Abstract
Background
Recent evidence suggests that measures of maternal gut enteropathy are associated with unfavorable fetal outcomes. It is, therefore, crucial to identify and treat the features of intestinal enteropathy among reproductive-age women living in areas where enteropathy is highly prevalent. However, there is a lack of non-invasive diagnostic tests to determine EED, making it difficult to identify the disease in field settings. In this study, we tested the potential of fecal pH as a biomarker of gut enteropathy and investigated its relationship with fecal biomarkers of intestinal enteropathy in reproductive-age women living in resource-limited environments.
Methods
Data on socio-demographic information, anthropometry, and biological samples were collected from 78 apparently healthy women aged between 20 and 27 years from November 2018 to December 2019. The association of stool pH with two fecal biomarkers of gut enteropathy (i.e., intestinal alkaline phosphatase [IAP] and fecal lipocalin-2 [LCN-2] was investigated using multiple linear regression models after adjusting for relevant covariates.
Results
In the adjusted models, alkaline stool pH (pH > 7.2) was found to be significantly associated with a decrease in the fecal IAP level by 1.05 unit (95% CI: -1.68, -0.42; p < 0.001) in the log scale, and acidic stool pH (pH < 6) was found to be significantly associated with an increase in the fecal LCN-2 level by 0.89 units (95% CI: 0.12, 1.67; p < 0.025) in the log scale.
Conclusions
The study findings demonstrated an association of fecal pH with biomarkers of gut enteropathy indicating its applicability as a simple tool for understanding intestinal enteropathy among reproductive-age women living in resource-limited settings.
Similar content being viewed by others
Introduction
The growth and development of the offspring are interlinked with maternal health and nutritional status [1,2,3,4,5]. The World Health Assembly’s 2012 global nutrition target places a strong emphasis on interventions that aim to prevent undernutrition in the first 1000 days of life. Therefore, the global interest in maternal determinants of childhood undernutrition has recently increased. These determinants include maternal education, maternal age, and maternal health status. Addressing these factors can significantly improve the nutritional status of children in their early years of life [6]. Emerging data indicate that measures of maternal gut enteropathy are associated with unfavorable perinatal outcomes, including preterm birth, low birth weight (LBW), and small gestational age (SGA) at birth [7,8,9]. During pregnancy, maternal gut microbiota can be affected by factors such as maternal diet, obesity, stress and depression, infection, and medicines which are correlated with gender-specific differences in fetal development [10]. When maternal gut microbial homeostasis is disrupted, it leads to an imbalance of bacterial composition defined as dysbiosis. Dysbiosis can be due to the loss of beneficial bacteria, overgrowth of potentially pathogenic bacteria, and loss of overall bacterial diversity [11]. The dysbiosis in the maternal gut microbial community can alter offspring’s microbiota and immunity through vertical transmission which later contributes to the risk of non-communicable diseases in adulthood [12,13,14,15]. Thus, early detection of maternal gut inflammation might be helpful in early intervention resulting in better nutritional outcomes in the offspring.
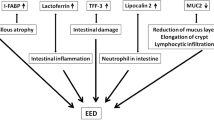
Studies have shown a linkage between gut microbiota dysbiosis and intestinal inflammation caused by environmental enteric dysfunction (EED) [16, 17]. EED is a clinical condition marked by subclinical inflammation in the small intestine, blunting of the villi, and a decrease in the capacity of the intestines to absorb food. This condition is frequently found among individuals chronically exposed to enteropathogens due to residing in a contaminated environment with improper water, sanitation, and hygiene (WASH) conditions [18]. The Malnutrition and Enteric Disease (MAL-ED) study showed a high prevalence of EED among children in LMICs [19]. This condition in children can be carried into adulthood. In adults, EED usually presents only with sub-acute weight loss and is, therefore, hard to diagnose. The gold standard for the diagnosis of EED involves a biopsy of the intestinal tissue and confirmation by histopathology. The Bangladesh Environmental Enteric Dysfunction (BEED) study revealed that 95% of adults residing in slums, who are asymptomatic, exhibit chronic non-specific intestinal inflammation, a hallmark feature of EED [20]. However, collecting endoscopy-guided small intestinal biopsy samples from clinically asymptomatic adults for routine diagnosis of EED is technically infeasible and, in some instances, unethical [20,21,22].
