than PFMT alone [31]. Mazur et al. [23] investigated the efficacy of preventive and therapeutic physiotherapy in women with UI and AI after childbirth. The results showed that physiotherapeutic interventions, including PFMT, biofeedback, and/or electrical stimulation can be effective in reducing incontinence symptoms [23].
To the authors’ knowledge, there is no systematic review that specifically reports the effect of PFMT with feedback from a physiotherapist or biofeedback in UI and AI after childbirth. Previously mentioned studies have not focused on only feedback and/or biofeedback [17, 23]. Additional studies have been published since these reviews were performed, and there is a need for an overview that compiles the current evidence in the field.
The objective of this systematic review was to examine the scientific evidence from 2012 to 2022 regarding the impact of pelvic floor muscle training (PFMT) with feedback from a physiotherapist and/or biofeedback on UI and AI in women during the first six months following vaginal delivery, compared to treatment without feedback.
Method
A systematic literature search was carried out based on previously established selection criteria to achieve the purpose of this systematic review (see Table 1). The Cochrane handbook [32] and PRISMA guidelines [33] were followed. The inclusion criteria were expanded after the protocol was registered due to the low number of relevant randomised controlled trials.
The systematic literature search was conducted independently by AH and JS on 30 September 2022 in the databases PubMed, Cochrane, and CINAHL. To ensure the inclusion of recent studies, the time limit 2012–2022 was used. In the search, five search blocks were used with keywords from MeSH terms and synonyms for these. Search block #1 included the population. Search block #2 consisted of pelvic floor terms. Search block #3 included the intervention. Search block #4 consisted of the term postpartum, and search block #5 included a description of the study design. The same search blocks were used in all databases (see Table 2). A complementary search to present date, was performed in October 2023, where the term ‘physical therapy’ was added, with no additional findings included. For the full search strategy, see Additional file 1.
The selection process began with AH and JS individually reading and reviewing all titles. The titles that were consistent with the purpose of the study were retained. The authors individually assessed the titles, and differences were discussed until a consensus was reached. After agreement on relevant titles, duplicates were excluded. In the next step, the authors reviewed abstracts individually based on inclusion and exclusion criteria. AH and JS discussed the assessment until a consensus was reached. In case of uncertainty, the title was kept to the next step. The following step included individual readings of the full texts, where the studies were reviewed based on the inclusion and exclusion criteria. The assessment was then discussed until a consensus was reached (including EL), and the remaining studies were included in the systematic review.
A manual search was performed in the reference lists of the included studies and in relevant systematic reviews. Review of the reference lists was done using a process similar to the previous selection process in the database search. In each step the authors assessed the studies individually and thereafter discussed until a consensus was reached.
Methodological quality
The PEDro scale was used to assess methodological quality [34]. It aims to assess the methodological quality of clinical studies based on eleven criteria [35]. The first criterion consists of external validity. Criteria 2–9 consist of internal validity and criteria 10–11 of statistical reporting. The maximum score was 10 since external validity is not included in the overall assessment. The assessment was performed individually by AH, and JS and a single score was agreed up on. Studies with a total score of < 4 were considered to have a low methodological quality [35]. Total points of 4–5 were considered medium quality, and studies with 6–8 points were considered high methodological quality. Studies with 9–10 points were considered to have excellent quality [35].
Probative value
After assessment of methodological quality, the studies were reviewed for probative value [36]. The probative value of the studies was graded as high, medium, or low. When assessing the probative value of studies, no absolute limits were applied [36]. The grading was based on the methodological quality of the study, as well as the appropriateness of the study design and the size of the study. The authors considered aspects such as randomisation, the similarity of the groups at baseline, and the number of dropouts when assessing the appropriateness of the study design. The size of the study was considered adequate if a power calculation or sample size analysis had been performed and a sufficient number of participants were included.
Assessment of clinical relevance
The clinical relevance of the results was assessed based on five questions [37] (see Table 3). The questions were answered yes, no, or unclear [37]. Questions 1–3 assess applicability, and questions 4–5 assess clinical relevance [38]. To assess descriptions of interventions, particular descriptions of specific exercises where sought. As this review was not restricted to specific outcome measures, effect sizes were calculated manually, and effect size cut-offs were used according to Cohen’s d [37]. At a Cohen’s d below 0.5, the effect was considered to be small; at 0.5 < 0.8, the effect was considered to be moderate; and at Cohen’s d ≥ 0.8, the effect was considered to be large [37]. The effect size was calculated manually with a calculator (Effect size calculators, University of Colorado, US) according to the formula Cohen’s d = M 1 - M 2 / s pooled where s pooled = √[ (s 1 2 + s 2 2 ) / 2]. At least moderate effect was required for the study to be considered clinically relevant in this systematic review. The studies were considered clinically relevant with five yes replies.