Thus, interest has grown in non-invasive indirect biomarker evaluation of intestinal inflammation utilizing enzyme tests (ELISA). Recently, fecal biomarkers have been evaluated as a feasible noninvasive tool to assess intestinal inflammation [23,24,25], and studies have established the association of several fecal biomarkers with intestinal inflammation [26,27,28,29]. IAP and fecal LCN-2 are among the promising fecal biomarkers of interest [30, 31]. IAP is an endogenous protein and a member of the alkaline phosphatase family. It plays a vital role in regulating intestinal inflammation [32]. LCN-2, also known as neutrophil gelatinase-associated lipocalin, is a bacteriostatic peptide. It might be regarded as a broadly dynamic marker of intestinal inflammation, as according to a study, the levels of fecal LCN-2 were found to increase by more than ten times in response to Dextran Sodium Sulfate (DSS) levels that caused mild or low-grade inflammation, and by over ten thousand times in response to DSS levels where the presence of colitis is histopathologically apparent [33]. These biomarkers have the potential to be used in predicting or diagnosing EED [34].
A study examined the factors associated with undernutrition among slum-dwelling adults in Bangladesh. The results show that adults living in Bangladeshi slums are more likely to be malnourished and have a number of physiological and sociodemographic problems, including gastrointestinal inflammation and changes in intestinal permeability. Biomarkers of intestinal inflammation were also higher in both male and female adults [35]. However, these newer biomarker assays require a sophisticated lab and trained technicians, resulting in few investigations on intestinal inflammation in asymptomatic adults in LMICs. There is a constant quest to identify potentially inexpensive, noninvasive, simple-to-do, and sensitive assays to detect maternal gut inflammation and EED in its preclinical stage.
Studies have demonstrated that a simple technique like stool pH estimation can give us a good surrogate estimation of the overall gut environment [36, 37]. According to studies, variations in fecal pH can be linked to a variety of disease states, and extreme deviation from the normal range is associated with increased morbidity and mortality [38,39,40,41].
Therefore, measuring fecal pH can be a simple and cost-effective way to monitor gut health and potentially identify individuals at risk for certain diseases. However, further research is needed to determine the optimal range of fecal pH values for maintaining overall health and preventing disease. A low-cost, ready-to-use technique to identify many intestinal illnesses is stool pH. The relationship between stool pH and fecal indicators of gut inflammation in women of reproductive age has to be further investigated. In this study, slum-dwelling women were examined for their stool pH and two fecal indicators of gut inflammation, IAP and LCN-2.
Methods
Study site, participants, and data collected
The data for the current study were obtained from a clinical trial conducted from November 2018 to December 2019 in a slum in the Mirpur area of Dhaka city, Bangladesh. This place was selected as the study site because it is inhabited by poor and lower middle-income families, densely populated, and frequently lacks access to essential services like clean water, sanitation, electricity, and healthcare, which can be compared to any typical congested urban settlement. The trial titled “The microbiota-directed complementary food formulation (MDCF) primary MAM study (Clinical Trial Registration Number NCT04015999) was a community-based clinical trial where nutritional interventions (MDCF and ready to use supplementary food) were given to 124 moderately acute malnourished (MAM) children for a period of 3 months with an aim to improve their nutritional status and gut microbiota composition. The details of the study design and the results of the trial have been published elsewhere [42]. All 124 mothers of the children included in the primary MAM study were approached to participate in this study. However, only 80 of them consented to take part, and written informed consent was obtained from each of them. Out of the total sample size of 80 participants, 78 non-pregnant, non-lactating women of reproductive age were included in the data analysis. Two participants were excluded from the data analysis as later it was found out that they were pregnant at that particular time. As a part of the MDCF primary MAM trial, we obtained socio-demographic, WASH, hand hygiene practice-related information, and information on toilet facilities. Sanitation facilities include a pit latrine with a slab, a ventilated improved pit latrine, or a water-sealed septic tank to avoid underground infiltration considered as improved toilet facility. We have also performed anthropometric assessments, and collected fecal samples once at baseline from the women. The weight, height and BMI of the women were measured using a standard protocol [43].