Grading of evidence
Grading of evidence was based on Britton’s model [36]. The level of evidence was graded as strong, moderately strong, limited, or insufficient (see Table 4). When assessing the level of evidence, the authors accounted for the probative value and consensus in the results, as well as the clinical relevance of the studies. In the event of contradictory results in the studies, the level of evidence was lowered.
Results
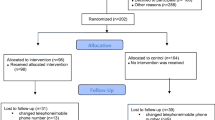
A total of 329 records were selected in the database searches. After removing irrelevant titles and duplicates, 35 abstracts were reviewed. Of these, 16 studies were selected for full-text reading (List of articles not included after full-text reading are presented in Additional file 2), resulting in eight studies [39,40,41,42,43,44,45,46] that were included in the systematic review (see Fig. 1). The manual search was carried out in reference lists of the included studies [39,40,41,42,43,44,45,46] and from seven systematic reviews [21, 23, 25, 31, 47,48,49]. The manual search did not lead to the inclusion of any additional studies.
Flow chart of the selection process reported according to PRISMA [33]
Characteristics of included studies
A total of 765 women who underwent a vaginal delivery participated in the included studies (see Table 5 for details). There were five studies that included women with perineal lacerations and levator ani injuries [40, 42, 43, 45, 46]. The remaining studies included women with incontinence diagnosed based on self-assessment questionnaires [39, 40, 42]. Of the included studies, three studies had the self-assessment of both UI and AI as outcome measures [41, 44, 45]. Three studies studied only UI [39, 40, 46] and two studies only AI [41, 43]. The mean age of the women in the studies was 28.5 (± 4.8) − 32.1 (± 4.9). Six of eight studies only included primiparous women [39, 40, 42,43,44, 46], in one study the mean number of pregnancy was 1.4 [41] and in one it was 1.1 [45].
Of the eight studies included, two studies reported using biofeedback devices only [42, 43], two studies reported using a combination of biofeedback devices and feedback from a physiotherapist [44, 46] while four reported participants receiving feedback from a physiotherapist [39,40,41, 45]. Initially, all participants, except those included in the study by Peirce et al. [43], were given instructions to perform correct pelvic floor contraction. Three studies reported using biofeedback in the daily training at home [42, 43, 46], and one study used biofeedback during clinical training sessions [44]. The studies used different devices, Myotrac Infiniti Vaginal Sensor [42], CombiStim XP Neurotech® [43], NeuroTrack Simplex [44] and Enraf-Nonius Myomed134 [46], which all provide visual feedback on muscle contractions with electromyography (EMG) biofeedback. In the other four studies, participants received feedback from a physiotherapist [39,40,41, 45]. In all intervention groups, participants received a home exercise program with PFMT. Participants in two of the studies used biofeedback at home in their daily training [43, 46]. The duration of the interventions ranged from 6 weeks to 6 months and differed slightly between studies. The intervention included follow-up with the participants, but the timing varied from weekly to every six weeks. For a more detailed description of the interventions used in the studies, see Table 5.
In five of the studies, participants in the control group received instructions to perform a correct pelvic floor contraction [39,40,41, 44, 46]. Of these, three studies provided the control groups with recommendations to continue carrying out PFMT [39, 41, 46]. In one of the included studies, the control groups only received recommendations about PFMT [43]. The participants in the control groups of Oakley et al. [42] and Von Bargen et al. [45] received standard care, which was not specified.
Results of included studies
Three of eight studies reported a significant difference between the groups, with participants in the intervention groups showing reduced incontinence symptoms [41, 44, 45]. All three studies evaluated AI symptoms [41, 44, 45], where two reported a significant difference between the groups [41, 45]. Two studies evaluated UI symptoms and both reported a significant difference between the groups [44, 45]. The included studies used different self-reported questionnaires to evaluate outcomes ( Table 5). In all questionnaires, a lower total score indicated less symptoms of incontinence, and all questionnaires were reported to be validated. One of the included studies compared standard care with an individualised daily home PFMT program and weekly verbal and tactile feedback on correct pelvic floor contraction from a physiotherapist [45]. After three months, a significant difference was observed between the groups in terms of urinary and AI symptoms [45]. The second study examined an individualised and progressive daily home exercise program delivered by a physiotherapist that included advice on PFMT. AI symptoms were evaluated after six months, and a significant difference between the groups was observed [41]. The third study examined an individualised daily home exercise program and 12 training sessions with biofeedback compared to PFMT instructions [44]. After six months, there was a significant between-group difference in symptoms of UI, but no difference in AI symptoms. After 12 months, no significant difference was reported between the groups [44]. In five of the studies, no significant between-group differences were reported [39, 40, 42, 43, 46]. Three of the included articles reported a significant difference in the reduction of incontinence symptoms within both the intervention group and the control group after three [42] and six months [39, 41], respectively.