Biological sample collection and assay
Fecal samples were collected from the participants in sterile stool collection pots and were transferred to the laboratory, maintaining a cold chain. Stool pH was measured from the freshly collected stools. A portable stool pH meter (Hanna Instruments, Woonsocket, Rhode Island, USA) was used for the stool pH measurement. For measuring stool pH, 1 gm of stool was transferred to a separate sterile container, and a homogenized stool solution was prepared by adding ten milliliters of deionized water. The pH meter probe was then submerged in the solution and kept for a minute.
The remaining stool aliquots were kept at -80oC until further analysis. For measuring the fecal biomarkers, stool samples were weighed, and double-distilled water (ddH2O) was added at a specified ratio. A homogenized stool suspension was prepared by mixing 1 mg of stool with 50 µL of stool dilution buffer and then shaking the mixture vigorously. The suspension was then centrifuged for 20 min at a rate of 10,000Xg, and the IAP-containing supernatant was collected. Alkaline Phosphatase Diethanolamine Activity Kit was used to measure IAP activity following the manufacturer’s instructions (Sigma-Aldrich, St. Louis, USA). The stool IAP values are reported in U/ml. Fecal LCN-2 levels were detected using available ELISA kits (R&D system, Minneapolis, USA). The stool LCN-2 values are reported in ng/ml unit.
All laboratory analyses were conducted at the parasitology laboratory of icddr,b.
Covariates
We have identified the covariates based on literature search and biological plausibility. A number of variables were considered including age, BMI, level of education, religion, monthly family income, the number of family members, and variables related to hand hygiene and wash practices [29, 35, 44, 45].
Statistical analysis
We presented the characteristics of the women using mean and standard deviation for continuous variables and frequency measures for categorical variables. IAP activity and LCN-2 concentration was log-transformed for further analysis because they were log-normally distributed. Based on evidence from published articles, stool pH was categorized into three groups, acidic (pH < 6.0), normal range (6.0 ≤ pH ≤ 7.2), and alkaline (pH > 7.2) [46]. We visualized the distribution of log-transformed IAP activity and LCN-2 concentration in the women’s fecal samples using box plots across the ranges of stool pH. Separate simple and multiple linear regression models were built for each of the biomarkers (log-transformed IAP and LCN-2) to assess the association of each fecal biomarker with stool pH. Strength of association was expressed as β (mean difference) with 95% confidence interval (CI). In the multivariable models, all the covariates of a priori interest were included. Statistical significance was set at p < 0.05. All the analyses were performed using STATA V.13.
Results
Women’s characteristics are reported in Table 1.
The mean age of the women was 24.1 years, ranging from 20 to 27 years. A large majority of the women were Muslim (96.2%). The mean family income was 163.7 USD per month. More than a quarter of the participants had secondary-level education (25.6%). Most of the participants do not treat water before drinking (62.8%). Among the women, 14.1% used soap for hand washing after defecation. More than half of the participants used improved toilet facilities. The women’s stool pH was abnormal (35.9% had acidic pH and 21.8% had alkaline pH).
The level of the fecal biomarkers seemed to vary with the change in stool pH level (Fig. 1).
In the adjusted models, alkaline stool pH (pH > 7.2) was found to be significantly associated with decrease in the fecal IAP level by 1.05 unit (95% CI: -1.68, -0.42; p < 0.001) in the log scale (Table 2) and.
acidic stool pH (pH < 6) was found to be significantly associated with increase in fecal LCN-2 level by 0.89 units (95% CI: 0.12, 1.67; p < 0.025) in the log scale. (Table 3).
Discussion
The findings of our investigation indicate a significant association between alterations in fecal pH levels and biomarkers of gut enteropathy, specifically LCN-2 and IAP, demonstrating either a drop or rise from neutral level of stool pH, respectively. While previous research demonstrated a notable correlation between fecal pH and severe acute malnutrition [47], a comprehensive examination of the existing literature did not provide any findings regarding the connection between fecal pH and indicators of EED in humans.