Methodological quality
Methodological quality was assessed with the PEDro scale (see Table 5). Three studies were considered to have high methodological quality [40, 44, 45], four were considered to have medium methodological quality [39, 41,42,43], and one was judged to have low methodological quality [46]. Seven studies performed a randomisation [30,31,32,33,34,35,36,37,38,39,40,41,42, 44,45,46], and six of these were concealed [39, 40, 42,43,44,45]. Five studies reported similarities between the groups at baseline [39, 41, 44,45,46]. None of the studies had blinded participants, therapists, or assessors. Four studies had more than 15% participant dropout [39, 41, 45, 46]. Three studies carried out an intention-to-treat analysis [40, 41, 45]. For a full presentation of the assessment of methodological quality, see Additional file 3.
Probative value
One of the studies was considered to have a high probative value [44]. Two studies were considered to have a medium level of evidence [40, 45], and five studies had a low level of evidence [39, 41,42,43, 46]. Two study was considered to have high methodological quality, but a medium level of evidence due to number of dropouts [45] or because the groups were not equal at baseline [40]. Four studies were considered to have medium methodological quality, but low evidentiary value [39, 41,42,43] due to not reporting or not homogeneous baseline values [42, 43], problem with the power calculation [39, 43] and an excessive number of dropouts [39, 41]. In addition, one study considered having low methodological quality was also classified as low level of evidence due to not perform a power calculation, lack of random allocation and high number dropouts [46] (See Table 5).
Clinical relevance
Based on the assessment of clinical relevance, no study achieved a score of “yes” on all five of Furlan’s questions (see Table 7) [37]. Seven studies had sufficient reporting of population and relevant outcome measures to be clinically relevant [39,40,41, 44,45,46]. The interventions were described sufficiently by five studies [39,40,41, 44, 46]. The effect size could be calculated with Cohen’s d in two studies, which resulted in a low effect size [41, 46]. The other studies did not present applicable data that could be used to calculate clinical relevance, nor did these studies report any corresponding measure for clinical relevance [39, 40, 42,43,44,45]. No study reported a comparison between the benefit of the intervention with potential risks and costs. As five yes replies were required for a study to be considered clinically relevant, no study was assessed as clinically relevant.
Grading of evidence
Three studies reported significant between-group differences [41, 44, 45], with reduced incontinence symptoms observed among participants in the intervention groups. One of these was considered to have a high value of evidence [44], one was considered to have a medium value of evidence [45], and one was considered to have a low value of evidence [41]. Five studies did not report any significant difference between groups [39, 40, 42, 43, 46]. Of these, one study had a medium level of evidence [40], and four studies had a low level of evidence [39, 41, 43, 46]. There are not enough studies with a high level of evidence, and no study was assessed as clinically relevant. In addition, the results in the included studies are contradictory; the scientific basis is therefore considered to be insufficient.
Discussion
This systematic review demonstrated that there is a lack of sufficient scientific evidence for the effect of PFMT with feedback from a physiotherapist and/or biofeedback for postpartum UI and AI.
The majority of the included studies in this systematic review had a medium to low level of evidence, which presents the risk of systematic error [32]. Five studies recruited the number of participants acquired to reach power [40,41,42, 44, 45]. However, four of the eight studies had a large participant dropout rate [39, 41, 45, 46]. An inadequate number of participants and high dropout rate leads to an increased risk of imprecision [32]. Dropouts could indicate difficulties adhering to the intervention; Pierce et al. [43] report that one of the reasons for low compliance is the time-consuming nature of the intervention.
In physiotherapy studies with active interventions, it is difficult to blind participants and therapists, which is the case in all reviewed studies. It has been suggested that lack of blinding increases the risk of treatment errors, because the expectations of the participant and the therapist can unintentionally affect the outcome. However, the effect of a lack of blinding in rehabilitation research is still inconclusive [50]. In one of the included studies, there is insufficient reported data and unclear reporting of baseline [43], which increases the risk of reporting errors [32]. This lowers the clinical relevance and transferability is reduced.
Despite the heterogeneity in the population and intervention groups in the included studies, the studies with significant between-group differences account for the heterogeneity [41, 44, 45]. This could indicate that women with different types of problems can benefit from feedback from a physiotherapist and/or biofeedback. Even so, the results are not specific, and none of the feedback types differed in terms of effect or non-effect. Results that are less specific are more difficult to apply clinically. Since the basis for physiotherapy is functional limitation and disability rather than medical diagnosis [51], the heterogeneity in included diagnosis might not be a limitation for the application of the results. On the other hand, it would have been beneficial to investigate UI and AI separately, as this likely would have provided a more consistent result. A Cochrane review, which evaluated the effect of pelvic floor training in adults with and without biofeedback on AI unrelated to childbirth, shows that the intervention has some scientific support [52]. The mechanisms that reduce symptoms in the general adult population may differ from the mechanisms in postpartum women, but the intervention nevertheless has scientific support. The included studies differ in how instructions for PFMT were provided in both intervention- and control groups, were some received verbal, some received written instructions, and some received both. To date, it is not clarified which type of instructions are most effective, and it has been suggested that there is no difference [53].
The included studies tended to evaluate the effect with varying time spans, which makes the results difficult to compare. One possible reason to lack of significant effects on incontinence symptoms postpartum could be time to follow up. If the time interval is too short between follow up points, the time interval may be too short to evaluate the effect of PMFT. Significant between-group differences were observed after three [45] and six [41, 45] months, respectively. Sigurdardottir et al. [44] reported that no differences between intervention and control groups could be observed regarding UI and AI after 12 months. This may indicate that PFMT with feedback from a physiotherapist and/or biofeedback accelerates natural healing in the first stage after delivery but does not produce a significant difference compared to the control group after one year [44]. The natural history tends to reduce incontinence symptoms in the first six months after childbirth [9]. The prevalence of UI has been shown to increase again after one year [9]. An overview by Mørkved et al. [54] reports that the research in the field is contradictory regarding the efficacy of pelvic floor training one year after childbirth. Furthermore, Mørkved et al. [54] conclude that the flattening of the effect may be due to the discontinuation of exercises among participants. A long-term effect cannot be expected if pelvic floor exercises are discontinuous [55]. Another factor leading to increased UI problems may be an increased demand on pelvic floor muscles through increased physical activity [56]. When the increase in abdominal pressure exceeds the threshold of the urinary sphincter, UI may occur. This applies to stress UI specifically [57].
Three studies showed statistically significant within-group differences in both the intervention and control groups [39, 41, 42]. Both groups performed PFMT, which may explain the within-group differences. The fact that all participants completed pelvic floor training [22] and underwent natural processes that reduce incontinence symptoms in the first six months after childbirth [9] can explain the occurrence of within group differences in both the intervention and control group, and thus explain why no significant between group differences were observed.
The instruments used were self-assessments, which are a measure of subjective experiences. Although all questionnaires were validated, there may be a discrepancy between patient-reported UI symptoms and objectively measured incontinence [58]. Such a discrepancy may relate to human support at physical appointments, where a relationship can be created between physiotherapist and study participant, which further increases the risk of bias, as neither participants nor therapists were blinded [59]. The self-estimate can also be influenced by internal factors, such as health status on the current day, and the individual’s subjective experience of symptoms and level of discomfort [59]. The results of the included studies would benefit from validation through other assessments [58].
Application of the results
Together with the natural history [9] and continuous independent pelvic floor training, several of the participants in the included studies [39, 41, 42] experienced reduced discomfort within the first six months without receiving feedback from a physiotherapist and/or biofeedback. PFMT without any form of feedback may be suitable for patients who are confident practicing the exercises on their own and who find their symptoms less troublesome [21]. It can also be considered suitable for patients who prefer the flexibility of home exercises. Pelvic floor training with feedback can be particularly suitable for women who have difficulty performing a pelvic floor contraction correctly [60]. A lack of confidence in performing the PFMT properly or uncertainty about doing the right exercise are known barriers to compliance to PFMT [61, 62], and feedback could offer support to overcome that barrier. Feedback, from a physiotherapist and/or biofeedback, is important for successful pelvic floor therapy, since it can provide confirmation that the patient is correctly contracting muscles and ensure that the dosage of exercise is adapted to the individual [24]. It was not an inclusion criterion in this study, and in none of the included studies, that the women in the included studies could perform a correct pelvic floor contraction from start, which reflect the studied population, where difficulties to correctly contract is common [60]. Patients may also see a positive effect by spending more time with healthcare professionals [24]. PFMT has been found to be effective when supervised training is conducted [54]. Cochrane suggests that, in certain groups of women, it is possible that the effects of PFMT would be greater with targeted treatment approaches [21]. Physiotherapists should therefore continue to provide individualised treatment.