The results obtained from this study could have significant implications for developing countries where access to expensive diagnostic tools is limited. As sample collection and preparation is very simple and stable, in settings with limited resources, stool pH testing may provide a cost-effective way and can be used as a potentially affordable and simple first-line investigation to identify intestinal inflammation, leading to earlier treatment and improved outcomes. The expenditure associated with conducting ELISA using commercially available kits for the quantification of EED biomarkers is around two to three times more in comparison to using a portable pH meter for measuring the stool pH. Furthermore, the feasibility of conducting this stool pH testing directly at the site of stool collection is an advantage, as it may be performed by individuals with minimal training. In contrast, the ELISA method necessitates the involvement of highly skilled staff.
Our current study revealed that highly acidic stool pH (< 6) was significantly associated with increased fecal LCN-2 level after adjusting for other covariates. LCN-2 plays a role in the body’s host immune system by limiting the growth of pathogenic bacteria in the gut [48]. Studies have shown that LCN-2 expression is increased in patients with intestinal inflammatory disorders, and its levels were significantly associated with the degree of severity of gut inflammation [49,50,51,52,53]. On the other hand, results from published studies also showed that inflammation in the gut villus caused by an increase in pathogenic gut bacteria resulted in a decrease in stool pH by interfering with the carbohydrate absorption pathway [54,55,56,57]. As there is a scarcity of research linking fecal LCN-2 level with the stool pH directly, it is therefore, difficult for us to compare our findings directly with other study findings. However, based on the above-mentioned findings, it can be said that the association of elevated levels of fecal LCN-2 with highly acidic stool pH are, in fact, in line with the published scientific works. Moreover, as fecal LCN-2 can be a promising biomarker to predict EED [34], stool pH might also be a low-cost tool to detect intestinal inflammation (EED) in asymptomatic adult individuals.
The findings of our study further showed that fecal levels of IAP were significantly negatively associated with highly alkaline stool pH (> 7.2) after adjusting for other covariates. In inflammatory conditions of the gut, expression of IAP is found to be decreased, and fecal IAP concentration is found to be lower in individuals suffering from chronic enteropathy. The relationship between IAP deficiency and overproduction and their effect on tight-junction protein (TJP) levels and function were studied. Results from studies suggested that IAP is a chief regulator of gut mucosal permeability and may act by improving TJP levels and localization [58]. IAP is a potential biomarker to monitor colitis in a mouse model of inflammatory bowel diseases (IBD). It was also reported that gut inflammation improved after treatment with synthetic IAP [59,60,61,62,63]. Even though there is a scarcity of human IAP-related studies, animal studies have shown that intestinal and fecal pH was higher among the IAP-knockout mice compared to their healthy counterparts [64], which is similar to our study findings.
Intestinal inflammatory disorders, which were previously believed to be the disease of the western world, are gradually increasing among the people of the developing world [65]. Many of the asymptomatic adults (both male and female) living in the unhygienic conditions of LMICs were suffering from intestinal inflammation. It is evident from published research that chronic low-grade inflammation in the gut can be a precursor for many health conditions, including adverse pregnancy outcomes in females [66, 67]. Early diagnosis and initiation of treatment are, therefore, pivotal for a better outcome of the disease. However, assessing gut inflammation using current techniques (Endoscopy, fecal biomarkers assessment, etc.) requires highly technical skills and a costly setup that is inappropriate for low-resource settings. Therefore, using stool pH as a proxy for fecal biomarkers (LCN-2, IAP) of intestinal inflammation would be highly beneficial for the early detection of enteric inflammation and assessing overall gut health in a resource-poor setting. In that context, we anticipate that the outcomes of our research will have a significant public health impact, especially for LMICs.
The findings of this study should be interpreted in light of its limitations since they are anticipated to have high public health importance for the early detection of enteric inflammation in women living in LMICs using a low-cost technique. The first drawback is the small sample size, and the second is that the study participants were not subjected to the gold standard confirmatory test for identifying intestinal inflammation (intestinal biopsy and histopathology) because of moral concerns. Besides, information on dietary intake and other co-morbidities were not collected from the study participants. Future studies with larger sample sizes and confirmatory tests could further validate these findings.