A systematic review [61] was performed that focused on prenatal and postnatal PFMT in prevention and treatment for pelvic organ prolapse and other pelvic floor dysfunction. The results showed a positive effect of pelvic-floor muscle training in prepartum and postpartum periods on pelvic-floor dysfunction prevention, particularly in UI symptoms. Based on their results, the authors argue for that there should be national strategies for pregnancy and postpartum rehabilitation programs due to a high prevalence of pelvic floor dysfunction in the general female population. In relation to that study, the present study further emphasises the importance of tailoring PFMT to suit the needs of the individual.
Future studies
There is a great need for additional high-quality randomised controlled trials that evaluate the effect of pelvic floor muscle training with different kinds of feedback on UI and AI separately [21, 31]. Considering the natural history and increased UI symptoms one year after childbirth, there is also a need for a longer follow-up period. Future studies could benefit from supplementing the evaluation with objective measures, such as the Pad Test provocation test [62]. Studies comparing feedback from a physiotherapist with biofeedback will be necessary to further optimise PFMT in post-partum women. To date, there is, as far as the authors know, no studies making such comparison.
Strengths and limitations
A strength of the current review is its systematic performance according to guidelines [32]. The three databases used hold a wide range of medical research. As maternity care overlaps several research fields, the databases were selected to obtain a large range of literature. In terms of methodology, the extensive search strategy was a strength, as it ensures that available research was found. To reduce the risk of bias, the selection process and all assessments were done by at least two authors independently [32]. The standardised PEDro scale was used in the assessment of methodological quality as an additional factor to reduce subjectivity [32].
This systematic review has limitations regarding the literature search method and selection criteria. The study aims to focus on the role of physiotherapists in the maternity care. It may increase the risk of bias, as the research question and selection criteria could have resulted in excluding relevant studies. The focus was chosen to highlight the competence of physiotherapists in the field. Despite this, the interventions can be used by several skilled professionals depending on national guidelines. The collection of literature was done in English only, as the authors did not have the resources to obtain text from other languages and guarantee a correct translation and interpretation. The language limitations could increase the risk of systematic bias and the risk of excluding relevant studies [63]. Studies published earlier than 2012 were excluded. A wider time span could result in a larger base and thus enable a narrower question and a more specific result. To reduce the risk of comparing different forms of technological equipment and to conduct an updated conclusion based on recent research, the inclusion criteria was chosen [64].
A limitation of the current systematic review is the use of the Britton model of probative value and grading of evidence, which is not internationally established [36]. The Britton model aims to assess the trustworthiness of an individual study’s conclusions and to generate a compilation of all conclusions. Despite the fact that the Britton model is not an established model, it considers several aspects and aims to identify systematic limitations. The current study aims to describe the method transparently.
Another limitation is the width and variation in population, intervention, control, and assessment measures, which generates results that are less specific. This also rules out the performance of a meta-analysis. The wide inclusion criteria was required to gather a sufficiently large base, since there are few randomised controlled studies in the area. However, the broad population of women with and without injury may reflect the patient group encountered in the clinic, as there is a large number of undetected injuries [65]. It is not possible to draw specific conclusions about the effect of the intervention on a specific population. However, with a focus on functional limitations and disability rather than medical diagnosis [51], the broad population could be considered adequate.
Conclusion
There is insufficient scientific evidence for the effect of PFMT with feedback from a physiotherapist or biofeedback in postpartum incontinence compared to pelvic floor training recommendations alone. Based on the results of this systematic review, it is not possible to draw new conclusions about the evidence for pelvic floor training with feedback. In individualised treatment, the use of feedback from a physiotherapist could fill a need for certain patients. Additional high-quality studies are needed to draw scientifically based conclusions about this treatment option for postpartum incontinence.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files, together with all included full texts (no 39–46 in the reference list).
Abbreviations
- UI:
-
urinary incontinence
- AI:
-
anal incontinence
- PFMT:
-
pelvic floor muscle training
- RCT:
-
randomised controlled trial
- CCT:
-
controlled clinical trial
- ICIQ-UI SF:
-
The international consultation on incontinence questionnaire urinary incontinence short form
- UDI – 6:
-
Urinary distress inventory short form
- FISI:
-
The fecal incontinence severity index
- APFQ:
-
Australian pelvic floor questionnaire
- CRADI-8:
-
Colorectal-anal distress inventory 8
- N/A:
-
not applicable
References
Weber-Rajek M, Strączyńska A, Strojek K, Piekorz Z, Pilarska B, Podhorecka M, et al. Assessment of the effectiveness of pelvic floor muscle training (PFMT) and extracorporeal magnetic innervation (ExMI) in treatment of stress urinary incontinence in women: a Randomized Controlled Trial. BioMed Res Int. 2020;2020:1–7.
Macmillan AK, Merrie AE, Marshall RJ, Parry BR. The prevalence of fecal incontinence in community-dwelling adults: a systematic review of the literature. Dis Colon Rectum. 2004;47(8):1341–9.
Boreham MK, Richter HE, Kenton KS, Nager CW, Gregory WT, Aronson MP, et al. Anal incontinence in women presenting for gynecologic care: prevalence, risk factors, and impact upon quality of life. Am J Obstet Gynecol. 2005;192(5):1637–42.
Blomquist JL, Muñoz A, Carroll M, Handa VL. Association of Delivery Mode with Pelvic Floor disorders after Childbirth. JAMA. december 2018;18(23):2438.
Schei B, Johannessen HH, Rydning A, Sultan A, Mørkved S. Anal incontinence after vaginal delivery or cesarean section. Acta Obstet Gynecol Scand. 2019;98(1):51–60.
Wesnes S, Hunskaar S, Bo K, Rortveit G. The effect of urinary incontinence status during pregnancy and delivery mode on incontinence postpartum. A cohort study. BJOG Int J Obstet Gynaecol. 2009;116(5):700–7.
Morkved S, Bo K. Prevalence of urinary incontinence during pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:394–8.
Viktrup L, Lose G, Rolff M, Barfoed K. The symptom of stress incontinence caused by pregnancy or delivery in primiparas. Obstet Gynecol. 1992;79:945–9.
Patel K, Long JB, Boyd SS, Kjerulff KH. Natural history of urinary incontinence from first Childbirth to 30-months postpartum. Arch Gynecol Obstet. 2021;304(3):713–24.
Robinson D. Relationship between patient reports of urinary incontinence symptoms and quality of life measures. Obstet Gynecol Februari. 1998;91(2):224–8.
Papanicolaou S, Hunskaar S, Lose G, Sykes D. Assessment of bothersomeness and impact on quality of life of urinary incontinence in women in France, Germany, Spain and the UK. BJU Int. 2005;96(6):831–8.
Goldberg RP, Kwon C, Gandhi S, Atkuru LV, Sand PK. Urinary incontinence after multiple gestation and delivery: impact on quality of life. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(5):334–6.
Hatem M, Fraser W, Lepire E. Postpartum urinary and anal incontinence: a population-based study of quality of life of primiparous women in Quebec. J Obstet Gynaecol Can. 2005;27(7):682–8.
Lo J, Osterweil P, Li H, Mori T, Eden KB, Guise JM. Quality of life in Women with Postpartum Anal Incontinence. Obstet Gynecol. 2010;115(4):809–14.
Cheng S, Lin D, Hu T, Cao L, Liao H, Mou X, et al. Association of urinary incontinence and depression or anxiety: a meta-analysis. J Int Med Res. 2020;48(6):030006052093134.
Nam JY, Park EC, Cho E. Does urinary Incontinence and Mode of Delivery Affect Postpartum Depression? A Nationwide Population-based Cohort Study in Korea. Int J Environ Res Public Health. 2021;18(2):437.
Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44(1):77–80.
Malik RD, Hess DS, Christie A, Carmel ME, Zimmern PE. Domain comparison between 6 validated questionnaires administered to women with urinary incontinence. Urology. 2019;132:75–80.
Nygaard I, Girts T, Fultz NH, Kinchen K, Pohl G, Sternfeld B. Is urinary incontinence a barrier to Exercise in women? Obstet Gynecol. 2005;106(2):307–14.
Nygaard IE, Shaw JM. Physical activity and the pelvic floor. Am J Obstet Gynecol. 2016;214(2):164–71.
Woodley SJ, Lawrenson P, Boyle R, Cody JD, Mørkved S, Kernohan A, Hay-Smith EJC. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;5(5):CD007471.
Wu Y, McInnes N, Leong Y. Pelvic floor muscle training versus watchful waiting and pelvic floor disorders in postpartum women. Female Pelvic Med Reconstr Surg. 2018;24(2):142–9.
Mazur-Bialy AI, Kołomańska-Bogucka D, Opławski M, Tim S. Physiotherapy for Prevention and Treatment of Fecal Incontinence in women—systematic review of methods. J Clin Med. 2020;9(10):3255.