Conclusion
The study findings demonstrated an association of fecal pH with biomarkers of gut enteropathy among reproductive-age women. This result suggests that fecal pH could serve as a potential measure for assessing enteropathy in individuals residing in resource-poor settings. Considering the low-cost and non-invasiveness of the test, stool pH can be assessed in any settings where enteropathy is highly prevalent. However, although the findings are promising, sufficiently powered and well-designed future studies are necessary to confirm the association of stool pH with fecal biomarkers of intestinal health and gut enteropathy (LCN-2, IAP), particularly among women of reproductive age.
Data Availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Di Gesù CM, Matz LM, Buffington SA. Diet-induced dysbiosis of the maternal gut microbiome in early life programming of neurodevelopmental disorders. Neurosci Res. 2021;168:3–19.
Wopereis H, et al. The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. 2014;25(5):428–38.
Clemente JC, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–70.
Cirulli F, Musillo C, Berry A. Maternal obesity as a risk factor for Brain Development and Mental Health in the offspring. Neuroscience. 2020;447:122–35.
Vuong HE, et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586(7828):281–6.
Victora CG, et al. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. 2021;397(10282):1388–99.
Lauer JM, et al. Biomarkers of maternal environmental enteric dysfunction are associated with shorter gestation and reduced length in newborn infants in Uganda. Am J Clin Nutr. 2018;108(4):889–96.
Abdullah A, et al. The relationship between biomarkers of environmental enteric dysfunction with Vulvovaginal Candidiasis. Pregnant mothers and pregnancy outcome (stunting) A literature review. Volume 8. NVEO-Natural Volatiles & Essential Oils; 2021. pp. 13–20. 4.
Padhi BK, et al. Risk of adverse pregnancy outcomes among women practicing poor sanitation in rural India: a Population-based prospective cohort study. PLoS Med. 2015;12(7):e1001851.
Sajdel-Sulkowska EM. The impact of maternal gut microbiota during pregnancy on fetal gut-brain Axis Development and Life-Long Health outcomes. Microorganisms, 2023. 11(9).
DeGruttola AK, et al. Current understanding of Dysbiosis in Disease in Human and Animal models. Inflamm Bowel Dis. 2016;22(5):1137–50.
Sato Y, et al. Maternal gut microbiota is associated with newborn anthropometrics in a sex-specific manner. J Dev Orig Health Dis. 2019;10(6):659–66.
Soderborg TK, et al. Microbial transmission from mothers with obesity or Diabetes to infants: an innovative opportunity to interrupt a vicious cycle. Diabetologia. 2016;59(5):895–906.
Hsu CN et al. Hypertension programmed by Perinatal High-Fat Diet: Effect of maternal gut microbiota-targeted therapy. Nutrients, 2019. 11(12).
Kozyrskyj AL, et al. Fetal programming of overweight through the microbiome: boys are disproportionately affected. J Dev Orig Health Dis. 2016;7(1):25–34.
Terefe Y, et al. Co-occurrence of Campylobacter species in Children from Eastern Ethiopia, and their Association with Environmental Enteric Dysfunction, Diarrhea, and host Microbiome. Front Public Health. 2020;8:99.
Chen RY, et al. Duodenal microbiota in stunted undernourished children with Enteropathy. N Engl J Med. 2020;383(4):321–33.
Mahfuz M, et al. Bangladesh Environmental Enteric Dysfunction (BEED) study: protocol for a community-based intervention study to validate non-invasive biomarkers of environmental enteric dysfunction. BMJ Open. 2017;7(8):e017768.
Kosek M, et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88(2):390–6.
Hossain MS, et al. Alterations in the histological features of the intestinal mucosa in malnourished adults of Bangladesh. Sci Rep. 2021;11(1):2355.
Rogawski ET, Guerrant RL. The Burden of Enteropathy and subclinical Infections. Pediatr Clin North Am. 2017;64(4):815–36.
Crane RJ, Jones KD, Berkley JA. Environmental enteric dysfunction: an overview. Food Nutr Bull. 2015;36(1 Suppl):S76–87.
Ministro P, Martins D. Fecal biomarkers in inflammatory bowel Disease: how, when and why? Expert Rev Gastroenterol Hepatol. 2017;11(4):317–28.