Herderschee R, Hay-Smith EJ, Herbison GP, Roovers JP, Heineman MJ. Feedback or biofeedback to augment pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev. 2011;7. CD009252.
Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10(10):CD005654.
Dannecker C, Wolf V, Raab R, Hepp H, Anthuber C. EMG-biofeedback assisted pelvic floor muscle training is an effective therapy of stress urinary or mixed incontinence: a 7-year experience with 390 patients. Arch Gynecol Obstet. 2005;273(2):93–7.
Shumway-Cook A, Woollacott MH. Motor control: theory and practical applications. Baltimore: Williams & Wilkins; 1995.
Bo K, Frawley HC, Haylen BT, Abramov Y, Almeida FG, Berghmans B, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the Conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36(2):221–44.
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. Quantity and quality of Exercise for developing and maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in apparently healthy adults: Guidance for Prescribing Exercise. Med Sci Sports Exerc. 2011;43(7):1334–59.
Schwartz GE, Beatty J. Biofeedback, theory and research. New York: Academic; 1977.
Zhu D, Xia Z, Yang Z. Effectiveness of physiotherapy for lower urinary tract symptoms in postpartum women: systematic review and meta-analysis. Int Urogynecol J. 2022;33(3):507–21.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 29 mars 2021;n71.
PEDro scale [Internet]. Pedro. [cited 221228]. Retrieved from: https://pedro.org.au/english/resources/pedro-scale/.
Cashin AG, McAuley JH. Clinimetrics: Physiotherapy evidence database (PEDro) scale. J Physiother. 2020;66(1):59.
Britton M. Grading of a study’s evidence value and the strength of the conclusions. Läkartidningen. 2000;97(40):4415.
Furlan AD, Pennick V, Bombardier C, van Tulder M. 2009 Updated Method Guidelines for Systematic Reviews in the Cochrane Back Review Group: Spine. 2009;34(18):1929–41.
Malmivaara A, Koes BW, Bouter LM, van Tulder MW. Applicability and clinical relevance of results in Randomized controlled trials: the Cochrane Review on Exercise Therapy for Low Back Pain as an Example. Spine. 2006;31(13):1405–9.
Ahlund S, Nordgren B, Wilander EL, Wiklund I, Fridén C. Is home-based pelvic floor muscle training effective in treatment of urinary incontinence after birth in primiparous women? A randomized controlled trial. Acta Obstet Gynecol Scand. 2013;92(8):909–15.
Hilde G, Stær-Jensen J, Siafarikas F, Ellström Engh M, Bø K. Postpartum pelvic floor muscle training and urinary incontinence: a Randomized Controlled Trial. Obstet Gynecol. 2013;122(6):1231–8.
Johannessen H, Wibe A, Stordahl A, Sandvik L, Mørkved S. Do pelvic floor muscle exercises reduce postpartum anal incontinence? A randomised controlled trial. BJOG Int J Obstet Gynaecol. 2017;124(4):686–94.
Oakley SH, Ghodsi VC, Crisp CC, Estanol MV, Westermann LB, Novicki KM, et al. Impact of pelvic floor physical therapy on quality of life and function after Obstetric Anal Sphincter Injury: a Randomized Controlled Trial. Female Pelvic Med Reconstr Surg. 2016;22(4):205–13.
Peirce C, Murphy C, Fitzpatrick M, Cassidy M, Daly L, O’Connell P, et al. Randomised controlled trial comparing early home biofeedback physiotherapy with pelvic floor exercises for the treatment of third-degree tears (EBAPT Trial). BJOG Int J Obstet Gynaecol. 2013;120(10):1240–7.
Sigurdardottir T, Steingrimsdottir T, Geirsson RT, Halldorsson TI, Aspelund T, Bø K. Can postpartum pelvic floor muscle training reduce urinary and anal incontinence? Am J Obstet Gynecol. 2020;222(3):247. .e1-247.e8.
Von Bargen E, Haviland MJ, Chang OH, McKinney J, Hacker MR, Elkadry E. Evaluation of Postpartum Pelvic Floor Physical Therapy on Obstetrical Anal Sphincter Injury: a Randomized Controlled Trial. Female Pelvic Med Reconstr Surg. 2021;27(5):315–21.
Wu TF, Huang LH, Lai YF, Chen GD, Ng SC. Early postpartum biofeedback assisted pelvic floor muscle training in primiparous women with second degree perineal laceration: Effect on sexual function and lower urinary tract symptoms. Taiwan J Obstet Gynecol. 2021;60(1):78–83.