Kopylov U, et al. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel Disease. Inflamm Bowel Dis. 2014;20(4):742–56.
Chen J, et al. DNA methylation biomarkers in stool for early screening of Colorectal cancer. J Cancer. 2019;10(21):5264–71.
Lopez RN, et al. Fecal biomarkers in inflammatory bowel Disease. J Gastroenterol Hepatol. 2017;32(3):577–82.
Lee K-M. Fecal biomarkers in inflammatory bowel Disease. Intestinal Res, 2013. 11(2).
Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel Disease: current practices and recent advances. Transl Res. 2012;159(4):313–25.
Campbell RK, et al. Biomarkers of environmental enteric dysfunction among children in Rural Bangladesh. J Pediatr Gastroenterol Nutr. 2017;65(1):40–6.
Santos GM et al. Intestinal alkaline phosphatase: a review of this enzyme role in the intestinal barrier function. Microorganisms, 2022. 10(4).
Chang YL et al. Lipocalin 2: a New Antimicrobial in mast cells. Int J Mol Sci, 2019. 20(10).
Heath M, et al. Association of Intestinal Alkaline Phosphatase with Necrotizing enterocolitis among premature infants. JAMA Netw Open. 2019;2(11):e1914996.
Chassaing B, et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS ONE. 2012;7(9):e44328.
Hasan MM, et al. Association of lipocalin-2 and low-density lipoprotein receptor-related protein-1 (LRP1) with biomarkers of environmental enteric dysfunction (EED) among under 2 children in Bangladesh: results from a community-based intervention study. BMJ Paediatr Open. 2021;5(1):e001138.
Fahim SM, et al. Evidence of gut enteropathy and factors associated with undernutrition among slum-dwelling adults in Bangladesh. Am J Clin Nutr. 2020;111(3):657–66.
Nugent SG, et al. Intestinal luminal pH in inflammatory bowel Disease: possible determinants and implications for therapy with aminosalicylates and other Drugs. Gut. 2001;48(4):571–7.
Ilhan ZE et al. pH-Mediated Microbial and metabolic interactions in Fecal Enrichment cultures. mSphere, 2017. 2(3).
Madanagopalan N, Nadar SA, Subramaniam R. Variation in the p-H of faeces in Disease. Gut. 1970;11(4):355–7.
Hossain MS, et al. Association of faecal pH with childhood stunting: results from a cross-sectional study. BMJ Paediatr Open. 2019;3(1):e000549.
Chin EL, et al. Machine learning identifies Stool pH as a predictor of bone Mineral density in healthy multiethnic US adults. J Nutr. 2021;151(11):3379–90.
Shimizu K, et al. Altered gut flora and environment in patients with severe SIRS. J Trauma. 2006;60(1):126–33.
Mostafa I, et al. Proof-of-concept study of the efficacy of a microbiota-directed complementary food formulation (MDCF) for treating moderate acute Malnutrition. BMC Public Health. 2020;20(1):242.
World Health Organization. Global database on body Mass Index: BMI classification. 2006. Switzerland: Geneva; 2015.
Delmondes LM, et al. Clinical and sociodemographic aspects of inflammatory bowel Disease patients. Gastroenterol Res. 2015;8(3–4):207–15.
Chen D, et al. Campylobacter colonization, Environmental Enteric Dysfunction, Stunting, and Associated Risk factors among Young Children in Rural Ethiopia: a cross-sectional study from the Campylobacter Genomics and Environmental Enteric Dysfunction (CAGED) project. Front Public Health. 2020;8:615793.
Osuka A, et al. Prognostic impact of fecal pH in critically ill patients. Crit Care. 2012;16(4):R119.
Kvissberg MA, et al. Carbohydrate malabsorption in acutely malnourished children and infants: a systematic review. Nutr Rev. 2016;74(1):48–58.
Goetz DH, et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10(5):1033–43.
Nielsen BS, et al. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel Diseases. Gut. 1996;38(3):414–20.
Nielsen OH, et al. Rectal dialysate and fecal concentrations of neutrophil gelatinase-associated lipocalin, interleukin-8, and Tumor necrosis factor-alpha in ulcerative Colitis. Am J Gastroenterol. 1999;94(10):2923–8.