Todhunter-Brown A, Hazelton C, Campbell P, Elders A, Hagen S, McClurg D. Conservative interventions for treating urinary incontinence in women: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2022;9(9):CD012337.
Ma Xxing, Liu A. Effectiveness of electrical stimulation combined with pelvic floor muscle training on postpartum urinary incontinence. Med (Baltim). 2019;98(10):e14762.
Alouini S, Memic S, Couillandre A. Pelvic floor muscle training for urinary incontinence with or without Biofeedback or Electrostimulation in women: a systematic review. Int J Environ Res Public Health. 2022;19(5):2789.
Armijo-Olivo S, Dennett L, Arienti C, Dahchi M, Arokoski J, Heinemann AW, et al. Blinding in Rehabilitation Research: empirical evidence on the Association between Blinding and Treatment Effect estimates. Am J Phys Med Rehabil. 2020;99(3):198–209.
Jiandani MP, Mhatre BS. Physical therapy diagnosis: how is it different? J Postgrad Med. 2018;64(2):69–72.
Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev. 2012;7CD002111.
Mateus-Vasconcelos ECL, Ribeiro AM, Antônio FI, de Oliveira LG, Ferreira B, Ferreira C. Physiotherapy methods to facilitate pelvic floor muscle contraction: a systematic review. Physiother Theory Pract. 2018;34(6):420–32.
Mørkved S, Bø K. Effect of pelvic floor muscle training during pregnancy and after Childbirth on prevention and treatment of urinary incontinence: a systematic review. Br J Sports Med. 2014;48(4):299–310.
Olivier B, Bø K, Berghmans B, Mørkved S, Van Kampen M. Evidence-based physical therapy for the pelvic floor: Bridging science and clinical practice. Rev 2. Amsterdam: Elsevier Ltd; 2015. Chapter 7, Measurement of pelvic floor muscle function and strength, and pelvic organ prolapse; 132–266. Phys Ther Sport. 2016;20:79.
Chisholm L, Delpe S, Priest T, Reynolds WS. Physical activity and Stress Incontinence in women. Curr Bladder Dysfunct Rep. 2019;14(3):174–9.
Yang J, Cheng JW, Wagner H, Lohman E, Yang SH, Krishingner GA, et al. The effect of high impact crossfit exercises on stress urinary incontinence in physically active women. Neurourol Urodyn. 2019;38(2):749–56.
Lozo S, Botros C, Iyer S, Gafni-Kane A, Sand P. Can patients independently identify their urinary incontinence symptoms? Int Urogynecol J. 2021;32(2):381–5.
Jette AM. Measuring subjective clinical outcomes. Phys Ther. 1989;69(7):580–4.
Neels H, De Wachter S, Wyndaele JJ, Van Aggelpoel T, Vermandel A. Common errors made in attempt to contract the pelvic floor muscles in women early after delivery: a prospective observational study. Eur J Obstet Gynecol Reprod Biol. 2018;220:113–7.
Romeikienė KE, Bartkevičienė D. Pelvic-floor dysfunction Prevention in Prepartum and Postpartum Periods. Med (Kaunas). 2021;57(4):387.
Krhut J, Zachoval R, Smith PP, Rosier PFWM, Valanský L, Martan A, et al. Pad weight testing in the evaluation of urinary incontinence: Pad Weight Testing. Neurourol Urodyn. 2014;33(5):507–10.
Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, Mierzwinski-Urban M, Clifford T, Hutton B, Rabb D. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–44.
Patsopoulos NA, Ioannidis JP. The use of older studies in meta-analyses of medical interventions: a survey. Open Med. 2009;3(2):e62–8.
Guzmán Rojas RA, Shek KL, Langer SM, Dietz HP. The prevalence of anal sphincter injury in primiparous women: OASIS in primiparae. Ultrasound Obstet Gynecol. 2013.
Acknowledgements
We would like to acknowledge colleagues that has offered feedback on our manuscript throughout the writing process.
Funding
The project was funded by the Research, Education, Development and Innovation Primary Health Care, Region Västra Götaland.
Open access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Contributions
AH and JS were responsible for the conception and performed the data selection and all assessments together. EL was responsible for the design. AH, JS, MF and EL interpreted the data. AH and JS drafted the manuscript, and EL and MF substantially revised the manuscript. All authors worked on revisions and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Höder, A., Stenbeck, J., Fernando, M. et al. Pelvic floor muscle training with biofeedback or feedback from a physiotherapist for urinary and anal incontinence after childbirth - a systematic review. BMC Women's Health 23, 618 (2023). https://doi.org/10.1186/s12905-023-02765-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02765-7