Mishra J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac Surgery. Lancet. 2005;365(9466):1231–8.
Oikonomou KA, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in inflammatory bowel Disease: association with pathophysiology of inflammation, established markers, and Disease activity. J Gastroenterol. 2012;47(5):519–30.
Yeşil A, et al. Relationship between neutrophil gelatinase-associated lipocalin (NGAL) levels and inflammatory bowel Disease type and activity. Dig Dis Sci. 2013;58(9):2587–93.
Pomare EW, Branch WJ, Cummings JH. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J Clin Invest. 1985;75(5):1448–54.
Eherer AJ, Fordtran JS. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103(2):545–51.
Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology. 1999;116(6):1464–86.
Heyman MB. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118(3):1279–86.
Bilski J et al. The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract Mediators Inflamm, 2017. 2017: p. 9074601.
Lallès JP. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev. 2014;72(2):82–94.
Martínez-Moya P, et al. Exogenous alkaline phosphatase treatment complements endogenous enzyme protection in colonic inflammation and reduces bacterial translocation in rats. Pharmacol Res. 2012;66(2):144–53.
Lukas M, et al. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative Colitis. Inflamm Bowel Dis. 2010;16(7):1180–6.
Peters E, et al. Pharmacokinetic modeling and dose selection in a Randomized, Double-Blind, placebo-controlled trial of a human recombinant alkaline phosphatase in healthy volunteers. Clin Pharmacokinet. 2016;55(10):1227–37.
Peters E, et al. Study protocol for a multicentre randomised controlled trial: safety, tolerability, efficacy and quality of life of a human recombinant alkaline phosphatase in patients with sepsis-associated Acute kidney Injury (STOP-AKI). BMJ Open. 2016;6(9):e012371.
M’Koma AE. Inflammatory bowel Disease: an expanding global health problem. Clin Med Insights Gastroenterol. 2013;6:33–47.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7.
Chassaing B, Gewirtz AT. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol Pathol. 2014;42(1):49–53.
Zietek T, Rath E. Inflammation meets metabolic Disease: gut feeling mediated by GLP-1. Front Immunol. 2016;7:154.
Acknowledgements
This research work is derived from a study funded by the Bill & Melinda Gates Foundation (BMGF). icddr,b acknowledges with gratitude the commitment of BMGF to its research efforts. icddr, b is also grateful to the Governments of Bangladesh and Canada, for providing core/unrestricted support.
Funding
This work was supported by the Bill and Melinda Gates Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization: Ishita Mostafa, Kazi Nazmus Saqeeb. Data curation: Md. Ashraful Alam. Formal analysis: Md. Ashraful Alam, Kazi Nazmus Saqeeb. Investigation: Ishita Mostafa, Kazi Nazmus Saqeeb. Methodology: Md. Amran Gazi, Ishita Mostafa, Shah Mohammad Fahim. Project administration: Ishita Mostafa, Tahmeed Ahmed. Resources: Shah Mohammad Fahim. Software: Md. Ashraful Alam. Supervision: Tahmeed Ahmed. Validation: S. M. Tafsir Hasan, Shah Mohammad Fahim. Visualization: Kazi Nazmus Saqeeb, Md. Ashraful Alam. Writing – original draft: Ishita Mostafa. Writing – review & editing: S. M. Tafsir Hasan, Tahmeed Ahmed.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the ethical guidelines of the Helsinki Declaration and all procedures involving human subjects were approved by the Research Review Committee (RRC) and Ethical Review Committee (ERC) of International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), (PR#18079). Informed written consent was obtained from all the participants, and anonymity and confidentiality were strictly maintained.
Consent for publication
Not Applicable.
Competing interests
The authors have declared that no competing interests exist with respect to the research, authorship, and publication of this article
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mostafa, I., Hasan, S.M.T., Gazi, M.A. et al. Alteration of stool pH and its association with biomarkers of gut enteropathy among slum-dwelling women of reproductive age in Bangladesh. BMC Women's Health 23, 661 (2023). https://doi.org/10.1186/s12905-023-02758-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02758-6